|
|
市場調査レポート
商品コード
1403327
静脈血栓塞栓症治療市場:デバイス別、ストッキング別、用途別、エンドユーザー別 - 世界予測(~2030年)Venous Thromboembolism Treatment Market by Device (Thrombectomy, Inferior Vena Cava Filter [Retrievable, Permanent], Stockings, Compression Pump) Application (DVT, Pulmonary Embolism) End User (Hospital, Ambulatory Care Center) - Global Forecast to 2030 |
||||||
カスタマイズ可能
|
|||||||
| 静脈血栓塞栓症治療市場:デバイス別、ストッキング別、用途別、エンドユーザー別 - 世界予測(~2030年) |
|
出版日: 2024年01月09日
発行: Meticulous Research
ページ情報: 英文 158 Pages
納期: 即納可能
|
全表示
- 概要
- 図表
- 目次
世界の静脈血栓塞栓症治療の市場規模は、2023~2030年にかけてCAGR 7.2%で成長し、2030年には44億米ドルに達すると予測されます。
広範な一次および二次調査と市場シナリオの詳細な分析を実施した後、本レポートは静脈血栓塞栓症治療市場内の主要促進要因・制約・課題・機会に関する洞察を提供します。
静脈血栓塞栓症の高い有病率、がんの罹患率の上昇、糖尿病や肥満などの二次的危険因子の有病率の増加、整形外科手術の増加、低侵襲治療手技に対する需要の高まりは、静脈血栓塞栓症治療市場の成長を促進する主な要因です。さらに、治療オプションの入手しやすさと手頃な価格の増加、VTE診断と治療方法の進歩、新興国における市場拡大が、市場成長の機会を生み出すと期待されています。
しかし、製品の不具合や製品回収の事例が市場の成長を抑制する可能性があります。さらに、静脈血栓塞栓症に対する認識不足や圧迫衣の副作用に対する認識の高まりは、市場成長にとって大きな課題です。
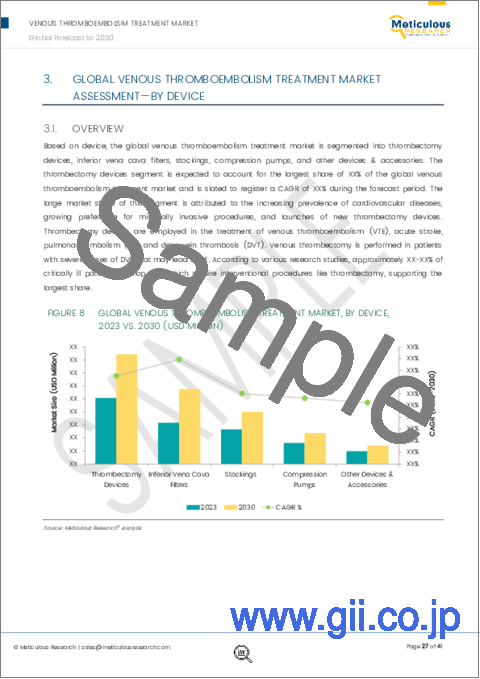
本レポートでカバーするデバイスのうち、2023年には血栓摘出デバイスセグメントが静脈血栓塞栓症治療市場で最大のシェアを占めると予想されます。このセグメントの市場シェアが大きいのは、心血管疾患の有病率の増加、低侵襲手技への嗜好の高まり、新しい血栓摘出デバイスの発売によるものです。
本レポートで対象とする用途のうち、2023年には深部静脈血栓症分野が静脈血栓塞栓症治療市場で最大のシェアを占めると予想されます。このセグメントの市場シェアが大きいのは、肥満の有病率の増加、高齢者人口の増加、生活習慣病による深部静脈血栓症の症例の増加、過度のアルコール摂取、DVTに対する意識の高まりなどが背景にあります。
本レポートでカバーするエンドユーザーのうち、2023年には病院セグメントが静脈血栓塞栓症治療市場で最大のシェアを占めると予想されます。この大きな市場シェアは、静脈血栓塞栓症の有病率の増加、病院や診療所の数の増加、高度に熟練した専門家の存在、病院の高い購買力などに起因しています。病院へのアクセスが容易であること、1カ所での診断、治療、手術を含む包括的なヘルスケアサービスを求める患者が多いことも、このセグメントの大きな市場シェアに寄与しています。
世界の静脈血栓塞栓症治療市場の地域別シナリオを詳細に分析することで、主要5地域に関する詳細な質的・量的洞察が得られます:北米(米国、カナダ)、欧州(ドイツ、フランス、英国、イタリア、スペイン、その他の欧州)、アジア太平洋(中国、日本、インド)、ラテンアメリカ、その他の中東・アフリカです。
2023年には、北米が世界の静脈血栓塞栓症治療市場で最大のシェアを占め、次いで欧州、アジア太平洋、ラテンアメリカ、中東・アフリカが続くと予想されます。しかし、アジア太平洋は予測期間中に最大のCAGRを記録する予定です。この地域市場の成長は、慢性疾患の有病率の増加、座りがちなライフスタイルを送る人の増加、医療費の増加、ヘルスケアインフラの改善を目的とした政府の支援策、静脈血栓塞栓症に関する意識の高まりなどの要因によるものと考えられます。
目次
第1章 イントロダクション
第2章 調査手法
- 調査の前提条件
第3章 エグゼクティブサマリー
第4章 市場洞察
- 概要
- 促進要因
- 静脈血栓塞栓症の高い有病率
- がん罹患率の上昇
- 抑制要因
- 製品の不具合とリコールの発生
- 機会
- 治療オプションの入手しやすさと手頃な価格の増加
- VTE診断および治療法の進歩
- 課題
- 静脈血栓塞栓症に対する認識不足
第5章 静脈血栓塞栓症治療市場評価:デバイス別
- 概要
- 血栓除去デバイス
- 下大静脈フィルター
- 回収可能フィルター
- 永久フィルター
- ストッキング
- 圧迫ポンプ
- その他の機器・付属品
第6章 静脈血栓塞栓症治療市場評価:用途別
- 概要
- 深部静脈血栓症(DVT)
- 肺塞栓症(PE)
第7章 静脈血栓塞栓症治療市場評価:エンドユーザー別
- 概要
- 病院
- 外来診療センター
- その他のエンドユーザー
第8章 静脈血栓塞栓症治療市場評価:地域別
- 概要
- 北米
- 米国
- カナダ
- 欧州
- ドイツ
- フランス
- 英国
- イタリア
- スペイン
- その他の欧州
- アジア太平洋
- 中国
- 日本
- インド
- その他のアジア太平洋
- ラテンアメリカ
- 中東・アフリカ
第9章 競合分析
- 概要
- 主要成長戦略
- 競合ベンチマーキング
- 競合ダッシュボード
- 業界リーダー
- 市場差別化要因
- 先行企業
- 新興企業
- 市場シェア分析(2022年)
- Boston Scientific Corporation(U.S.)
- Stryker Corporation(U.S.)
- Cardinal Health, Inc.(U.S.)
- Enovis Corporation(U.S.)
- Medtronic plc(Ireland)
- Cook Group Incorporated(U.S.)
第10章 企業プロファイル
- ALN Implants chirurgicaux(ALN Surgical Implants)
- AngioDynamics, Inc.
- Argon Medical Devices, Inc.
- ArjoHuntleigh AB
- Boston Scientific Corporation
- Cardinal Health, Inc.
- Cook Group Incorporated
- Koninklijke Philips N.V.
- DS MAREF Co., Ltd
- Enovis Corporation
- LifeTech Scientific Corporation
- Medtronic plc
- Stryker Corporation
第11章 付録
- Table 1 Share of Population Aged 65 Years or Above, by Region, 2022 Vs. 2030 Vs. 2050
- Table 2 Number of New Cancer Cases, by Type, 2020 Vs. 2030
- Table 3 Global Venous Thromboembolism Treatment Market, by Device, 2021-2030 (USD Million)
- Table 4 Global Venous Thromboembolism Treatment Market for Thrombectomy Devices, by Country/Region, 2021-2030 (USD Million)
- Table 5 Global Venous Thromboembolism Treatment Market for Inferior Vena Cava Filters, by Type, 2021-2030 (USD Million)
- Table 6 Global Venous Thromboembolism Treatment Market for Inferior Vena Cava Filters, by Country/Region, 2021-2030 (USD Million)
- Table 7 Global Retrievable Filters Market, by Country/Region, 2021-2030 (USD Million)
- Table 8 Global Permanent Filters Market, by Country/Region, 2021-2030 (USD Million)
- Table 9 Global Venous Thromboembolism Treatment Market for Stockings, by Country/Region, 2021-2030 (USD Million)
- Table 10 Global Venous Thromboembolism Treatment Market for Compression Pumps, by Country/Region, 2021-2030 (USD Million)
- Table 11 Global Venous Thromboembolism Treatment Market for Other Devices & Accessories, by Country/Region, 2021-2030 (USD Million)
- Table 12 Global Venous Thromboembolism Treatment Market, by Application, 2021-2030 (USD Million)
- Table 13 Global Venous Thromboembolism Treatment Market for Deep Vein Thrombosis (DVT), by Country/Region, 2021-2030 (USD Million)
- Table 14 Global Venous Thromboembolism Treatment Market for Pulmonary Embolism (PE), by Country/Region, 2021-2030 (USD Million)
- Table 15 Venous Thromboembolism Treatment Market, by End User, 2021-2030 (USD Million)
- Table 16 Global Venous Thromboembolism Treatment Market for Hospitals, by Country/Region, 2021-2030 (USD Million)
- Table 17 Global Venous Thromboembolism Market for Ambulatory Care Centers, by Country/Region, 2021-2030 (USD Million)
- Table 18 Global Venous Thromboembolism Market for Other End Users, by Country/Region, 2021-2030 (USD Million)
- Table 19 Global Venous Thromboembolism Treatment Market, by Country/Region, 2021-2030 (USD Million)
- Table 20 North America: Venous Thromboembolism Treatment Market, by Country, 2021-2030 (USD Million)
- Table 21 North America: Venous Thromboembolism Treatment Market, by Device, 2021-2030 (USD Million)
- Table 22 North America: Inferior Vena Cava Filters Market, by Type, 2021-2030 (USD Million)
- Table 23 North America: Venous Thromboembolism Treatment Market, by Application, 2021-2030 (USD Million)
- Table 24 North America: Venous Thromboembolism Treatment Market, by End User, 2021-2030 (USD Million)
- Table 25 U.S.: Venous Thromboembolism Treatment Market, by Device, 2021-2030 (USD Million)
- Table 26 U.S.: Inferior Vena Cava Filters Market, by Type, 2021-2030 (USD Million)
- Table 27 U.S.: Venous Thromboembolism Treatment Market, by Application, 2021-2030 (USD Million)
- Table 28 U.S.: Venous Thromboembolism Treatment Market, by End User, 2021-2030 (USD Million)
- Table 29 Canada: Prevalence of Diseases/Conditions in The Geriatric (Age 65 Years And Above) Population (2019-2020)
- Table 30 Canada: Venous Thromboembolism Treatment Market, by Device, 2021-2030 (USD Million)
- Table 31 Canada: Inferior Vena Cava Filters Market, by Type, 2021-2030 (USD Million)
- Table 32 Canada: Venous Thromboembolism Treatment Market, by Application, 2021-2030 (USD Million)
- Table 33 Canada: Venous Thromboembolism Treatment Market, by End User, 2021-2030 (USD Million)
- Table 34 Europe: Venous Thromboembolism Treatment Market, by Country/Region, 2021-2030 (USD Million)
- Table 35 Europe: Venous Thromboembolism Treatment Market, by Device, 2021-2030 (USD Million)
- Table 36 Europe: Inferior Vena Cava Filters Market, by Type, 2021-2030 (USD Million)
- Table 37 Europe: Venous Thromboembolism Treatment Market, by Application, 2021-2030 (USD Million)
- Table 38 Europe: Venous Thromboembolism Treatment Market, by End User, 2021-2030 (USD Million)
- Table 39 Germany: Venous Thromboembolism Treatment Market, by Device, 2021-2030 (USD Million)
- Table 40 Germany: Inferior Vena Cava Filters Market, by Type, 2021-2030 (USD Million)
- Table 41 Germany: Venous Thromboembolism Treatment Market, by Application, 2021-2030 (USD Million)
- Table 42 Germany: Venous Thromboembolism Treatment Market, by End User, 2021-2030 (USD Million)
- Table 43 France: Venous Thromboembolism Treatment Market, by Device, 2021-2030 (USD Million)
- Table 44 France: Inferior Vena Cava Filters Market, by Type, 2021-2030 (USD Million)
- Table 45 France: Venous Thromboembolism Treatment Market, by Application, 2021-2030 (USD Million)
- Table 46 France: Venous Thromboembolism Treatment Market, by End User, 2021-2030 (USD Million)
- Table 47 U.K.: Venous Thromboembolism Treatment Market, by Device, 2021-2030 (USD Million)
- Table 48 U.K.: Inferior Vena Cava Filters Market, by Type, 2021-2030 (USD Million)
- Table 49 U.K.: Venous Thromboembolism Treatment Market, by Application, 2021-2030 (USD Million)
- Table 50 U.K.: Venous Thromboembolism Treatment Market, by End User, 2021-2030 (USD Million)
- Table 51 Italy: Venous Thromboembolism Treatment Market, by Device, 2021-2030 (USD Million)
- Table 52 Italy: Inferior Vena Cava Filters Market, by Type, 2021-2030 (USD Million)
- Table 53 Italy: Venous Thromboembolism Treatment Market, by Application, 2021-2030 (USD Million)
- Table 54 Italy: Venous Thromboembolism Treatment Market, by End User, 2021-2030 (USD Million)
- Table 55 Spain: Venous Thromboembolism Treatment Market, by Device, 2021-2030 (USD Million)
- Table 56 Spain: Inferior Vena Cava Filters Market, by Type, 2021-2030 (USD Million)
- Table 57 Spain: Venous Thromboembolism Treatment Market, by Application, 2021-2030 (USD Million)
- Table 58 Spain: Venous Thromboembolism Treatment Market, by End User, 2021-2030 (USD Million)
- Table 59 Number of People Aged 56 And Above, by Country, 2012- 2022
- Table 60 Estimated Number of New Cancer Cases, by Country, 2020 Vs. 2030
- Table 61 RoE: Venous Thromboembolism Treatment Market, by Device, 2021-2030 (USD Million)
- Table 62 RoE: Inferior Vena Cava Filters Market, by Type, 2021-2030 (USD Million)
- Table 63 RoE: Venous Thromboembolism Treatment Market, by Application, 2021-2030 (USD Million)
- Table 64 RoE: Venous Thromboembolism Treatment Market, by End User, 2021-2030 (USD Million)
- Table 65 Asia-Pacific: Venous Thromboembolism Treatment Market, by Country/Region, 2021-2030 (USD Million)
- Table 66 Asia-Pacific: Venous Thromboembolism Treatment Market, by Device, 2021-2030 (USD Million)
- Table 67 Asia-Pacific: Inferior Vena Cava Filters Market, by Type, 2021-2030 (USD Million)
- Table 68 Asia-Pacific: Venous Thromboembolism Treatment Market, by Application, 2021-2030 (USD Million)
- Table 69 Asia-Pacific: Venous Thromboembolism Treatment Market, by End User, 2021-2030 (USD Million)
- Table 70 China: Venous Thromboembolism Treatment Market, by Device, 2021-2030 (USD Million)
- Table 71 China: Inferior Vena Cava Filters Market, by Type, 2021-2030 (USD Million)
- Table 72 China: Venous Thromboembolism Treatment Market, by Application, 2021-2030 (USD Million)
- Table 73 China: Venous Thromboembolism Treatment Market, by End User, 2021-2030 (USD Million)
- Table 74 Japan: Venous Thromboembolism Treatment Market, by Device, 2021-2030 (USD Million)
- Table 75 Japan: Inferior Vena Cava Filters Market, by Type, 2021-2030 (USD Million)
- Table 76 Japan: Venous Thromboembolism Treatment Market, by Application, 2021-2030 (USD Million)
- Table 77 Japan: Venous Thromboembolism Treatment Market, by End User, 2021-2030 (USD Million)
- Table 78 India: Venous Thromboembolism Treatment Market, by Device, 2021-2030 (USD Million)
- Table 79 India: Inferior Vena Cava Filters Market, by Type, 2021-2030 (USD Million)
- Table 80 India: Venous Thromboembolism Treatment Market, by Application, 2021-2030 (USD Million)
- Table 81 India: Venous Thromboembolism Treatment Market, by End User, 2021-2030 (USD Million)
- Table 82 RoAPAC: Venous Thromboembolism Treatment Market, by Device, 2021-2030 (USD Million)
- Table 83 RoAPAC: Inferior Vena Cava Filters Market, by Type, 2021-2030 (USD Million)
- Table 84 RoAPAC: Venous Thromboembolism Treatment Market, by Application, 2021-2030 (USD Million)
- Table 85 RoAPAC: Venous Thromboembolism Treatment Market, by End User, 2021-2030 (USD Million)
- Table 86 Latin America: Venous Thromboembolism Treatment Market, by Device, 2021-2030 (USD Million)
- Table 87 Latin America: Inferior Vena Cava Filters Market, by Type, 2021-2030 (USD Million)
- Table 88 Latin America: Venous Thromboembolism Treatment Market, by Application, 2021-2030 (USD Million)
- Table 89 Latin America: Venous Thromboembolism Treatment Market, by End User, 2021-2030 (USD Million)
- Table 90 Middle East & Africa: Venous Thromboembolism Treatment Market, by Device, 2021-2030 (USD Million)
- Table 91 Middle East & Africa: Inferior Vena Cava Filters Market, by Type, 2021-2030 (USD Million)
- Table 92 Middle East & Africa: Venous Thromboembolism Treatment Market, by Application, 2021-2030 (USD Million)
- Table 93 Middle East & Africa: Venous Thromboembolism Treatment Market, by End User, 2021-2030 (USD Million)
- Table 94 Recent Developments, by Company (2020-2023)
LIST OF FIGURES
- Figure 1 Research Process
- Figure 2 Secondary Sources Referenced for this Study
- Figure 3 Primary Research Techniques
- Figure 4 Key Executives Interviewed
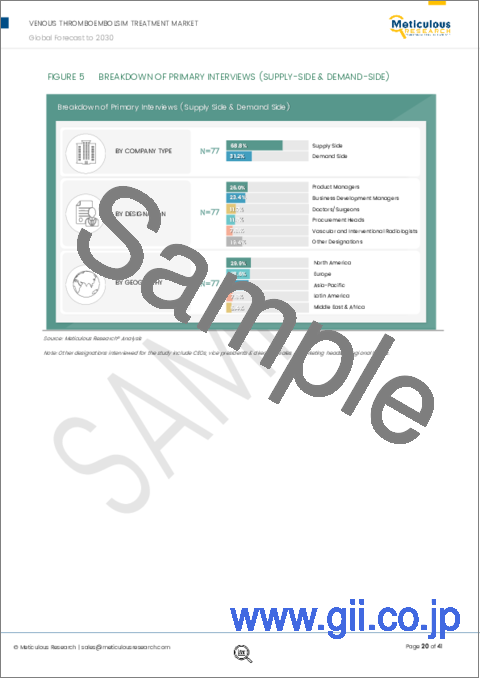
- Figure 5 Breakdown of Primary Interviews (Supply-Side & Demand-Side)
- Figure 6 Market Sizing and Growth Forecast Approach
- Figure 7 Global Venous Thromboembolism Treatment Market, by Device, 2023 Vs. 2030 (USD Million)
- Figure 8 Global Venous Thromboembolism Treatment Market, by Application, 2023 Vs. 2030 (USD Million)
- Figure 9 Global Venous Thromboembolism Treatment Market, by End User, 2023 Vs. 2030 (USD Million)
- Figure 10 Global Venous Thromboembolism Treatment Market, by Geography
- Figure 11 Impact Analysis of Market Dynamics
- Figure 12 Global Venous Thromboembolism Treatment Market, by Device, 2023 Vs. 2030 (USD Million)
- Figure 13 Global Venous Thromboembolism Treatment Market, by Application, 2023 Vs. 2030 (USD Million)
- Figure 14 Venous Thromboembolism Treatment Market, by End User, 2023 Vs. 2030 (USD Million)
- Figure 15 Global Venous Thromboembolism Treatment Market, by Geography, 2023 Vs. 2030 (USD Million)
- Figure 16 North America: Venous Thromboembolism Treatment Market Snapshot
- Figure 17 Canada: Estimated Number of New Cancer Cases, 2020-2035
- Figure 18 Europe: Venous Thromboembolism Treatment Market Snapshot
- Figure 19 France: Percentage of Population Aged 65 Years and Above (2016-2022)
- Figure 20 France: Prevalence of Chronic Diseases in People Aged 65 And Above (2020)
- Figure 21 U.K.: Number of Diabetes And Urogenital, Blood, and Endocrine Disease Cases - DALYs (Disability-Adjusted Life Years), 2017-2020 (In Thousands)
- Figure 22 Spain: Estimated Number of New Cancer Cases (2020-2035)
- Figure 23 Switzerland: Number of New Cancer Cases Reported (2020-2040)
- Figure 24 Asia-Pacific: Venous Thromboembolism Treatment Market Snapshot
- Figure 25 Japan: Percentage Share of the Geriatric Population (Aged 65 Years And Above) (2015-2022)
- Figure 26 Saudi Arabia: Number of People Aged 65 Years and Above, 2017-2021 (In Thousands)
- Figure 27 South Africa: Number of People with Diabetes, 2000-2045 (In Thousands)
- Figure 28 Key Growth Strategies Adopted by Leading Players 2020-2023
- Figure 29 Venous Thromboembolism Treatment Market: Competitive Benchmarking, by Device
- Figure 30 Venous Thromboembolism Treatment Market: Competitive Benchmarking, by Region
- Figure 31 Competitive Dashboard: Venous Thromboembolism Treatment Market
- Figure 32 Global Venous Thromboembolism Treatment Market Share Analysis, by Key Players (2022)
- Figure 33 AngioDynamics, Inc.: Financial Overview (2023)
- Figure 34 ArjoHuntleigh Ab: Financial Overview (2022)
- Figure 35 Boston Scientific Corporation: Financial Overview (2022)
- Figure 36 Cardinal, Inc.: Financial Overview (2022)
- Figure 37 Koninklijke Philips N.V.: Financial Overview (2022)
- Figure 38 Enovis Corporation: Financial Overview (2022)
- Figure 39 LifeTech Scientific Corporation: Financial Overview (2022)
- Figure 40 Medtronic plc: Financial Overview (2023)
- Figure 41 Stryker Corporation: Financial Overview (2022)
Venous Thromboembolism Treatment Market by Device (Thrombectomy, Inferior Vena Cava Filter [Retrievable, Permanent], Stockings, Compression Pump) Application (DVT, Pulmonary Embolism) End User (Hospital, Ambulatory Care Center) - Global Forecast to 2030.
The global venous thromboembolism treatment market is projected to reach $4.40 billion by 2030, at a CAGR of 7.2% from 2023 to 2030.
After conducting an extensive primary and secondary study and an in-depth analysis of the market scenario, this report offers insights into the key drivers, constraints, challenges, and opportunities within the venous thromboembolism treatment market.
The high prevalence of venous thromboembolism, rising incidence of cancer, growing prevalence of secondary risk factors such as diabetes and obesity, increase in orthopedic surgical procedures, and the rising demand for minimally invasive treatment procedures are the key factors driving the growth of the venous thromboembolism treatment market. Furthermore, the growing accessibility and affordability of treatment options, advancements in VTE diagnosis and treatment methods, and the market expansion in emerging economies are expected to create market growth opportunities.
However, instances of product failure and product recalls may restrain the market's growth. Additionally, lack of awareness about venous thromboembolism and increasing awareness of probable side effects of compression garments are major challenges for market growth.
Among the devices covered in this report, in 2023, the thrombectomy devices segment is expected to account for the largest share of the venous thromboembolism treatment market. The large market share of this segment is attributed to the increasing prevalence of cardiovascular diseases, growing preference for minimally invasive procedures, and launches of new thrombectomy devices.
Among the applications covered in this report, in 2023, the deep vein thrombosis segment is expected to account for the largest share of the venous thromboembolism treatment market. The large market share of this segment is attributed to the increasing prevalence of obesity, a growing geriatric population, rising cases of deep vein thrombosis due to lifestyle-related diseases, excessive alcohol consumption, and heightened awareness of DVT.
Among the end users covered in this report, in 2023, the hospitals segment is expected to account for the largest share of the venous thromboembolism treatment market. The large market share is attributed to the increasing prevalence of venous thromboembolism, the rising number of hospitals and clinics, the presence of highly skilled professionals, and the high purchasing power of hospitals. The easy accessibility to hospitals, coupled with a large patient population seeking comprehensive healthcare services, including diagnosis, treatment, and surgeries at a single location, also contributes to the significant market share of this segment.
An in-depth analysis of the geographical scenario of the global venous thromboembolism treatment market provides detailed qualitative and quantitative insights into the five major geographies: North America (U.S., Canada), Europe (Germany, France, U.K., Italy, Spain, Rest of Europe), Asia-Pacific (China, Japan, India), Latin America, and the Middle East & Africa.
In 2023, North America is expected to account for the largest share of the global venous thromboembolism treatment market, followed by Europe, Asia-Pacific, Latin America, and the Middle East & Africa. However, Asia-Pacific is slated to register the largest CAGR during the forecast period. The growth of this regional market can be attributed to factors such as the growing prevalence of chronic diseases, the increasing number of people living sedentary lifestyles, growing health expenditures, supportive government initiatives aimed at improving healthcare infrastructure, and rising awareness regarding venous thromboembolism.
The key players operating in the global venous thromboembolism treatment market are ALN Implants chirurgicaux (ALN Surgical Implants) (France), AngioDynamics, Inc. (U.S.), Argon Medical Devices, Inc. (U.S.), ArjoHuntleigh AB (Sweden), Boston Scientific Corporation (U.S.), Cardinal Health, Inc. (U.S.), Cook Group Incorporated (U.S.), Koninklijke Philips N.V. (Netherlands), DS MAREF Co., LTD (South Korea), Enovis Corporation (U.S.), LifeTech Scientific Corporation (China), Medtronic plc (Ireland), and Stryker Corporation (U.S.).
Scope of the Report:
Venous Thromboembolism Treatment Market Assessment-by Device
- Thrombectomy Devices
- Inferior Vena Cava Filters
- Retrievable Filters
- Permanent Filters
- Stockings
- Compression Pumps
- Other Devices & Accessories
Note: Other devices & accessories include extraction and repositioning devices for vena cava filters and garments for compression therapy (excluding stockings).
Venous Thromboembolism Treatment Market Assessment-by Application
- Deep Vein Thrombosis
- Pulmonary Embolism
Venous Thromboembolism Treatment Market Assessment-by End User
- Hospitals
- Ambulatory Care Centers
- Other End Users
Note: Other end users include nursing care centers, long-term care centers, and military health centers.
Venous Thromboembolism Treatment Market Assessment-by Geography
- North America
- U.S.
- Canada
- Europe
- Germany
- U.K.
- France
- Italy
- Spain
- Rest of Europe (RoE)
- Asia-Pacific (APAC)
- China
- Japan
- India
- Rest of APAC (RoAPAC)
- Latin America
- Middle East & Africa
TABLE OF CONTENTS
1. Introduction
- 1.1. Market Definition & Scope
- 1.2. Market Ecosystem
- 1.3. Currency & Limitations
- 1.4. Key Stakeholders
2. Research Methodology
- 2.1. Research Process
- 2.2. Process of Data Collection & Validation
- 2.2.1. Secondary Research
- 2.2.2. Primary Research/Interviews With Key Opinion Leaders of the Industry
- 2.3. Market Sizing and Forecasting
- 2.3.1. Market Size Estimation
- 2.3.1.1. Bottom-Up Approach
- 2.3.1.2. Top-Down Approach
- 2.3.1.3. Growth Forecast Approach
- 2.3.2. Market Share Analysis
- 2.3.1. Market Size Estimation
- 2.4. Assumptions for the Study
3. Executive Summary
4. Market Insights
- 4.1. Overview
- 4.2. Drivers
- 4.2.1. High Prevalence of Venous Thromboembolism
- 4.2.2. Rising Incidence of Cancer
- 4.3. Restraints
- 4.3.1. Instances of Product Failure and Product Recalls
- 4.4. Opportunities
- 4.4.1. Growing Accessibility and Affordability of Treatment Options
- 4.4.2. Advancements in VTE Diagnosis and Treatment Methods
- 4.5. Challenges
- 4.5.1. Lack of Awareness About Venous Thromboembolism
5. Venous Thromboembolism Treatment Market Assessment-by Device
- 5.1. Overview
- 5.2. Thrombectomy Devices
- 5.3. Inferior Vena Cava Filters
- 5.3.1. Retrievable Filters
- 5.3.2. Permanent Filters
- 5.4. Stockings
- 5.5. Compression Pumps
- 5.6. Other Devices & Accessories
6. Venous Thromboembolism Treatment Market Assessment-by Application
- 6.1. Overview
- 6.2. Deep Vein Thrombosis (DVT)
- 6.3. Pulmonary Embolism (PE)
7. Venous Thromboembolism Treatment Market Assessment-by End User
- 7.1. Overview
- 7.2. Hospitals
- 7.3. Ambulatory Care Centers
- 7.4. Other End Users
8. Venous Thromboembolism Treatment Market Assessment-by Geography
- 8.1. Overview
- 8.2. North America
- 8.2.1. U.S.
- 8.2.2. Canada
- 8.3. Europe
- 8.3.1. Germany
- 8.3.2. France
- 8.3.3. U.K.
- 8.3.4. Italy
- 8.3.5. Spain
- 8.3.6. Rest of Europe
- 8.4. Asia-Pacific
- 8.4.1. China
- 8.4.2. Japan
- 8.4.3. India
- 8.4.4. Rest of Asia-Pacific
- 8.5. Latin America
- 8.6. Middle East & Africa
9. Competition Analysis
- 9.1. Overview
- 9.2. Key Growth Strategies
- 9.3. Competitive Benchmarking
- 9.4. Competitive Dashboard
- 9.4.1. Industry Leaders
- 9.4.2. Market Differentiators
- 9.4.3. Vanguards
- 9.4.4. Emerging Companies
- 9.5. Market Share Analysis (2022)
- 9.5.1. Boston Scientific Corporation (U.S.)
- 9.5.2. Stryker Corporation (U.S.)
- 9.5.3. Cardinal Health, Inc. (U.S.)
- 9.5.4. Enovis Corporation (U.S.)
- 9.5.5. Medtronic plc (Ireland)
- 9.5.6. Cook Group Incorporated (U.S.)
10. Company Profiles
- 10.1. ALN Implants chirurgicaux (ALN Surgical Implants)
- 10.2. AngioDynamics, Inc.
- 10.3. Argon Medical Devices, Inc.
- 10.4. ArjoHuntleigh AB
- 10.5. Boston Scientific Corporation
- 10.6. Cardinal Health, Inc.
- 10.7. Cook Group Incorporated
- 10.8. Koninklijke Philips N.V.
- 10.9. DS MAREF Co., Ltd
- 10.10. Enovis Corporation
- 10.11. LifeTech Scientific Corporation
- 10.12. Medtronic plc
- 10.13. Stryker Corporation
11. Appendix
- 11.1. Available Customization
- 11.2. Related Reports