
|
市場調査レポート
商品コード
1849803
CAR T細胞療法市場:業界動向と世界の予測 - 標的抗原別、適応症別、主要地域別、医薬品売上予測、主要参入企業CAR T-Cell Therapy Market: Industry Trends and Global Forecasts - Distribution by Target Antigens, Target Indication, Key Geographies, Sales Forecast of Drugs and Leading Players |
||||||
カスタマイズ可能
|
|||||||
| CAR T細胞療法市場:業界動向と世界の予測 - 標的抗原別、適応症別、主要地域別、医薬品売上予測、主要参入企業 |
|
出版日: 2025年10月22日
発行: Roots Analysis
ページ情報: 英文 598 Pages
納期: 即日から翌営業日
|
概要
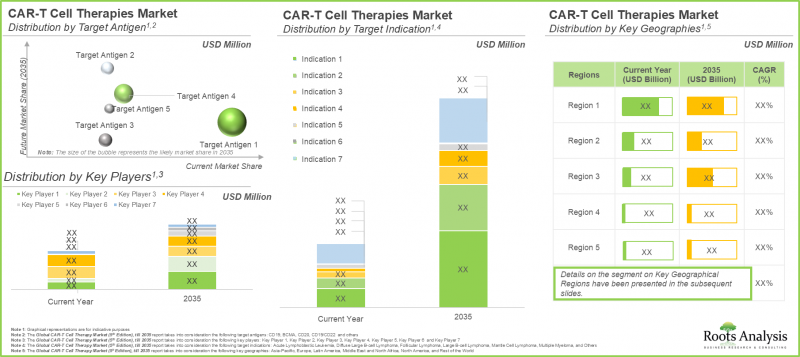
世界のCAR T細胞療法の市場規模は、2035年までの予測期間中に11.4%のCAGRで拡大し、現在の46億米ドルから2035年までに152億米ドルに成長すると予測されています。
市場セグメンテーションでは、市場規模と市場機会を以下のパラメータで区分しています。
適応症
- 急性リンパ芽球性白血病
- びまん性大細胞型B細胞リンパ腫
- 濾胞性リンパ腫
- 大細胞型B細胞リンパ腫
- マントル細胞リンパ腫
- 多発性骨髄腫
- その他
標的抗原
- CD19
- BCMA
- CD20
- CD19/CD22
- その他
主な地域
- 北米
- 米国
- カナダ
- 欧州
- ドイツ
- フランス
- イタリア
- スペイン
- 英国
- その他の欧州
- アジア太平洋
- 日本
- インド
- 中国
- オーストラリア
- 韓国
- その他のアジア太平洋地域
- ラテンアメリカ
- 中東・北アフリカ
- その他の地域
主要医薬品
- イエスカルタ
- キムリア
- アベックマ
- ブリアンジ
- カルヴィクティ
- テカルタス
- その他
CAR T細胞療法市場:成長と動向
がん研究は、製薬業界において医薬品開発が最も活発な分野のひとつです。実際、近年、USFDAはさまざまながん治療に対して70以上の医薬品を認可しています。従来の治療では困難が伴うため、製薬研究者はより焦点を絞った抗がん剤治療を積極的に模索しています。その中で、CAR T細胞療法が有望な選択肢として登場しています。CAR T細胞治療は、身体の自然な免疫反応を利用してがん細胞を特定し、破壊します。CAR T細胞療法は、疾患の完全寛解を達成し、さらなる治療の必要性をなくす能力を示しています。さらに、多くの研究によって、これらの治療法の有効性も確認されています。CAR T細胞療法に関連する臨床試験は、過去8年間で1,000件近く登録されており、多大な研究努力がなされていることがわかる。現在、230以上の企業がさまざまながん、非がん、その他の疾患の治療のためにCAR T細胞療法の開発に携わっています。
CAR T細胞療法市場の成長は、分子研究の技術的進歩、個別化がん治療に対する需要の増加、臨床試験数の増加、複数の治療法の承認によってもたらされます。さらに、投資家からの継続的な資金援助が、中長期的なCAR T細胞療法開発の着実な成長を促進すると期待されています。
CAR T細胞療法市場主要インサイト
当レポートでは、CAR T細胞療法市場の現状を掘り下げ、業界内の潜在的な成長機会を特定しています。当レポートの主な調査結果は以下の通りです。
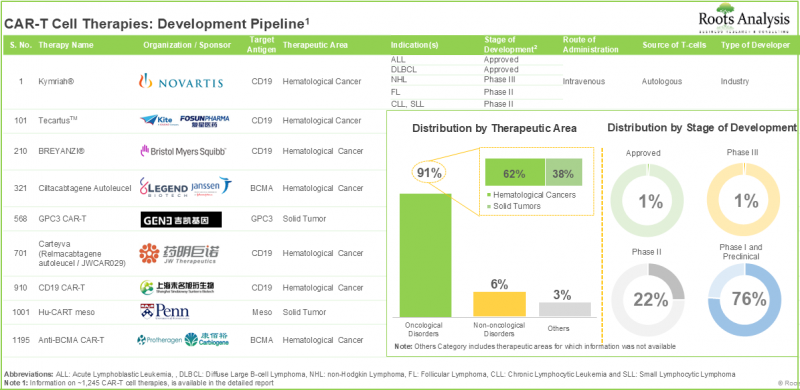
- CAR T細胞療法は、1,240以上の前臨床/臨床製品候補を有し、医薬品領域で最も活発な分野の一つです。
- 様々な疾患を対象に開発されている治療候補の75%近くが自己由来であり、特にCD19とBCMAが最も人気のある標的抗原として浮上しています。
- CARコンストラクトを世代を超えて改良するため、さまざまな遺伝子導入ベクターを用いてscFv領域に変更を加えるなど、広範な努力が続けられています。
- さまざまなタイプの細胞療法を製造するために必要な能力を有すると主張する企業は200社近くあり、そのような企業は製品開発のさまざまな段階にわたって幅広いサービスも提供しています。
- 過去8年間で、CAR T細胞療法に関する臨床試験は、さまざまな地域で970件以上登録されています。
- 現在、有名大学の375人以上の科学者がCAR T細胞療法の臨床開発に携わっており、これらのKOLは主に米国と中国に拠点を置いています。
- 最近、国際的な利害関係者と現地の利害関係者の両方が関与する提携が増加しており、この分野への関心が高まっていることを裏付けています。
- がん免疫療法というこれからの分野でのビジネスチャンスに気づいた複数の投資家が、370件以上にわたって320億米ドル以上を投資しています。
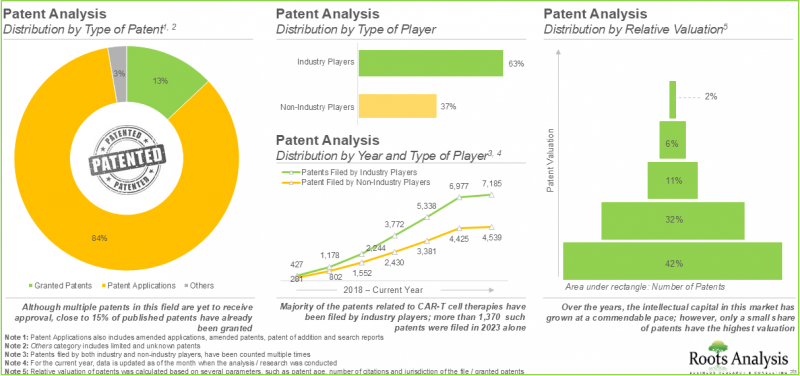
- CAR T細胞療法に関連する特許は11,900件以上出願され、この分野で生まれた知的財産を保護しています。
- これらの治療を効率的に推進し、CAR T細胞療法市場での地位を維持するため、医薬品開発企業は積極的に多様なプロモーション戦略を模索しています。
- CAR T細胞療法市場は、がん罹患率の増加、技術開発、進行中の承認を考慮すると、予測される将来において着実な成長を示すことになるとみられています。
- 開発パイプラインへの注目の高まりと有望な臨床結果により、市場は今後10年間、年率11.4%以上の成長が見込まれています。
CAR T細胞療法市場主要セグメント
予測期間中、多発性骨髄腫がCAR T細胞療法市場を独占する可能性が高い
適応症別では、市場は急性リンパ芽球性白血病、びまん性大細胞型B細胞リンパ腫、濾胞性リンパ腫、大細胞型B細胞リンパ腫、マントル細胞リンパ腫、多発性骨髄腫、その他に区分されます。大細胞型B細胞リンパ腫の治療を目的としたCAR T療法市場は、予測期間中、年間成長率14%で成長すると予測されていることは注目に値します。
予測期間中、CD19標的治療薬がCAR T細胞療法市場で最大シェアを獲得する見込み
標的抗原別では、市場はCD19、BCMA、CD20、CD19/CD22、その他に区分されます。注目すべきは、CD19のセグメントが今後数年間で市場全体の65%以上を占める可能性が高いことです。これは、Kymriah(R)(tisagenlecleucel)やYescarta(R)(axicabtagene ciloleucel)などのCD19を標的とした治療薬の成功に起因しています。
北米が市場の最大シェアを占める
主要地域別に見ると、市場は北米、欧州、アジア太平洋、ラテンアメリカ、中東・北アフリカ、その他世界のその他の地域に区分されます。特筆すべきは、アジア太平洋の市場が今後高いCAGRで成長すると予想されることです。
CAR T細胞療法市場の参入企業例
- Autolus
- Bluebird Bio
- Bristol Myers Squibb
- Carsgen Therapeutics
- Cellectis, Gilead Sciences
- Innovative Cellular Therapeutics
- Noile-Immune Biotech
- Novartis
- Shanghai GeneChem
- Sinobioway Cell Therapy
- Takara Bio
- Wellington Zhaotai Therapies
1次調査の概要
本調査で提示した意見や洞察は、複数の利害関係者との議論によって影響を受けたものです。本調査レポートでは、以下の業界利害関係者とのインタビューの詳細な記録を掲載しています:
- A社最高経営責任者
- B社最高経営責任者
- C社最高執行責任者
- D社サイエンティフィック・アフェアーズ担当副社長
- E社免疫腫瘍学担当副社長
- F社ワイスマン・バイオマニュファクチャリング社戦略・事業開発担当競合情報マネージャー兼事業開発マネージャー
- G社医学部教授兼腫瘍内科部長
- H社医学助教授
CAR T細胞療法市場調査対象
- 市場予測と機会分析:この調査レポートは、CAR T細胞療法市場を詳細に分析し、[A]標的抗原、[B]適応症、[C]主要地域分析、[D]薬剤の売上予測、[E]主要企業などの主要市場セグメントに焦点を当てています。
- 市場への影響分析:市場の成長に影響を与える[A]促進要因・[B]抑制要因・[C]市場促進要因・[D]課題など様々な要因を分析しています。
- 市場情勢:A]開発企業のタイプ、[B]開発フェーズ、[C]治療領域、[D]主要な適応症、[E]主要な標的抗原、[F]T細胞の供給源、[G]投与経路、[H]投与回数、[I]患者セグメント、[J]治療法のタイプ、[K]設立年、[L]企業規模、[M]本社所在地などの様々なパラメータを考慮し、CAR T細胞療法の現在の市場状況を包括的に評価します。
- 臨床試験分析:A]臨床試験登録年、[B]登録患者数、[C]臨床試験募集状況、[D]臨床試験フェーズ、[E]対象患者セグメント、[F]スポンサー/共同研究者のタイプ、[G]最も活発なプレーヤー、[H]臨床試験の地域分布などのパラメータに基づき、完了済み、進行中、計画中の臨床試験を詳細に分析。
- 主要な洞察血液悪性腫瘍および固形がんに関連する一般的な標的抗原に重点を置いた競合分析を特徴とする研究から得られた主要考察の分析。さらに、[A]CARの世代、[B]結合ドメインのタイプ、[C]使用されるウイルスのタイプ、[D]使用される遺伝子導入法のタイプ、[E]使用される共刺激ドメインのタイプに基づく、臨床段階のCAR T細胞療法のCARコンストラクト分析を含みます。
- KOL:Key Opinion Leaders:この分野におけるキー・オピニオン・リーダー(KOL)の詳細な分析には、CAR T療法に関連する臨床試験を監督する様々な主任研究者の評価が含まれます。これらの治験責任医師は、CAR T細胞療法の研究開発に積極的に関与していることから、KOLとして認識されています。さらに本章では、第三者評価別評価とともに、独自のスコアリングシステムを活用したKOLの専門知識の比較分析も行っています。
- 企業プロファイル:A]企業概要、[B]CAR T療法に特化した製品ポートフォリオ、[C]技術ポートフォリオ(ある場合)、[D]CAR T細胞療法に関連する最近の動向、企業の製造能力に焦点を当てたCAR T細胞療法市場の主要企業の詳細なプロファイル。さらに、これらの企業に対する戦略的/ベンチャーキャピタル投資の詳細も含まれています。
- パートナーシップとコラボレーション:A]提携の年、[B]提携のタイプ、[C]提携先のタイプ、[D]地域分布など、いくつかの関連パラメータに基づいて、この領域で確立されたパートナーシップを分析。
- 資金調達と投資の分析:A]資金調達年、[B]資金調達の種類、[C]投資額、[D]地域分布など、いくつかの関連パラメータに基づく、この領域で報告された資金調達と投資の分析。
- 特許分析:A]特許の種類、[B]公開年、[C]地域分布、[D]特許協力分類(CPC)記号、[E]新たな重点領域、[F]出願人の種類、[G]主要プレーヤー(特許出願/付与数ベース)、[H]特許評価、[I]特許ベンチマーキングに基づいて、CAR T細胞療法に関連する出願または付与された11,900件以上の特許を詳細に分析。
- ケーススタディ細胞治療製品の製造に焦点を当てたケーススタディで、課題を強調し、この領域に関わる受託サービスプロバイダーと自社製造業者の包括的なリストを提供します。
- コスト価格分析:細胞療法の価格設定に寄与する様々な要因を包括的に評価。近い将来に上市が見込まれるCAR T細胞療法の価格を決定する際に、製薬会社が検討しうるさまざまなモデルや戦略も含まれています。
目次
第1章 序文
第2章 調査手法
第3章 経済的およびその他のプロジェクト特有の考慮事項
第4章 エグゼクティブサマリー
第5章 イントロダクション
- 章の概要
- T細胞免疫療法の概要
- 歴史的進化
- T細胞免疫療法の開発における重要な考慮事項
- T細胞の方向転換に用いられる戦略
- 改変T細胞の製造
- キメラ抗原受容体T細胞(CAR T)療法
- 開発の歴史
- CARの構造
- CAR T細胞の開発
- ユニバーサルCAR T細胞
- 投与経路
- CAR T細胞療法に伴う課題
- 結論
第6章 CAR T細胞療法:市場情勢
- 章の概要
- CAR T細胞療法:市場情勢
- CAR T細胞療法:開発企業の全体情勢
第7章 重要な洞察
- 章の概要
- CAR T細胞療法:人気の標的抗原
- 造血悪性腫瘍の標的
- 固形腫瘍の一般的な標的
- CAR T療法:CAR構造解析
第8章 臨床試験の分析
- 章の概要
- 範囲と調査手法
- CAR T細胞療法:臨床試験分析
第9章 主要なオピニオンリーダー
- 章の概要
- 前提と主要なパラメータ
- 調査手法
- CAR T細胞療法:主要オピニオンリーダー
第10章 企業プロファイル
- 章の概要
- Autolus
- Bluebird Bio
- Bristol Myers Squibb
- CARsgen Therapeutics
- Cellectis
- Gilead Sciences
- Innovative Cellular Therapeutics
- Noile-Immune Biotech
- Novartis
- Sinobioway Cell Therapy
- Takara Bio
- Wellington Zhaotai Therapies
第11章 パートナーシップとコラボレーション
- 章の概要
- パートナーシップモデル
- CAR T細胞療法:パートナーシップとコラボレーション
第12章 資金調達と投資分析
- 章の概要
- 資金調達の種類
- CAR T細胞療法:資金調達と投資分析
第13章 特許分析
- 章の概要
- 範囲と調査手法
- CAR T細胞療法:特許分析
- 特許ベンチマーク分析
- 特許評価
- 引用数の多い特許
第14章 ケーススタディ:細胞治療の製造
- 章の概要
- 細胞治療製造の概要
- 細胞治療製造モデル
- 集中型製造モデル
- 分散型製造モデル
- 細胞治療製造プロセスのスケーラビリティ
- 細胞治療メーカーの種類
- 細胞治療の製造に関連する主な課題
- 細胞治療薬製造における重要な考慮事項
- 細胞治療製造プロセスの自動化
- 細胞治療製造サプライチェーン
- 自社製造能力を持つ企業と委託製造企業の比較
- 規制状況
- 将来の展望
第15章 原価分析
- 章の概要
- 細胞・遺伝子治療の高価格化に寄与する要因
- T細胞免疫療法の価格モデル
- T細胞免疫療法の償還に関する考慮事項
第16章 市場影響分析:促進要因、制約要因、機会、課題
第17章 世界のCAR T細胞療法市場
第18章 CAR T細胞療法市場(標的抗原別)
第19章 CAR T細胞療法市場(適応症別)
第20章 CAR T細胞療法市場(主要地域別)
第21章 CAR T療法市場、医薬品の売上予測
- 章の概要
- 主要な前提と調査手法
- 市販されているCAR T細胞療法:売上予測
- 臨床CAR T細胞療法:売上予測
- データの三角測量と検証
第22章 CAR T細胞療法市場:主要企業の売上予測
- 章の概要
- 主要な前提と調査手法
- CAR T細胞療法:主要参入企業
- Gilead Sciencesの売上予測
- Bristol Myers Squibbの売上予測
- Novartisの売上予測
- Janssenの売上予測
- JW Therapeuticsの売上予測
- データの三角測量と検証
第23章 プロモーション分析
- 章の概要
- プロモーションキャンペーンに使用されるチャネル
- 製品ウェブサイト分析のサマリー
- 患者サポートサービスと情報ダウンロードのサマリー
- Kymriah:プロモーション分析
- Yescarta:プロモーション分析
- Tecartus:プロモーション分析
- Breyanzi:プロモーション分析
- Abecma:プロモーション分析
- Carvykti:プロモーション分析


