
|
市場調査レポート
商品コード
1920861
細胞治療製造市場(第7版):2035年までの動向と予測 - 細胞治療タイプ別、細胞由来別、事業規模別、製造業者タイプ別、地域別Cell Therapy Manufacturing Market (7th Edition): Trends and Forecast Till 2035 - Distribution by Type of Cell Therapy, Source of Cells, Scale of Operation, Type of Manufacturer, and Geography |
||||||
カスタマイズ可能
|
|||||||
| 細胞治療製造市場(第7版):2035年までの動向と予測 - 細胞治療タイプ別、細胞由来別、事業規模別、製造業者タイプ別、地域別 |
|
出版日: 2026年01月23日
発行: Roots Analysis
ページ情報: 英文 656 Pages
納期: 即日から翌営業日
|
概要
細胞治療製造市場:概要
Roots Analysisの調査によると、細胞治療製造の市場規模は、2035年までの予測期間においてCAGR7.7%で成長し、現在の71億7,200万米ドルから2035年までに140億1,700万米ドルに達すると推定されています。
細胞治療製造市場 - 成長と動向
長年にわたり、数多くの技術革新と発見が医療分野を変革してまいりました。特に顕著な進歩の一つが、細胞ベース療法の登場です。これらは大きな治療的可能性を示しており、従来の薬理学的治療に伴う多くの毒性問題からほぼ自由であります。研究者らは、幅広い疾患適応症を対象とした1,000件以上の細胞・遺伝子治療候補を調査中です。このうち約45件の細胞ベース療法は、既に世界各国の規制当局から承認を得ています。さらに、複数の組織が製造上の課題に対処し、これらの革新的治療法の持続的成功を確保するための取り組みを実施しています。
細胞・遺伝子治療の製造は、厳密に管理された無菌環境下で行われる複雑なプロセスであり、T細胞や幹細胞を含む免疫細胞の分離・改変・増殖など、複数の重要な段階を含みます。各段階では、最終製品の安全性と有効性を保証するため、専用の閉鎖系システムの使用と規制基準への厳格な遵守が求められます。企業は、細胞処理・加工装置の高度化、補助材料や細胞計数技術の改善、現行の物流課題への対応に注力しています。その結果、細胞療法開発企業にとって、これらの先進的治療候補物質の製造過程における人的ミスに関連するリスクを軽減するため、柔軟で自動化された技術への投資がますます重要になってきています。こうした改善により、企業は製品の品質と一貫性を維持しつつ、コストを管理可能な範囲に抑えることが期待されています。
成長の促進要因:市場拡大の戦略的基盤
細胞治療分野は、臨床試験の増加と規制当局の承認拡大を特徴とする急速な成長を遂げています。初期調査から後期臨床試験へ進む治療法が増えるにつれ、患者様がこれらの治療にアクセスする機会も拡大しています。米国遺伝子・細胞治療学会(ASGCT)の報告書によれば、現在進行中の遺伝子・細胞・RNA治療パイプラインは合計4,418件(うち遺伝子治療2,155件、非遺伝子改変細胞治療966件)に上り、新規遺伝子治療試験は79件(57%が腫瘍学分野に集中)実施されています。およびRNA療法のパイプラインには、2,155件の遺伝子治療と966件の非遺伝子改変細胞治療が含まれており、さらに当該四半期中に開始された新規遺伝子治療試験79件(57%が腫瘍学分野)および新規細胞治療試験27件(74%が非腫瘍学分野)が加わっています。この進展は、細胞治療のための現地製造施設の設立によってさらに強化される見込みです。
市場の課題:進展を阻む重大な障壁
需要が堅調であるにもかかわらず、細胞治療製造分野では、細胞ベースの治療製品を研究室から臨床規模へ移行させるにあたり、複数の課題に直面しています。これらの障壁には、熟練労働者の不足、規制順守の問題、そして先進的な施設やインフラの不足が含まれます。細胞治療の製造プロセスには、製品の完全性とコンプライアンスを確保するため、無菌の高グレードクリーンルームと厳格な封じ込めレベルの監視が不可欠です。こうした複雑な手順のため、細胞治療製造のスケールアップはかなり困難となる可能性があります。
細胞治療製造市場:主要な洞察
当レポートは、細胞治療製造市場の現状を詳細に分析し、業界内の潜在的な成長機会を特定しています。レポートからの主な知見は以下の通りです。
- 現在、約200社の業界関係者が細胞治療製造に携わっており、そのうち約45%の企業が米国に本社を置いています。
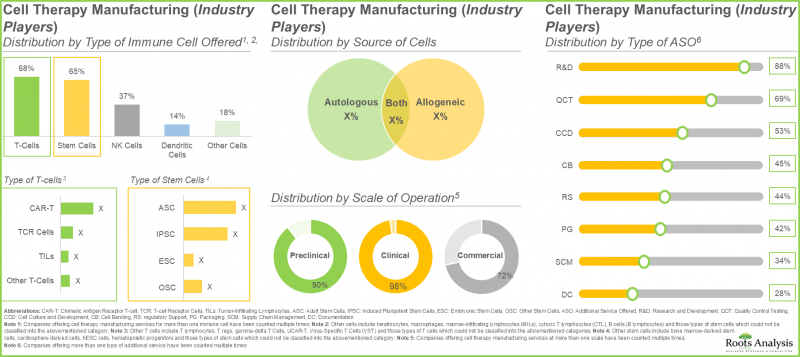
- 業界関係者の40%以上がT細胞と幹細胞の両方を提供しています。これは、T細胞が拒絶反応のリスクが低い一方、幹細胞には損傷した細胞や組織を再生・修復する独自の能力があるためです。
- 現在、80社以上の非業界参入企業が細胞治療の製造に従事しており、そのうち約55%が米国に拠点を置いています。
- 非業界の細胞治療製造企業の約30%は2001年以前に設立されており、そのうち60%以上の企業が自己由来細胞と他家由来細胞の両方を活用しています。
- 免疫細胞療法を評価する複数の臨床試験がこれまでに登録されており、これらの研究の大半(65%)はアジア太平洋地域の様々な試験施設で実施されています。
- この市場への関心の高まりは、近年さまざまな利害関係者間で結ばれた多様な提携関係にも表れています。実際、提携契約の55%以上が2022年以降に締結されています。
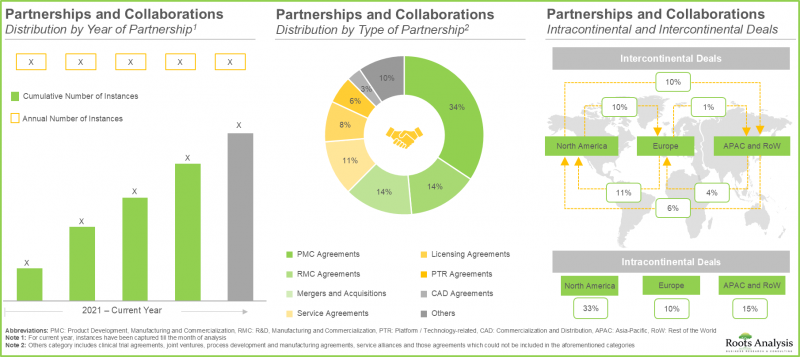
- 業界関係者の70%が新施設の設立により既存の生産能力と技術力を拡大しており、40%以上の施設がT細胞の製造に特化しています。
- この分野における主要大手製薬企業の取り組みの55%以上は、提携や共同研究を通じて推進されています。そのうちかなりの割合はBristol-Myers Squibbにより実施されました。
- 現在稼働中の細胞治療製造能力(クリーンルーム数ベース)の約95%は業界関係者が保有しており、最大規模の設備は北米に設置されています。
- 細胞ベースの治療法に対する需要の高まりは、製品承認の増加、治療法の進歩、医療インフラの拡大、患者意識の高まりに起因しています。
- 細胞治療製造分野は、高い資本コストと先進技術により、CAGR8.25%で成長が見込まれています。製造活動の約60%はCMO(受託製造機関)への外部委託が予想されます。
- がん罹患率の上昇、患者様の個別化細胞治療への嗜好の変化、ならびに細胞治療製造技術の進歩により、市場は今後も着実な成長が見込まれます。
- 北米地域では技術革新が急速に進んでいることから、本年度は細胞治療製造市場において大きなシェアを獲得すると見込まれます。
- 米国の有力企業別開発された承認済み細胞治療の成功に牽引され、商業用細胞治療製造市場はCAGR12.93%で成長すると予測されています。
- 臨床での急速な成功と細胞療法への需要の高まり、さらに血液がんなどの疾患治療における広範な可能性を考慮すると、この業界は将来の成長に向けて良好な位置にあると言えます。
細胞治療製造市場
市場規模および機会分析は以下のパラメータ別にセグメント化されています:
細胞療法タイプ別
- CAR-T細胞療法
- 幹細胞療法
- TCR療法
- TIL療法
- 樹状細胞療法
- その他
細胞由来別
- 自己由来細胞
- 同種細胞
- 未特定細胞
事業規模別
- 臨床規模
- 商業規模
製造業者タイプ別
- 自社製造メーカー
- 受託製造機関
地域別
- 北米
- 米国
- カナダ
- 欧州
- フランス
- ドイツ
- イタリア
- スペイン
- 英国
- その他
アジア太平洋地域およびその他の地域
- アルゼンチン
- オーストラリア
- ブラジル
- 中国
- インド
- イスラエル
- 日本
- シンガポール
- 南アフリカ
- 韓国
- 台湾
細胞治療製造市場:主要セグメント
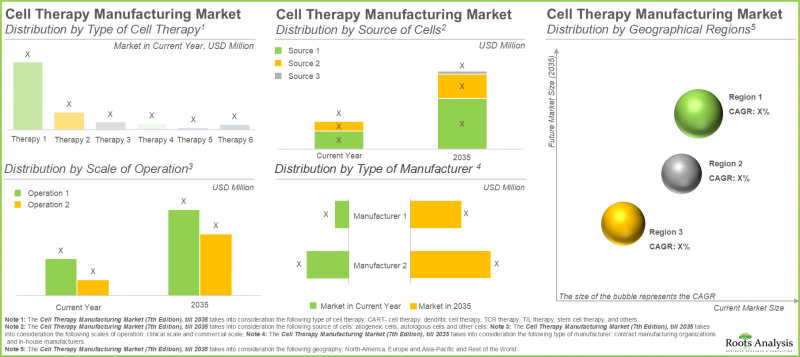
CAR-T細胞療法は細胞治療製造市場において最大のシェアを占めています
本年、CAR-T細胞療法セグメントは、より少ない点滴投与を必要とする短い治療レジメンに起因し、最大の市場シェア(約65%)を占めています。さらに、ナチュラルキラー細胞療法や遺伝子改変細胞療法を含むその他の細胞療法セグメントは、予測期間を通じて16.15%という高いCAGRを記録すると見込まれています。NK細胞の良好な安全性プロファイルは、移植片対宿主病などのリスクを低減します。加えて、既製療法としての機能可能性が、この成長を促進しています。
自己由来細胞が細胞治療製造市場で最大のシェアを占める
2035年までに、自家細胞セグメントは細胞治療製造市場において50%超のシェアを占め、より大きな割合を占めると予測されています。さらに、このセグメントは予測期間を通じて顕著な成長率を示す見込みです。これは主に、自家細胞治療製造が患者自身の細胞に焦点を当てていることに起因します。これにより、移植片対宿主病などの重篤な合併症を引き起こす可能性のある免疫拒絶反応のリスクが最小限に抑えられます。
北米が最大のシェアを確保し市場を独占
当社の市場予測に基づき、北米は2025年までに約45%を占め、細胞治療製造市場で最大のシェアを維持すると見込まれます。さらに、北米は2035年まで比較的高速な9.70%の成長率を達成すると予測されます。この市場の約60%が臨床細胞治療製造に由来している点は特筆すべきです。臨床細胞治療製造分野において、米国は革新を促進する規制枠組みと高度な技能を持つ労働力に支えられ、製造業者にとっての中心地となっています。この地域的な集中により、初期開発段階から商業供給に至るまで、細胞治療の生産を効率的に拡大することが可能となります。
細胞治療製造市場における代表的な参入企業
- Advanced Therapies
- AGC Biologics
- Astellas Pharma
- Catalent
- Charles River Laboratories
- City of Hope
- Clinical Cell and Vaccine Production Facility(CVPF)
- Evotec
- Fraunhofer Institute for Cell Therapy and Immunology
- ImmunityBio
- Lonza
- Merck Millipore
- Miltenyi Biotec
- Minaris Advanced Therapies
- Newcastle Advanced Therapies
- NHS Blood and Transplant
- OBiO Tech
- Pharmaron
- Rayne Cell Therapy Suite
- Resilience
- Sartorius
- Scottish National Blood Transfusion Service(SNBTS)Cellular Therapy Facility
- SK pharmteco(Subsidiary of SK Inc.)
- Takara Bio
- Thermo Fisher Scientific
細胞治療製造市場:調査範囲
- 市場規模と機会分析:当レポートでは、細胞治療製造市場について、[A]細胞治療タイプ、[B]細胞由来、[C]操業規模、[D]製造業者タイプ、[E]地理的地域といった主要市場セグメントに焦点を当てた詳細な分析を掲載しています。
- 業界参入企業- 市場情勢1:細胞治療製造分野に携わる企業別現在の市場情勢の詳細な概要を、[A]設立年、[B]企業規模、[C]本社所在地、[D]製造施設の所在地、[E]提供される免疫細胞タイプ、[F]細胞由来、[G]事業規模、[H]提供される追加サービスタイプなどの情報を含みます。
- 非業界参入企業- 市場情勢2:細胞治療製造分野における非業界参入企業の現在の市場情勢の詳細な概要と、以下の関連パラメータに関する情報:[A]設立年[B]企業規模[C]本社所在地[D]製造施設所在地[E]提供免疫細胞タイプ[F]細胞由来[G]事業規模[H]提供追加サービスタイプ
- 規制状況:北米(米国中心)、欧州、アジア(日本・中国中心)など、各地域における細胞治療製造関連の規制概要。世界各国の主要規制機関から製造施設に付与される多様な認証・認可の分析を含みます。
- 事例研究1:細胞治療製造プロセスの推進戦略を提供するため、世界各国の機関が公表した様々なロードマップの概要。
- ケーススタディ2:閉鎖系システムおよびシングルユースシステムを活用した現行製造工程の最適化における、技術自動化の具体的な役割について詳述します。さらに、自動化装置開発の段階を示すロードマップを、2つの事例研究を基に紹介いたします。
- 企業プロファイル:北米、欧州、アジア太平洋地域において細胞治療製造に携わる主要な業界関係者および非業界関係者の詳細なプロファイルを、[A]設立年、[B]本社所在地、[C]製品ポートフォリオ、[D]最近の動向、[E]将来展望に基づき提供します。
- 臨床試験分析:完了済み、進行中、計画中の臨床試験を、試験登録年、登録患者数、試験状況、試験段階、患者の性別、研究デザイン、割付方式、介入モデル、盲検化タイプ、スポンサー/協力者タイプ、臨床試験実施数に基づく主要参入企業、試験の地理的分布など、複数の関連パラメータに基づいて分析します。
- 提携・協力関係:細胞療法メーカー間で締結された提携・協力関係について、提携年、提携形態、提供免疫細胞タイプ、事業規模、提携件数に基づく主要参入企業など、複数の関連パラメータに基づく詳細な分析を行います。本章では、当該市場における提携活動の地域別分布についても取り上げます。
- 最近の拡張動向:当該分野で報告された拡張計画の詳細な分析に加え、[A]拡張実施年、[B]拡張進捗状況、[C]拡張タイプ、[D]施設所在地、[E]投資額、[F]提供される免疫細胞タイプ、[G]提供されるサービスタイプ、[H]最も活発な参入企業といった複数の関連パラメータに基づく分析を行います。
- 大手製薬企業の取り組み:大手製薬企業が実施した細胞治療製造に焦点を当てた様々な取り組みについて、[A]取り組み実施年、[B]取り組みタイプ、[C]取り組み件数、[D]提携タイプ、[E]提携件数、[F]施設拡張タイプ、[G]施設拡張の数、[H]施設拡張の地域、[I]合併・買収の数、[J]資金調達・投資の数、[K]提供される免疫細胞タイプ、[L]事業規模。
- 生産能力分析:細胞治療製造の総設備容量に関する推定値です。公開情報に基づき、利害関係者から報告された情報を基に算出され、クリーンルーム面積およびクリーンルーム数における利用可能容量の分布を明らかにします。
- 需要分析:細胞療法の年間商業需要および臨床需要に関する情報に基づいた推定値。以下の関連パラメータを横断的に分析:[A]細胞療法タイプ、[B]主要地理的地域。
- 原価分析:細胞治療の価格に影響を与える可能性のある様々な要因の詳細な分析。メーカーが自社製品の価格を決定するために採用している様々なモデル/アプローチを特徴としています。
- 自社製造か外部委託かの意思決定の枠組み:細胞治療開発者が自社製品を自社製造するか、CMO(受託製造機関)のサービスを利用するかを決定する際に考慮すべき様々な要素を明らかにする定性分析。
- 総所有コスト:細胞治療製造組織を対象に、企業規模に基づく20年間の総所有コストの詳細な分析。
- SWOT分析:強み、弱み、機会、脅威について議論し、各SWOTパラメータが市場力学に及ぼす相対的な影響を明らかにします。
目次
第1章 序文
第2章 調査手法
第3章 市場力学
- 章の概要
- 予測調査手法
- 市場評価フレームワーク
- 予測ツールとテクニック
- 重要な考慮事項
- 制限事項
第4章 マクロ経済指標
第5章 エグゼクティブサマリー
第6章 イントロダクション
- 章の概要
- 細胞療法入門
- 細胞治療製造の概要
- 細胞治療製造モデル
- 細胞治療製造プロセスのスケーラビリティ
- 細胞治療メーカータイプ
- 細胞治療薬の製造における主な課題
- 細胞治療の製造に影響を与える主な要因
- 細胞治療製造の自動化
- 細胞治療製造サプライチェーン
- 将来の展望
第7章 細胞治療メーカー(業界参入企業):市場情勢
- 章の概要
- 業界参入企業:市場情勢
第8章 細胞治療メーカー(非業界参入企業):市場情勢
- 章の概要
- 非業界参入企業:市場情勢
第9章 規制状況
- 章の概要
- 現在のシナリオ
- 細胞治療薬製造に関する規制当局
- 細胞治療の初期段階の製造に関するガイドラインのサマリー
- 細胞治療の初期段階における既存の課題
- 地域別規制ガイドラインのばらつき
第10章 ケーススタディ:細胞治療製造に関連する課題を克服するためのロードマップ
- 章の概要
- 米国のロードマップ
- 他の地域のロードマップ
第11章 ケーススタディ:細胞治療製造における自動化技術
- 章の概要
- 細胞治療製造プロセスの自動化
- 成長の原動力と障害
- ケーススタディ
第12章 企業プロファイル業界参入企業
- 章の概要
- 北米のサービスプロバイダー
- AGC Biologics
- Catalent
- Charles River Laboratories
- ImmunityBio
- Merck Millipore
- Minaris Advanced Therapies
- Resilience
- SK pharmteco(Subsidiary of SK Inc.)
- Thermo Fisher Scientific
- 欧州のサービスプロバイダー
- Evotec
- Lonza
- Miltenyi Biotec
- Sartorius
- アジア太平洋のサービスプロバイダー
- Astellas Pharma
- OBiO Tech
- Pharmaron
- Takara Bio
第13章 企業プロファイル:業界外企業
- 章の概要
- City of Hope
- Clinical Cell and Vaccine Production Facility(CVPF)
- Fraunhofer Institute for Cell Therapy and Immunology
- Newcastle Advanced Therapies
- NHS Blood and Transplant
- Rayne Cell Therapy Suite(King's College London)
- Scottish National Blood Transfusion Service(SNBTS)Cellular Therapy Facility
第14章 細胞治療製造における非営利団体
- 章の概要
- 細胞治療製造:非営利団体
- 細胞治療製造:国際学会
第15章 臨床試験の分析
- 章の概要
- 範囲と調査手法
- 細胞治療:臨床試験分析
第16章 パートナーシップとコラボレーション
第17章 最近の拡張
- 章の概要
- 拡張タイプ
- 細胞治療製造:最近の拡大
第18章 大手製薬会社の取り組み
- 章の概要
- 細胞治療薬製造:大手製薬企業一覧
- 細胞治療薬製造:大手製薬企業別取り組み一覧
第19章 容量分析
第20章 需要分析
第21章 原価分析
- 章の概要
- 細胞治療の高価格化に寄与する要因
- 細胞治療の価格モデル
- 細胞治療コストの最適化
- 細胞治療受託製造組織の役割
- 細胞治療の償還に関する考慮事項
第22章 製造か購入かの意思意思決定の枠組み
- 章の概要
- 前提と主要なパラメータ
- 細胞治療薬の製造:製造か購入かの意思決定


