|
|
市場調査レポート
商品コード
1677773
臨床試験インフラの地域市場分析:中東・北アフリカClinical Trial Infrastructure Regional Analysis Market: Middle East and North Africa |
||||||
|
|||||||
| 臨床試験インフラの地域市場分析:中東・北アフリカ |
|
出版日: 2025年03月05日
発行: BCC Research
ページ情報: 英文 81 Pages
納期: 即納可能
|
全表示
- 概要
- 図表
- 目次
当レポートでは、中東・北アフリカ地域の臨床試験インフラの市場を調査し、市場概要、市場影響因子および市場機会の分析、市場規模の推移・予測、主要国別の詳細分析、競合情勢などをまとめています。
目次
第1章 エグゼクティブサマリー
- 市場見通し
- 調査範囲
- この調査を行う理由
- 市場サマリー
第2章 中東・北アフリカ地域の臨床試験産業の展望
- 世界の臨床試験市場:概要
- 中東・北アフリカ地域のシェア
- 進化する中東・北アフリカ地域の臨床試験業界
- インフラと資源:新興市場におけるギャップを埋める
- 患者募集の可能性:多様な患者層と高い募集率
- コスト効率
- 規制環境:調和と地域適応
- 政府の支援と投資
- 地域政策の影響:臨床研究の奨励
- コラボレーションにより地域ネットワークが拡大
- 新たな動向:精密医療と希少疾患への焦点
- 腫瘍と希少疾患における需要の高まり
- 技術の進歩:臨床業務のデジタル化
- 中東・北アフリカ地域の臨床試験の情勢への理解
第3章 市場力学
- 市場力学
- 市場促進要因
- 試験の実現可能性を推進する規制の枠組み
- 患者数の増加による採用と多様性の強化
- 技術の進歩による業務の効率化
- コラボレーション
- 個別化医療の試験設計
- 市場の課題
- 複雑な規制状況
- 患者の募集と維持の限界
- 不十分なインフラとサイト容量
- デジタル技術の導入が限定的
- 主要治療領域をめぐる競合
- 市場機会
- 規制改革により承認の迅速化が可能に
- 腫瘍と希少疾患への新たな注目
- 官民連携による研究能力の強化
- 精密医療とゲノミクスへの焦点
第4章 市場セグメンテーション分析
- セグメンテーションの内訳
- 市場分析:スポンサータイプ別
- 市場分析:フェーズ別
- 市場分析:サービスタイプ別
- 市場分析:研究タイプ別
- 市場分析:治療領域別
第5章 新興市場
- エジプト
- 概要
- 臨床試験の情勢分析
- 観察
- BCCによる推奨事項
- サウジアラビア(KSA)
- 概要
- 臨床試験の情勢分析
- 観察
- BCCによる推奨事項
- レバノン
- 概要
- 臨床試験の情勢分析
- 観察
- BCCによる推奨事項
- UAE
- 概要
- 臨床試験の情勢分析
- 観察
- BCCによる推奨事項
- イラン
- 概要
- 臨床試験の情勢分析
- 観察
- BCCによる推奨事項
第6章 ロシア・ウクライナ戦争の影響分析
- 概要
- サプライチェーンの混乱
- 規制上の課題
- 患者の募集と維持
- 地政学的リスク
第7章 競合情勢
- 概要
- 競合情勢
- 国際的CROと製薬会社
- 現地CROおよび機関
- 学術研究機関
- 臨床試験製品・サービスのマッピング
- 中東・北アフリカ地域における臨床試験の最近の動向
- BCCによる総論
第8章 付録
List of Tables
- Summary Table : MENA Market for Clinical Trials, by Country, Through 2029
- Table 1 : MENA Clinical Trial Analysis, by Study Status, Through 2024
- Table 2 : Comparison of MENA Clinical Trial Regulations, Through 2024
- Table 3 : Comparison of MENA Clinical Trial Regulations, Through 2024
- Table 4 : MENA Clinical Trials Analysis, by Study Status, Phase, Type, Results and Sponsor Type, Through 2024
- Table 5 : Regulatory Bodies for Conducting Clinical Trials in the MENA Region
- Table 6 : Recent Technological Advances in the MENA Clinical Trial Market, 2023 and 2024
- Table 7 : Recent Collaborations in the MENA Clinical Trial Market, 2024
- Table 8 : Recent Developments in Clinical Trial Infrastructure in MENA, 2023 and 2024
- Table 9 : Leading Causes of Death in MENA, by Country, 2021
- Table 10 : Recent Developments in the MENA Region, 2023 and 2024
- Table 11 : Recent Collaborations in the MENA Region, 2024
- Table 12 : Leading MENA Countries for Clinical Trials, by Sponsor Type, Through 2024
- Table 13 : MENA Market for Clinical Trials, by Sponsor Type, Through 2029
- Table 14 : Leading MENA Countries for Clinical Trials, by Phase, Through 2024
- Table 15 : MENA Market for Clinical Trials, by Phase, Through 2029
- Table 16 : MENA Market for Clinical Trials, by Service Type, Through 2029
- Table 17 : Leading MENA Countries for Clinical Trials, by Study Type, Through 2024
- Table 18 : MENA Market for Clinical Trials, by Study Type, Through 2029
- Table 19 : MENA Market for Clinical Trials, by Therapeutic Area, Through 2029
- Table 20 : Egyptian Clinical Trials Analysis, by Study Status, Phase, Type, Results and Sponsor Type, Through 2024
- Table 21 : Companies and Research Institutes for Clinical Trials in Egypt
- Table 22 : Egyptian Market for Clinical Trials, by Value, Through 2029
- Table 23 : Saudi Arabian Clinical Trials Analysis, by Study Status, Phase, Type, Results and Sponsor Type, Through 2024
- Table 24 : Companies and Research Institutes for Clinical Trials in the KSA
- Table 25 : Saudi Arabian Market for Clinical Trials, by Value, Through 2029
- Table 26 : Lebanese Clinical Trials Analysis, by Study Status, Phase, Type, Results and Sponsor Type, Through 2024
- Table 27 : Companies and Research Institutes for Clinical Trials in Lebanon
- Table 28 : Lebanese Market for Clinical Trials, by Value, Through 2029
- Table 29 : UAE Clinical Trials Analysis, by Study Status, Phase, Type, Results and Sponsor Type, Through 2024
- Table 30 : Companies and Research Institutes for Clinical Trials in UAE
- Table 31 : UAE Market for Clinical Trials, by Value, Through 2029
- Table 32 : Iranian Clinical Trials Analysis, by Study Status, Phase, Type, Results and Sponsor Type, Through 2024
- Table 33 : Companies and Research Institutes for Clinical Trials in Iran
- Table 34 : Iranian Market for Clinical Trials, by Value, Through 2029
- Table 35 : MENA Clinical Trials Product/Services Mapping Analysis
- Table 36 : Recent Developments in the MENA Clinical Trial Market, 2020-2024
- Table 37 : Information Sources in this Report
- Table 38 : Abbreviations Used in this Report
List of Figures
- Summary Figure : MENA Market for Clinical Trials, by Country, 2023-2029
- Figure 1 : Global Market for Clinical Trials, by Value, 2023-2029
- Figure 2 : Share of the Global Clinical Trials Market Value, by Region, 2023
- Figure 3 : Key Considerations for MENA Clinical Trials
- Figure 4 : Market Dynamics of Clinical Trial Infrastructure in the MENA Region
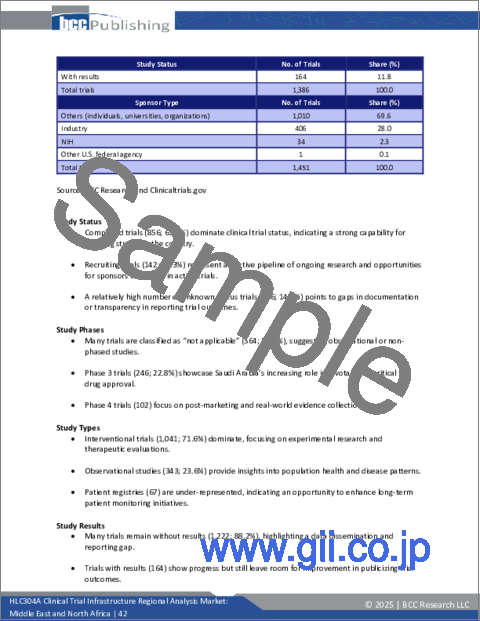
- Figure 5 : Share of Deaths, by Broad Cause, by Country, 2021
- Figure 6 : Share of MENA Market for Clinical Trials, by Sponsor Type, 2023
- Figure 7 : Share of MENA Market for Clinical Trials, by Phase, 2023
- Figure 8 : Share of MENA Market for Clinical Trials, by Service Type, 2023
- Figure 9 : Share of MENA Market for Clinical Trials, by Study Type, 2023
- Figure 10 : Share of MENA Market for Clinical Trials, by Therapeutic Area, 2023
- Figure 11 : Egyptian Market for Clinical Trials, by Value, 2023-2029
- Figure 12 : BCC Recommendations for the Egyptian Market for Clinical Trials
- Figure 13 : Saudi Arabian Market for Clinical Trials, by Value, 2023-2029
- Figure 14 : BCC Recommendations for Saudi Arabian Clinical Trials
- Figure 15 : Lebanese Market for Clinical Trials, by Value, 2023-2029
- Figure 16 : BCC Recommendations for Lebanese Clinical Trials
- Figure 17 : UAE Market for Clinical Trials, by Value, 2023-2029
- Figure 18 : BCC Recommendations for the UAE Clinical Trials
- Figure 19 : Iranian Market for Clinical Trials, by Value, 2023-2029
- Figure 20 : BCC Recommendations for the Iranian Clinical Trials
- Figure 21 : Share of MENA Market for Clinical Trials, by Stakeholder, 2024
- Figure 22 : Recommendations for Companies Looking to Enter the MENA Clinical Trial Market
- Figure 23 : Methodology Used in the MENA Clinical Trial Market
This report provides an overview of the clinical trial infrastructure markets in the Middle East and North Africa (MENA) region. It includes a detailed examination of the market dynamics and an understanding of the MENA clinical trial landscape by study status, phase, type, results and sponsor type.
Report Scope
This report analyzes the Middle East and North Africa (MENA) clinical trial markets, using 2023 as a benchmark year, and offers projections for the forecast period from 2024 to 2029, with estimates of compound annual growth rates (CAGR). The report covers many aspects of the market, including clinical advances, economic factors and business considerations, and outlines the market forces impacting the industry and its phases, study types and therapeutic areas. The report segments the clinical trial market into services, solutions and supplies, and examines the regional dynamics influencing the market.
This report excluded Turkey and Israel from its scope because their clinical trial markets are significantly more advanced than other countries in the MENA region. Including these markets would have skewed the analysis and not accurately reflected the opportunities and challenges the rest of the region faces. This approach ensures a focused examination of MENA's emerging clinical trial landscape, which may be more relevant for companies seeking to expand into less mature markets.
Report Includes
- Analysis of the Middle East and Northern Africa's (MENA) regional markets for clinical trial infrastructure
- Analyses of the market trends, with revenue data for 2023, estimates for 2024, and projected CAGRs through 2029
- Estimate of the current market size and revenue prospects, accompanied by a market share analysis by sponsor type, clinical trial phase, service type, study type, therapeutic area, and country
- An understanding of the opportunities and progressions in clinical trial studies by some select countries in emerging MENA markets
- Facts and figures pertaining to the current market dynamics, advancements in technology, regulations and prospects
- Insights of the ripple effects of the Ukraine-Russia war on clinical trial operations, including supply chain disruptions, regulatory challenges, and patient recruitment and retention
- Analysis of the industry structure, including stakeholders' market shares, clinical trial product/services mapping, and recent key developments
Table of Contents
Chapter 1 Executive Summary
- Market Outlook
- Scope of Report
- Reasons for Doing this Study
- Market Summary
Chapter 2 MENA Clinical Trial Industry Outlook
- Global Clinical Trial Market Overview
- MENA Share
- Evolving MENA Clinical Trial Industry
- Infrastructure and Resources: Bridging Gaps in Emerging Markets
- Patient Recruitment Potential: Diverse Populations and High Recruitment Rates
- Cost-Effectiveness
- Regulatory Environment: Harmonization and Local Adaptations
- Government Support and Investments
- Impact of Regional Policies: Incentivizing Clinical Research
- Collaborations Expand Regional Networks
- Emerging Trends: Focus on Precision Medicine and Rare Diseases
- Growing Demand in Oncology and Rare Diseases
- Technological Advances: Digitalizing Clinical Operations
- Understanding the MENA Clinical Trial Landscape
Chapter 3 Market Dynamics
- Market Dynamics
- Market Drivers
- Regulatory Frameworks Driving Trial Feasibility
- Growing Patient Population Enhancing Recruitment and Diversity
- Technological Advances Streamlining Operations
- Collaborations
- Personalized Medicine Shaping Trial Design
- Market Challenges
- Complex Regulatory Landscape
- Limited Patient Recruitment and Retention
- Insufficient Infrastructure and Site Capacity
- Limited Adoption of Digital Technologies
- Competition for Key Therapeutic Areas
- Market Opportunities
- Regulatory Reforms Enabling Faster Approvals
- Emerging Focus on Oncology and Rare Diseases
- Public-Private Partnerships Boosting Research Capacity
- Focus on Precision Medicine and Genomics
Chapter 4 Market Segmentation Analysis
- Segmentation Breakdown
- Market Analysis, by Sponsor Type
- Market Analysis, by Phase
- Market Analysis, by Service Type
- Market Analysis, by Study Type
- Market Analysis, by Therapeutic Area
Chapter 5 Emerging Markets
- Egypt
- Overview
- Clinical Trial Landscape Analysis
- Observations
- BCC Recommendations
- Kingdom of Saudi Arabia (KSA)
- Overview
- Clinical Trial Landscape Analysis
- Observations
- BCC Recommendations
- Lebanon
- Overview
- Clinical Trial Landscape Analysis
- Observations
- BCC Recommendations
- United Arab Emirates
- Overview
- Clinical Trial Landscape Analysis
- Observations
- BCC Recommendations
- Iran
- Overview
- Clinical Trial Landscape Analysis
- Observations
- BCC Recommendations
Chapter 6 Russia-Ukraine War Impact Analysis
- Overview
- Supply Chain Disruptions
- Regulatory Challenges
- Patient Recruitment and Retention
- Geopolitical Risks
Chapter 7 Competitive Landscape
- Overview
- Competitive Landscape
- International CROs and Pharmaceutical Companies
- Local CROs and Institutions
- Academic and Research Institutions
- Mapping of Clinical Trial Product Services
- Recent Developments in Clinical Trials in the MENA Region
- Concluding Remarks from BCC
Chapter 8 Appendix
- Methodology
- Research Steps
- Sources
- Abbreviations