|
|
市場調査レポート
商品コード
1590581
肢帯型筋ジストロフィー(LGMD)市場:競合情勢Limb-Girdle Muscular Dystrophy (LGMD): Competitive Landscape |
||||||
カスタマイズ可能
適宜更新あり
|
|||||||
| 肢帯型筋ジストロフィー(LGMD)市場:競合情勢 |
|
出版日: 2024年10月07日
発行: GlobalData
ページ情報: 英文 65 Pages
納期: 即納可能
|
全表示
- 概要
- 目次
2024年には、16カ国で111,000人以上が肢帯型筋ジストロフィー(LGMD)有病者と診断されると予測されています。
これまでのところ、LGMDの進行を変えることが証明された疾患修飾治療はないため、症状管理が治療の主軸となっています。
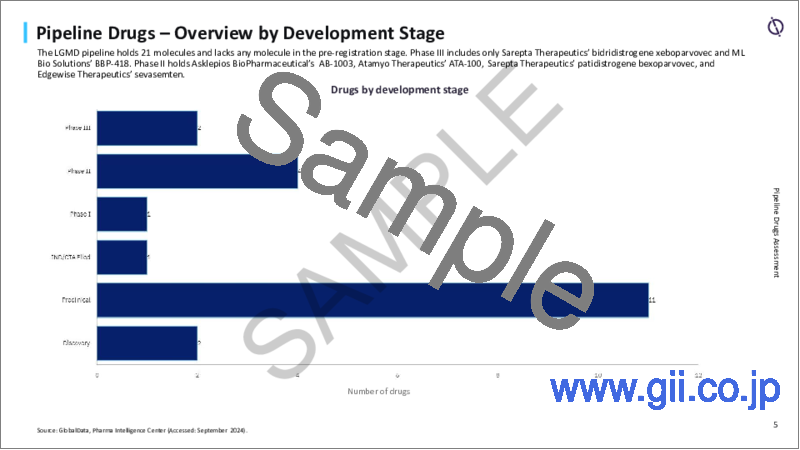
LGMDパイプラインは21分子を保有し、登録前段階のアセットはなく、第III相開発中のアセットはわずか2分子、フェーズIIのアセットは4分子です。
過去10年間、LGMDでは22件の臨床試験が実施されました。最も多くの試験が実施されたのは2016年(4試験)で、次いで2023年、2022年、2019年(各3試験)でした。
過去10年間、北米とアジア太平洋ではパートナーシップが最も頻繁に行われました。一方、欧州では合併とライセンシング契約が同数でした。
当レポートでは、世界の肢帯型筋ジストロフィー(LGMD)市場について調査し、疾患の概要とともに、治験動向、パイプライン概要、将来の見通しなどを提供しています。
目次
第1章 序文
第2章 主な調査結果
第3章 病気の情勢
- 疾患の概要
- 疫学概要
- 治療の概要
第4章 上市済み薬剤の評価
- 主な市販薬
- 作用機序別概要
- 分子タイプ別概要
- 製品プロファイルと売上予測
第5章 価格設定と償還評価
第6章 パイプライン薬剤の評価
第7章 臨床試験の評価
- 歴史的概要
- 相別概要
- ステータス別概要
- 進行中および計画中の試験の相別概要
- 仮想コンポーネントの試験
- 地域の試験概要
- 地域別の単一国および多国間試験
- 相別のスポンサー上位20社
- ステータス別のスポンサー上位20社
- エンドポイントステータス別概要
- 人種と民族別概要
- 登録データ
- 治験実施施設主要20カ国
- 世界の主要20施設
- 実現可能性分析- 地理的概要
- 実現可能性分析- ベンチマークモデル
第8章 取引情勢
第9章 商業的評価
- 主要な市場参入企業
第10章 将来の市場促進要因
第11章 付録
This reports provides a data-driven overview of the current and future competitive landscape in Limb-Girdle Muscular Dystrophy therapeutics.
In 2024, more than 111,000 diagnosed prevalent cases of LGMD are anticipated in the 16 countries covered in GlobalData's epidemiology forecast.
No disease-modifying treatment has proven to alter LGMD progression so far, and thus symptom management remains the mainstay of care.
The LGMD pipeline holds 21 molecules with no assets in the pre-registration stage, only two molecules in Phase III development, and four assets in Phase II.
Over the past decade, 22 clinical trials have been conducted in LGMD. The largest number of studies was conducted in 2016 (four trials) followed by 2023, 2022, and 2019 (three trials each).
During the past decade, partnerships were the most frequent type of deal in North America and Asia-Pacific. Meanwhile, in Europe, mergers and licensing agreements contributed to an equal number of deals.
Scope
GlobalData's Limb-Girdle Muscular Dystrophy: Competitive Landscape combines data from the Pharma Intelligence Center with in-house analyst expertise to provide a competitive assessment of the disease marketplace.
Components of the report include -
- Disease Landscape
- Disease Overview
- Epidemiology Overview
- Treatment Overview
- Marketed Products Assessment
- Breakdown by Mechanism of Action, Route of Administration
- Product Profiles with Sales Forecast
- Pricing and Reimbursement Assessment
- Annual Therapy Cost
- Time to Pricing and Time to Reimbursement
- Pipeline Assessment
- Breakdown by Development Stage, Mechanism of Action, Molecule Type, Route of Administration
- Product Profiles with Sales Forecast
- Late-to-mid-stage Pipeline Drugs
- Phase Transition Success Rate and Likelihood of Approval
- Clinical Trials Assessment
- Breakdown of Trials by Phase, Status, Virtual Components, Sponsors, Geography, and Endpoint Status
- Enrolment Analytics, Site Analytics, Feasibility Analysis
- Deals Landscape
- Mergers, Acquisitions, and Strategic Alliances by Region
- Overview of Recent Deals
- Commercial Assessment
- Key Market Players
- Future Market Catalysts
Reasons to Buy
- Develop and design your in-licensing and out-licensing strategies through a review of pipeline products and technologies, and by identifying the companies with the most robust pipeline.
- Develop business strategies by understanding the trends shaping and driving the Limb-Girdle Muscular Dystrophy market.
- Drive revenues by understanding the key trends, innovative products and technologies, and companies likely to impact the global Limb-Girdle Muscular Dystrophy market in the future.
- Formulate effective sales and marketing strategies by understanding the competitive landscape and analyzing the performance of various competitors.
- Identify emerging players with potentially strong product portfolios and create effective counter-strategies to gain a competitive advantage.
- Organize your sales and marketing efforts by identifying the market categories that present the maximum opportunities for consolidations, investments, and strategic partnerships.
Table of Contents
Table of Contents
1 Preface
- 1.1 Contents
- 1.2 Report Scope
- 1.3 List of Tables and Figures
- 1.4 Abbreviations
2 Key Findings
3 Disease Landscape
- 3.1 Disease Overview
- 3.2 Epidemiology Overview
- 3.3 Treatment Overview
4 Marketed Drugs Assessment
- 4.1 Leading Marketed Drugs
- 4.2 Overview by Mechanism of Action
- 4.3 Overview by Molecule Type
- 4.4 Product Profiles and Sales Forecast
5 Pricing and Reimbursement Assessment
- 5.1 Annual Cost of Therapy
- 5.2 Time to Pricing and Reimbursement
6 Pipeline Drugs Assessment
- 6.1 Mid-to-late-stage Pipeline Drugs
- 6.2 Overview by Development Stage
- 6.3 Overview by Mechanism of Action
- 6.4 Overview by Molecule Type
- 6.5 Drug Specific Phase Transition Success Rate (PTSR) and Likelihood of Approval (LoA)
- 6.6 Therapy Area and Indication-specific PTSR and LoA
7 Clinical Trials Assessment
- 7.1 Historical Overview
- 7.2 Overview by Phase
- 7.3 Overview by Status
- 7.4 Overview by Phase for Ongoing and Planned Trials
- 7.5 Trials with Virtual Components
- 7.6 Overview of Trials by Geography
- 7.7 Single-Country and Multinational Trials by Region
- 7.8 Top 20 Sponsors with Breakdown by Phase
- 7.9 Top 20 Sponsors with Breakdown by Status
- 7.10 Overview by Endpoint Status
- 7.11 Overview by Race and Ethnicity
- 7.12 Enrollment Data
- 7.13 Top 20 countries for Trial Sites
- 7.14 Top 20 Sites Globally
- 7.15 Feasibility Analysis - Geographic Overview
- 7.16 Feasibility Analysis - Benchmark Models
8 Deals Landscape
- 8.1 Mergers, Acquisitions, and Strategic Alliances by Region
- 8.2 Recent Mergers, Acquisitions, and Strategic Alliances
9 Commercial Assessment
- 9.1 Key Market Players
10 Future Market Catalysts
11 Appendix
- 11.1 Methodology
- 11.2 Methodology - Sales Forecast
- 11.3 Methodology - Pricing and Reimbursement
- 11.4 Methodology - PTSR and LoA Analysis
- 11.5 About the Authors
- 11.6 Contact Us
- 11.7 Disclaimer