|
|
市場調査レポート
商品コード
1402478
中南米の臨床試験市場の2028年までの予測-地域別分析-試験設計、フェーズ、適応症別South & Central America Clinical Trials Market Forecast to 2028 - Regional Analysis - by Study Design, Phase, and Indication |
||||||
| 中南米の臨床試験市場の2028年までの予測-地域別分析-試験設計、フェーズ、適応症別 |
|
出版日: 2023年10月26日
発行: The Insight Partners
ページ情報: 英文 125 Pages
納期: 即納可能
|
- 全表示
- 概要
- 図表
- 目次
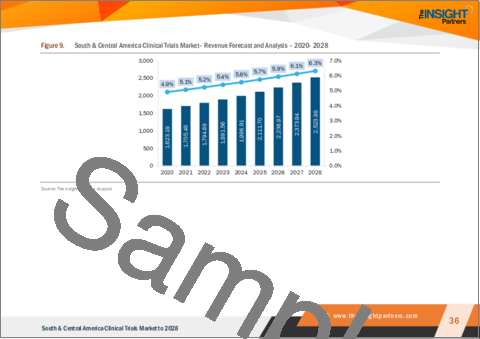
中南米の臨床試験市場は、2022年の17億9,469万米ドルから2028年には25億2,398万米ドルに成長すると予測されています。2022年から2028年までのCAGRは5.8%と推定されます。
分散型臨床試験とハイブリッド型臨床試験の採用が中南米の臨床試験市場を後押し
分散型臨床試験(DCT)では、病院を拠点とする臨床試験施設への患者の物理的なアクセスが減少または排除されます。DCTでは、患者の臨床研究へのアクセス、遠隔データ収集とモニタリング、治験責任医師と参加者間のコミュニケーションを可能にするためにデジタル技術が使用されます。ハイブリッド臨床試験のアプローチは、在宅と現場での活動を組み合わせることで、最高の患者体験をもたらし、複雑なプロトコールレジメニーを満たし、様々な治療領域や臨床試験フェーズのジャーニーで支持を集めています。当初は、患者のプライバシー、データセキュリティ、規制上の障壁、複雑なプロトコール制度などの課題により、DCTの採用は影響を受けていました。しかし、COVID-19の大流行により、臨床試験のスポンサーは医薬品開発のために分散型臨床技術やハイブリッド臨床技術を採用しました。従来の臨床試験は直接訪問する必要があったため、継続できなかったのです。出張が制限されるため、データを収集し、臨床試験を継続する唯一の方法は、遠隔地から働き、他の方法よりもはるかに早く技術を導入することでした。そのため、分散化によって治験へのアクセスが広がり、より多くの、そして潜在的に多様な患者にアプローチできるようになります。
さらに、ハイブリッド臨床試験により、スポンサーは戦略的に分散型臨床試験(DCT)の要素を試験設計に組み込むことができます。このような臨床試験モデルはこれまでにない柔軟性を提供するため、ハイブリッド臨床試験に関心を持つ企業が増え、その結果、業界の情勢が再定義されつつあります。ObvioHealthによると、FDAは2023年にDCT法の使用をサポートするプロトコルを発表する予定であり、将来の臨床研究に対するこれらの要素への信頼を鼓舞しています。このように、従来の臨床試験手法よりも分散型臨床試験やハイブリッド臨床試験の利用に注目が集まっていることから、予測期間中、臨床試験市場に有利な機会がもたらされると期待されています。
中南米の臨床試験市場概要
中南米の臨床試験市場は、ブラジル、アルゼンチン、その他の中南米に区分されます。中南米は臨床試験市場において重要な位置を占めており、市場推定・予測期間において堅調な成長率を記録すると予測されます。成長の背景には、臨床試験の増加、市場参入企業による臨床試験プロセスの主要な革新、臨床試験を支援する政府の取り組みなどがあり、市場の成長を後押ししています。
中南米の臨床試験市場の収益と2028年までの予測(金額)
中南米の臨床試験市場のセグメンテーション
中南米の臨床試験市場は、フェーズ、試験設計、適応症、国別に区分されます。
フェーズに基づき、中南米の臨床試験市場はフェーズI、フェーズII、フェーズIII、フェーズIVに区分されます。第III相セグメントは2022年に南米の臨床試験市場で最大のシェアを記録しました。
研究設計に基づいて、中南米の臨床試験市場は介入、観察、拡大アクセスに区分されます。2022年の中南米の臨床試験市場シェアは介入型が最大でした。
適応症に基づき、中南米の臨床試験市場は自己免疫/炎症、疼痛管理、腫瘍、中枢神経系疾患、糖尿病、肥満、心血管、その他に区分されます。2022年の中南米の臨床試験市場シェアは、がん領域が最大でした。
国別に見ると、中南米の臨床試験市場はブラジル、アルゼンチン、その他の南米に分類されます。その他の南米が2022年の中南米の臨床試験市場シェアを独占しました。
Charles River Laboratories International Inc、ICON Plc、IQVIA Holdings Inc、Laboratory Corp of America Holdings、Parexel International Corp、SGS SA、Syneos Health Inc、Thermo Fisher Scientific Inc.は、中南米の臨床試験市場で事業を展開する大手企業です。
目次
第1章 イントロダクション
第2章 キーポイント
第3章 調査手法
- カバー範囲
- 2次調査
- 1次調査
第4章 中南米の臨床試験市場:市場情勢
- イントロダクション
- PEST分析
- 中南米のPEST分析
- 専門家の見解
第5章 中南米の臨床試験市場:主要市場力学
- 市場の促進要因
- 臨床試験の採用とアウトソーシングの増加
- 製薬業界の繁栄と研究開発活動の活発化
- 市場抑制要因
- 高価で時間のかかるプロセス
- 市場機会
- 分散型臨床試験とハイブリッド臨床試験の採用
- 今後の動向
- AI主導治験
- 影響分析
第6章 臨床試験市場:中南米分析
- 中南米の臨床試験市場の収益予測と分析
第7章 中南米の臨床試験市場の収益と2028年までの予測:試験設計別
- イントロダクション
- 中南米の臨床試験市場:試験設計別、2022年と2028年(%)
- 介入試験
- 観察研究
- 拡大アクセス
第8章 中南米の臨床試験市場の収益と2028年までの予測:フェーズ別
- イントロダクション
- 中南米の臨床試験市場:フェーズ別、2022年と2028年(%)
- フェーズIII
- フェーズII
- フェーズIV
- フェーズi
第9章 中南米の臨床試験市場の収益と2028年までの予測:適応症別
- イントロダクション
- 中南米の臨床試験市場:適応症別、2022年と2028年(%)
- 自己免疫/炎症
- 疼痛管理
- 腫瘍学
- 中枢神経系疾患
- 糖尿病
- 肥満症
- 心血管
- その他
第10章 中南米の臨床試験市場:2028年までの収益と予測:国別分析
第11章 業界情勢
- イントロダクション
- 市場参入企業の成長戦略(%)
- 有機的展開
- 概要
- 無機的展開
- 概要
第12章 企業プロファイル
- IQVIA Holdings Inc
- Parexel International Corp
- Charles River Laboratories International Inc
- ICON Plc
- SGS SA
- Syneos Health Inc
- Thermo Fisher Scientific Inc
- Laboratory Corp of America Holdings
第13章 付録
List Of Tables
- Table 1. Brazil Clinical Trials Market, by Phase - Revenue and Forecast to 2028 (US$ Million)
- Table 2. Brazil Clinical Trials Market, by Study Design - Revenue and Forecast to 2028 (US$ Million)
- Table 3. Brazil Clinical Trials Market, by Indication - Revenue and Forecast to 2028 (US$ Million)
- Table 4. Argentina Clinical Trials Market, by Phase - Revenue and Forecast to 2028 (US$ Million)
- Table 5. Argentina Clinical Trials Market, by Study Design - Revenue and Forecast to 2028 (US$ Million)
- Table 6. Argentina Clinical Trials Market, by Indication - Revenue and Forecast to 2028 (US$ Million)
- Table 7. Rest of South & Central America Clinical Trials Market, by Phase - Revenue and Forecast to 2028 (US$ Million)
- Table 8. Rest of South & Central America Clinical Trials Market, by Study Design - Revenue and Forecast to 2028 (US$ Million)
- Table 9. Rest of South & Central America Clinical Trials Market, by Indication - Revenue and Forecast to 2028 (US$ Million)
- Table 10. Organic Developments Done by Companies
- Table 11. Inorganic Developments Done by Companies
- Table 12. Glossary of Terms
List Of Figures
- Figure 1. South & Central America Clinical Trials Market Segmentation
- Figure 2. South & Central America Clinical Trials Market, By Region
- Figure 3. South & Central America Clinical Trials Market Overview
- Figure 4. Phase III Segment Held Largest Share of Phase Segment in South & Central America Clinical Trials Market
- Figure 5. Argentina is Expected to Show Remarkable Growth During the Forecast Period
- Figure 6. South And Central America: PEST Analysis
- Figure 7. South & Central America Experts Opinion
- Figure 8. Impact Analysis
- Figure 9. South & Central America Clinical Trials Market- Revenue Forecast and Analysis - 2020- 2028
- Figure 10. South & Central America Clinical Trials Market Revenue Share, by Study Design (2022 and 2028)
- Figure 11. Interventional: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- Figure 12. Observational: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- Figure 13. Expanded Access: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- Figure 14. South & Central America Clinical Trials Market, by Phase 2022 and 2028 (%)
- Figure 15. Phase III: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- Figure 16. Phase II: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- Figure 17. Phase IV: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- Figure 18. Phase I: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- Figure 19. South & Central America Clinical Trials Market Revenue Share, by Indication (2022 and 2028)
- Figure 20. Autoimmune/Inflammation: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- Figure 21. Pain Management: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- Figure 22. Oncology: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- Figure 23. CNS Condition: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- Figure 24. Diabetes: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- Figure 25. Obesity: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- Figure 26. Cardiovascular: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- Figure 27. Other: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- Figure 28. South & Central America: Clinical Trials Market, by Key Country - Revenue (2022) (US$ Million)
- Figure 29. South & Central America: Clinical Trials Market, by Country, 2022 & 2028 (%)
- Figure 30. Brazil: Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- Figure 31. Argentina: Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- Figure 32. Rest of South & Central America: Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- Figure 33. Growth Strategies Done by the Companies in the Market, (%)
The South & Central America clinical trials market is expected to grow from US$ 1,794.69 million in 2022 to US$ 2,523.98 million by 2028. It is estimated to grow at a CAGR of 5.8% from 2022 to 2028.
Adoption of Decentralized Clinical Trials and Hybrid Clinical Trials Fuels South & Central America Clinical Trials Market
In decentralized clinical trials (DCT), patients' physical access to hospital-based trial sites is reduced or eliminated. In DCTs, digital technologies are used to enable access of patients to clinical research, remote data collection and monitoring, and communication between investigators and participants. A hybrid clinical trial approach combines home-based and on-site activities, bringing the best patient experience and meeting complex protocol regimes, gaining traction across various therapeutic areas and trial phase journeys. Initially, due to challenges such as patient privacy, data security, regulatory barriers, and complex protocol regimes, the adoption of DCT was affected. However, due to the COVID-19 pandemic, the sponsors of clinical trials adopted decentralized and hybrid clinical techniques for developing drugs. They could not continue with traditional trials as they required in-person visits. Due to the restrictions for travel, the only way to gather data and keep trials going was to work remotely and adopt technology much faster than they would have otherwise. Therefore, decentralization broadens trial access to reach a larger number and potentially a more diverse pool of patients.
Further, hybrid clinical trials allow sponsors to strategically incorporate decentralized clinical trial (DCT) elements into study designs. These trial models offer unprecedented flexibility, so more companies are interested in hybrid trials, and the resulting growth is redefining the industry landscape. According to ObvioHealth, the FDA plans to unveil protocols in 2023 to support the use of DCT methods, inspiring confidence in these components for future clinical research. Thus, the increasing focus on using decentralized and hybrid clinical trials over traditional clinical trial methods is expected to provide lucrative opportunities for the clinical trials market during the forecast period.
South & Central America Clinical Trials Market Overview
The South & Central America clinical trials market is segmented into Brazil, Argentina, and the Rest of South & Central America. South & Central America occupies a significant position in the clinical trials market and is estimated to register a robust growth rate over the forecast period. The growth is due to growing clinical trials, key innovation by market players of clinical trial process, and government initiatives to support the clinical trial fuels the market growth.
South & Central America Clinical Trials Market Revenue and Forecast to 2028 (US$ Million)
South & Central America Clinical Trials Market Segmentation
The South & Central America clinical trials market is segmented into phase, study design, indication, and country.
Based on phase, the South & Central America clinical trials market is segmented into phase I, phase II, phase III, and phase IV. The phase III segment registered the largest South & Central America clinical trials market share in 2022.
Based on study design, the South & Central America clinical trials market is segmented into interventional, observational, and expanded access. The interventional segment held the largest South & Central America clinical trials market share in 2022.
Based on indication, the South & Central America clinical trials market is segmented into autoimmune/inflammation, pain management, oncology, CNS condition, diabetes, obesity, cardiovascular, and others. The oncology segment held the largest South & Central America clinical trials market share in 2022.
Based on country, the South & Central America clinical trials market has been categorized into Brazil, Argentina, and the Rest of South & Central America. The Rest of South & Central America dominated the South & Central America clinical trials market share in 2022.
Charles River Laboratories International Inc, ICON Plc, IQVIA Holdings Inc, Laboratory Corp of America Holdings, Parexel International Corp, SGS SA, Syneos Health Inc, and Thermo Fisher Scientific Inc. are some of the leading companies operating in the South & Central America clinical trials market.
Reasons to Buy:
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players, and segments in the South & Central America clinical trials market.
- Highlights key business priorities in order to assist companies to realign their business strategies
- The key findings and recommendations highlight crucial progressive industry trends in the South & Central America clinical trials market, thereby allowing players across the value chain to develop effective long-term strategies
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets
- Scrutinize in-depth South & Central America market trends and outlook coupled with the factors driving the clinical trials market, as well as those hindering it
- Enhance the decision-making process by understanding the strategies that underpin commercial interest with respect to client products, segmentation, pricing, and distribution
Table Of Contents
1. Introduction
- 1.1 Scope of the Study
- 1.2 The Insight Partners Research Report Guidance
- 1.3 Market Segmentation
- 1.3.1 South & Central America Clinical Trials Market - By Phase
- 1.3.2 South & Central America Clinical Trials Market - By Study Design
- 1.3.3 South & Central America Clinical Trials Market - By Indication
- 1.3.4 South & Central America Clinical Trials Market - By Country
2. Key Takeaways
3. Research Methodology
- 3.1 Coverage
- 3.2 Secondary Research
- 3.3 Primary Research
4. South & Central America Clinical Trials Market - Market Landscape
- 4.1 Overview
- 4.2 PEST Analysis
- 4.2.1 South And Central America PEST Analysis
- 4.3 Experts Opinion
5. South & Central America Clinical Trials Market - Key Market Dynamics
- 5.1 Market Drivers
- 5.1.1 Increasing Adoption and Outsourcing of Clinical Trials
- 5.1.2 Flourishing Pharmaceutical Industry and Increasing R&D Activities in Pharmaceutical Industry
- 5.2 Market Restraints
- 5.2.1 Expensive and Time-Consuming Process
- 5.3 Market Opportunities
- 5.3.1 Adoption of Decentralized Clinical Trials and Hybrid Clinical Trials
- 5.4 Future Trends
- 5.4.1 AI-Driven Clinical Trials
- 5.5 Impact Analysis
6. Clinical Trials Market - South & Central America Analysis
- 6.1 South & Central America Clinical Trials Market Revenue Forecast and Analysis
7. South & Central America Clinical Trials Market Revenue and Forecast To 2028 - by Study Design
- 7.1 Overview
- 7.2 South & Central America Clinical Trials Market Revenue Share, by Study Design (2022 and 2028)
- 7.3 Interventional
- 7.3.1 Overview
- 7.3.2 Interventional: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- 7.4 Observational
- 7.4.1 Overview
- 7.4.2 Observational: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- 7.5 Expanded Access
- 7.5.1 Overview
- 7.5.2 Expanded Access: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
8. South & Central America Clinical Trials Market - By Phase
- 8.1 Overview
- 8.2 South & Central America Clinical Trials Market, by Phase, 2022 and 2028 (%)
- 8.3 Phase III
- 8.3.1 Overview
- 8.3.2 Phase III: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- 8.4 Phase II
- 8.4.1 Overview
- 8.4.2 Phase II: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- 8.5 Phase IV
- 8.5.1 Overview
- 8.5.2 Phase IV: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- 8.6 Phase I
- 8.6.1 Overview
- 8.6.2 Phase I: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
9. South & Central America Clinical Trials Market Revenue and Forecast To 2028 - Indication
- 9.1 Overview
- 9.2 South & Central America Clinical Trials Market Revenue Share, by Indication (2022 and 2028)
- 9.3 Autoimmune/Inflammation
- 9.3.1 Overview
- 9.3.2 Autoimmune/Inflammation: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- 9.4 Pain Management
- 9.4.1 Overview
- 9.4.2 Pain Management: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- 9.5 Oncology
- 9.5.1 Overview
- 9.5.2 Oncology: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- 9.6 CNS Condition
- 9.6.1 Overview
- 9.6.2 CNS Condition: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- 9.7 Diabetes
- 9.7.1 Overview
- 9.7.2 Diabetes: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- 9.8 Obesity
- 9.8.1 Overview
- 9.8.2 Obesity: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- 9.9 Cardiovascular
- 9.9.1 Overview
- 9.9.2 Cardiovascular: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- 9.10 Other
- 9.10.1 Overview
- 9.10.2 Other: South & Central America Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
10. South & Central America Clinical Trials Market - Revenue and Forecast to 2028 - Country Analysis
- 10.1 South & Central America Clinical Trials Market
- 10.1.1 Overview
- 10.1.2 South & Central America: Clinical Trials Market, by Country, 2022 & 2028 (%)
- 10.1.2.1 Brazil: Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- 10.1.2.1.1 Overview
- 10.1.2.1.2 Brazil: Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- 10.1.2.1.3 Brazil: Clinical Trials Market, by Phase, 2020-2028 (US$ Million)
- 10.1.2.1.4 Brazil: Clinical Trials Market, by Study Design, 2020-2028 (US$ Million)
- 10.1.2.1.5 Brazil: Clinical Trials Market, by Indication, 2020-2028 (US$ Million)
- 10.1.2.2 Argentina: Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- 10.1.2.2.1 Overview
- 10.1.2.2.2 Argentina: Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- 10.1.2.2.3 Argentina: Clinical Trials Market, by Phase, 2020-2028 (US$ Million)
- 10.1.2.2.4 Argentina: Clinical Trials Market, by Study Design, 2020-2028 (US$ Million)
- 10.1.2.2.5 Argentina: Clinical Trials Market, by Indication, 2020-2028 (US$ Million)
- 10.1.2.3 Rest of South & Central America: Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- 10.1.2.3.1 Overview
- 10.1.2.3.2 Rest of South & Central America: Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
- 10.1.2.3.3 Rest of South & Central America: Clinical Trials Market, by Phase, 2020-2028 (US$ Million)
- 10.1.2.3.4 Rest of South & Central America: Clinical Trials Market, by Study Design, 2020-2028 (US$ Million)
- 10.1.2.3.5 Rest of South & Central America: Clinical Trials Market, by Indication, 2020-2028 (US$ Million)
- 10.1.2.1 Brazil: Clinical Trials Market - Revenue and Forecast to 2028 (US$ Million)
11. Industry Landscape
- 11.1 Overview
- 11.2 Growth Strategies Done by the Companies in the Market, (%)
- 11.3 Organic Developments
- 11.3.1 Overview
- 11.4 Inorganic Developments
- 11.4.1 Overview
12. Company Profiles
- 12.1 IQVIA Holdings Inc
- 12.1.1 Key Facts
- 12.1.2 Business Description
- 12.1.3 Products and Services
- 12.1.4 Financial Overview
- 12.1.5 SWOT Analysis
- 12.1.6 Key Developments
- 12.2 Parexel International Corp
- 12.2.1 Key Facts
- 12.2.2 Business Description
- 12.2.3 Products and Services
- 12.2.4 Financial Overview
- 12.2.5 SWOT Analysis
- 12.2.6 Key Developments
- 12.3 Charles River Laboratories International Inc
- 12.3.1 Key Facts
- 12.3.2 Business Description
- 12.3.3 Products and Services
- 12.3.4 Financial Overview
- 12.3.5 SWOT Analysis
- 12.3.6 Key Developments
- 12.4 ICON Plc
- 12.4.1 Key Facts
- 12.4.2 Business Description
- 12.4.3 Products and Services
- 12.4.4 Financial Overview
- 12.4.5 SWOT Analysis
- 12.4.6 Key Developments
- 12.5 SGS SA
- 12.5.1 Key Facts
- 12.5.2 Business Description
- 12.5.3 Products and Services
- 12.5.4 Financial Overview
- 12.5.5 SWOT Analysis
- 12.5.6 Key Developments
- 12.6 Syneos Health Inc
- 12.6.1 Key Facts
- 12.6.2 Business Description
- 12.6.3 Products and Services
- 12.6.4 Financial Overview
- 12.6.5 SWOT Analysis
- 12.6.6 Key Developments
- 12.7 Thermo Fisher Scientific Inc
- 12.7.1 Key Facts
- 12.7.2 Business Description
- 12.7.3 Products and Services
- 12.7.4 Financial Overview
- 12.7.5 SWOT Analysis
- 12.7.6
- 12.7.7 Key Developments
- 12.8 Laboratory Corp of America Holdings
- 12.8.1 Key Facts
- 12.8.2 Business Description
- 12.8.3 Products and Services
- 12.8.4 Financial Overview
- 12.8.5 SWOT Analysis
- 12.8.6 Key Developments
13. Appendix
- 13.1 About The Insight Partners
- 13.2 Glossary of Terms