|
|
市場調査レポート
商品コード
1632465
DLL3標的治療薬の世界市場:承認用量、価格、臨床試験動向(2025年)Global DLL3 Targeted Therapies Market, Approved Drugs Dosage, Pricing & Clinical Trials Insight 2025 |
||||||
|
|||||||
| DLL3標的治療薬の世界市場:承認用量、価格、臨床試験動向(2025年) |
|
出版日: 2025年01月01日
発行: KuicK Research
ページ情報: 英文 140 Pages
納期: 即日から翌営業日
|
全表示
- 概要
- 図表
- 目次
デルタ様リガンド3(DLL3)は、小細胞肺がん(SCLC)のような神経内分泌腫瘍で優位に発現する一方で、正常な成人組織では最小限の発現しか示さない細胞表面タンパク質です。この選択的発現により、DLL3は治療選択肢が限られている攻撃的がんの魅力的な治療標的となります。DLL3を標的とすることで、新規治療薬は健康な組織へのダメージを抑えながら腫瘍細胞を効果的に標的とすることができ、プレシジョン・オンコロジーの原則に合致します。2024年にDLL3を標的とした最初の治療法が承認されたことは極めて重要な開発であり、この特徴的なバイオマーカーに焦点を当てた治療と診断の両方の進歩に対する関心が高まるきっかけとなっています。
2024年5月にAmgenからイムデルトラ(タルラタマブ)が承認されたことで、DLL3を標的とする治療法の展望は大きく変わっています。DLL3を標的とした最初の治療薬であるImdelltraは、腫瘍学分野における目覚ましい進歩を意味します。この二重特異性T細胞エンゲイジャー(BiTE)は、プラチナ製剤ベースの化学療法後に病状が進行した広範病期小細胞肺がん(ES-SCLC)の成人患者を対象に承認を受けた。臨床試験で観察された顕著な奏効率と奏効期間(DoR)に後押しされたこの早期承認は、その臨床的有望性を浮き彫りにしています。とはいえ、その治療効果をさらに立証するためには、現在進行中の確認試験が不可欠です。
商業的観点からは、Imdelltraは、米国市場のみで発売後数ヶ月で4,500万米ドルを超える売上を達成し、目覚しい初期成功を収めています。この初期の業績は、ES-SCLCにおける革新的治療に対する大きな需要を反映しており、Imdelltraをこの開発途上の治療カテゴリーにおける重要な競合薬として位置づけています。イムデルトラの商業的成功は、DLL3標的治療薬への関心と投資の急増を呼び起こし、主要企業がこの有望な市場での権益をめぐって競い合う中、多くの提携やライセンシング契約につながっています。
当レポートは、世界のDLL3標的治療薬市場について調査し、市場の概要とともに、薬剤動向、臨床試験動向、地域別動向、および市場に参入する企業の競合情勢などを提供しています。
目次
第1章 DLL3標的療法のイントロダクション
第2章 DLL3ターゲットのアプローチ、メカニズム、商業的側面
- 抗体薬物複合体
- 抗体
- 細胞療法
- 抗体放射性核種複合体
- 抗体光吸収剤/光増感剤複合体
第3章 DLL3標的療法の独自プラットフォーム
- DLL3標的治療の開発のために使用されるプラットフォーム
- DLL3標的治療の開発のための潜在的プラットフォーム
第4章 イムデルトラ- 最初に承認されたDLL3標的薬
- 臨床概要と特許の洞察
- 治療サイクルの価格設定と投与量分析
- 売上分析
第5章 DLL3標的療法市場と臨床動向の洞察
- 現在の市場動向、開発、臨床試験の評価
- 将来の商業化の機会
第6章 DLL3標的療法の調査と地域別の市場動向
- 米国
- 英国
- 欧州連合
- カナダ
- オーストラリア
- 中国
第7章 DLL3標的療法の臨床開発と適応症別の市場洞察
- 小細胞肺がん
- 大細胞神経内分泌がん
- 神経内分泌前立腺がん
- 消化管膵神経内分泌腫瘍
- 神経膠腫
第8章 DLL3標的薬の世界臨床試験の概要
- 企業別
- ファストトラックステータス
- 適応症別
- 場所別
- 患者セグメント別
- フェーズ別
第9章 DLL3標的薬の臨床試験(企業別、適応症別、相別)
- 調査
- 前臨床
- 第I相
- 第I/II相
- 第II相
第10章 市販されているDLL3標的薬の臨床試験(企業別、適応症別、相別)
第11章 DLL3標的療法市場力学
第12章 DLL3標的療法の研究開発に携わる主要企業
- Abdera Therapeutics
- Allogene Therapeutics
- Amgen
- Boehringer Ingelheim
- Chugai Pharmaceutical
- Gensun Biopharma
- Harpoon Therapeutics
- ImaginAb Inc
- Legend Biotech
- Molecular Partners
- Phanes Therapeutics
- Qilu Pharmaceutical
- Shanghai Affinity Biopharmaceutical
- ZAI Lab
List of Figures
- Figure 2-1: Antibody Drug Conjugates - Mechanism Of Action
- Figure 2-2: BiTE - Amgen
- Figure 2-3: HPN328 - Structure
- Figure 2-4: Rova-IR700 - Mechanism
- Figure 3-1: T-Charge Platform - Features
- Figure 3-2: BiTE(R) Antibody Construct
- Figure 3-3: TMALIN - ADC Advantages
- Figure 3-4: TMALIN - ADC Structure
- Figure 3-5: TriTAC - T-Cell Engagers Structure
- Figure 3-6: TriTAC - Advantages
- Figure 3-7: PACbody(TM) Platform
- Figure 3-8: ATACCbody(TM) Platform
- Figure 3-9: SPECpair(TM) Platform
- Figure 3-10: ROVEr Platform - Process
- Figure 3-11: RPT - Advantages
- Figure 3-12: Dual-Ig Technology - Antibody Structure & Mechanism
- Figure 3-13: AlloCAR T - Process
- Figure 3-14: AlloCAR T - Features
- Figure 3-15: Alluminox(TM) Platform - Drug Structure
- Figure 3-16: Rakuten Medical - Photoimmunotherapy Process
- Figure 4-1: Imdelltra - Approval Year By Region
- Figure 4-2: US - Price of Supply of Imdelltra Intravenous Powder (US$), January'2025
- Figure 4-3: EU - Price Of Imdelltra Intravenous Powder (US$), January'2025:
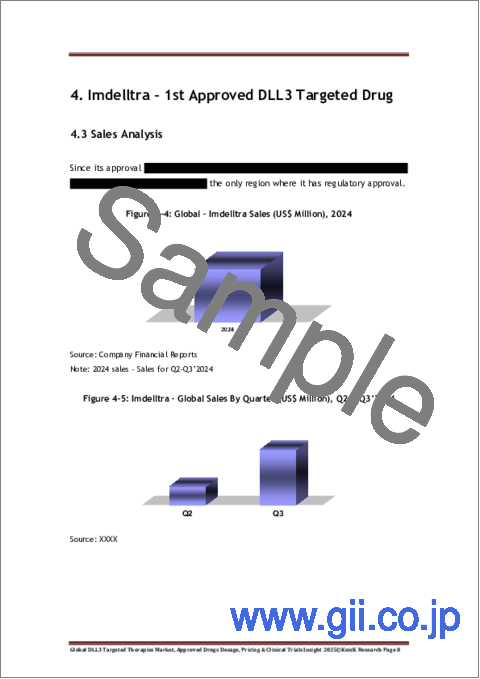
- Figure 4-4: Global - Imdelltra Sales (US$ Million), 2024
- Figure 4-5: Imdelltra - Global Sales By Quarter (US$ Million), Q2 & Q3'2024
- Figure 4-6: Imdelltra - US Sales (US$ Million), 2024
- Figure 4-7: Imdelltra- US Sales By Quarter (US$ Million), Q2 & Q3'2024
- Figure 5-1: Global - DLL3 Targeted Therapy Market (US$ Million), 2024
- Figure 5-2: Global - DLL3 Targeted Therapy Market Sales (US$ Million), Q2 & Q3'2024
- Figure 7-1: DLL3 - Small Cell Lung Cancer
- Figure 7-2: DeLLphi-301 Phase 2 Study - Initiation & Completion Year
- Figure 7-3: DeLLphi-304 Phase 2 Study - Initiation & Completion Year
- Figure 7-4: DAREON(TM)-5 Phase 2 Study - Initiation & Completion Year
- Figure 7-5: PT217X1101 Phase 1 Study - Initiation & Completion Year
- Figure 7-6: HPN328 Phase 1 Study - Initiation & Completion Year
- Figure 7-7: LB2102-1001 Phase 1 Study - Initiation & Completion Year
- Figure 7-8: NCT05507593 Phase 1 Study - Initiation & Completion Year
- Figure 7-9: DLL3 - Large Cell Neuroendocrine Cancer
- Figure 8-1: Global - DLL3 Targeted Drugs Clinical Trials By Company (Number), 2025
- Figure 8-2: Global - DLL3 Targeted Drugs Clinical Trials By Fast Track Status (Number), 2025
- Figure 8-3: Global - DLL3 Targeted Drugs Clinical Trials By Indication (Number), 2025
- Figure 8-4: Global - DLL3 Targeted Drugs Clinical Trials By Location (Number), 2025
- Figure 8-5: Global - DLL3 Targeted Drugs Clinical Trials By Patient Segment (Number), 2025
- Figure 8-6: Global - DLL3 Targeted Drugs Clinical Trials By Phase (Number), 2025
- Figure 11-1: DLL3 Targeted Therapies - Market Drivers & Opportunities
- Figure 11-2: DLL3 Targeted Therapies - Market Challenges & Restraints
List of Tables
- Table 6-1: US - FDA Designated DLL3 Targeted Therapies, 2025
- Table 7-1: Large Cell Neuroendocrine Cancer - Candidates In Clinical Trials, 2025
- Table 7-2: Neuroendocrine Prostate Cancer - Candidates In Clinical Trials, 2025
- Table 7 3: Gastroenteropancreatic Neuroendocrine Tumors - Candidates In Clinical Trials, 2025
- Table 7-4: Glioma - Candidates In Clinical Trials, 2025
Global DLL3 Targeted Therapies Market, Approved Drugs Dosage, Pricing & Clinical Trials Insight 2025 Report Finding and Highlights:
- Global & Regional Market Overview
- DLL3 Targeted Drugs Available In Market:1 Drug, Imdelltra (Tarlatamab)
- Approved Drug Dosage, Sales & Pricing Insight
- DLL3 Targeted Therapies Proprietary Platforms: > 10 Platform
- Role of DLL3 As Diagnostic & Prognostic Markers
- DLL3 Targeted Drugs Clinical Trials Insight By Company, Indication and Phase: > 10 Drugs
- Global DLL3 Targeted Therapies Clinical Development Trends By Indication
Delta-like ligand 3 (DLL3) is a cell surface protein that is predominantly expressed in neuroendocrine tumors, such as small cell lung cancer (SCLC), while exhibiting minimal presence in normal adult tissues. This selective expression renders DLL3 an attractive therapeutic target for aggressive cancers that have limited treatment alternatives. By targeting DLL3, novel therapies can effectively target tumor cells while reducing damage to healthy tissues, aligning with the principles of precision oncology. The approval of the first DLL3-targeted therapy in 2024 represented a pivotal development, sparking increased interest in both treatment and diagnostic advancements focused on this distinctive biomarker.
The approval of Imdelltra (tarlatamab) by Amgen in May 2024 significantly transformed the landscape for DLL3-targeted therapies. As the first therapy aimed at DLL3, Imdelltra signifies a remarkable progression in the field of oncology. This bispecific T-cell engager (BiTE) received approval for adult patients with extensive-stage small cell lung cancer (ES-SCLC) whose condition had progressed following platinum-based chemotherapy. Its accelerated approval, driven by notable response rates and duration of response (DoR) observed in clinical trials, highlights its clinical promise. Nevertheless, ongoing confirmatory studies are essential to further substantiate its therapeutic efficacy.
From a commercial perspective, Imdelltra has achieved impressive initial success, generating over US$ 45 Million in sales within its first few months solely in the US market. This early performance reflects the substantial demand for innovative therapies in ES-SCLC and positions Imdelltra as a significant contender in this developing therapeutic category. Its commercial success has sparked a surge of interest and investment in DLL3-targeting therapies, leading to numerous collaborations and licensing agreements as companies compete for a stake in this promising market.
Boehringer Ingelheim's Obrixtamig (BI 764532), a DLL3/CD3 bispecific antibody, represents the next most advanced clinical candidate in this domain. Developed using the OGAP(R) Discovery Platform, Obrixtamig has received FDA Fast Track designation for small cell lung cancer, underscoring its potential to offer a new therapeutic option for patients. Although clinical development is ongoing, its advancement signifies the increasing competition and variety of strategies within the DLL3-targeting sector.
The approval and initial success of Imdelltra have catalyzed a surge in licensing agreements aimed at expediting the development of innovative DLL3-targeted therapies. For example, in January 2025, Innovent Biologics entered into a collaboration and exclusive licensing agreement with Roche to further develop IBI3009, a novel DLL3-targeted antibody-drug conjugate (ADC). With IND approvals secured in Australia, China, and the US, and the first patient dosed in December 2024, this partnership highlights the potential of ADCs in enhancing DLL3-targeting strategies. Roche's intention to assume full development responsibilities after the initial phases further emphasizes the strategic significance of this collaboration, with Innovent poised to receive up to US$ 1 billion in milestone payments along with tiered royalties on net sales.
In addition to therapeutic uses, DLL3 is being utilized as a diagnostic tool, thereby increasing its overall market potential. Researchers at Memorial Sloan Kettering Cancer Center have created an imaging technology that employs a radioactive particle binding to DLL3, facilitating more accurate detection of DLL3-expressing lung and prostate cancers through PET scans. This diagnostic advancement could be pivotal in identifying patients likely to benefit from DLL3-targeted therapies, such as Imdelltra, thus optimizing treatment outcomes and broadening the market for these agents.
The integration of substantial clinical evidence, impressive commercial success, and continuous research and development efforts establishes the DLL3-targeting therapies market as a vibrant and swiftly expanding area within oncology. Numerous companies are progressing their development pipelines, forming strategic partnerships, and investigating novel diagnostic uses, indicating that the future of DLL3-targeted therapies is highly promising for both patients and stakeholders. As the market evolves, it is expected to become a fundamental component in the treatment of cancers associated with DLL3 expression.
Table of Contents
1. Introduction To DLL3 Targeted Therapies
- 1.1 Overview
- 1.2 History & Evolution Of DLL3 Targeted Therapies
- 1.3 Role Of DLL3 As Diagnostic & Prognostic Markers
2. DLL3 Targeting Approaches, Mechanism & Commercial Aspects
- 2.1 Antibody Drug Conjugates
- 2.2 Antibodies
- 2.3 Cell Therapies
- 2.4 Antibody Radionuclide Conjugates
- 2.5 Antibody Photoabsorber/Photosensitizer Conjugate
3. DLL3 Targeted Therapies Proprietary Platforms
- 3.1 Platforms Used To Develop DLL3 Targeted Therapies
- 3.2 Potential Platforms For DLL3 Targeted Therapies Development
4. Imdelltra - 1st Approved DLL3 Targeted Drug
- 4.1 Clinical Overview & Patent Insight
- 4.2 Treatment Cycle Pricing & Dosage Analysis
- 4.3 Sales Analysis
5. DLL3 Targeted Therapies Market & Clinical Trends Insights
- 5.1 Current Market Trends, Developments & Clinical Trials Assessment
- 5.2 Future Commercialization Opportunity
6. DLL3 Targeted Therapies Research & Market Trends by Region
- 6.1 US
- 6.2 UK
- 6.3 EU
- 6.4 Canada
- 6.5 Australia
- 6.6 China
7. Global DLL3 Targeted Therapies Clinical Development & Market Insight By Indication
- 7.1 Small Cell Lung Cancer
- 7.2 Large Cell Neuroendocrine Cancer
- 7.3 Neuroendocrine Prostate Cancer
- 7.4 Gastroenteropancreatic Neuroendocrine Tumors
- 7.5 Glioma
8. Global DLL3 Targeted Drugs Clinical Trials Overview
- 8.1 By Company
- 8.2 By Fast Track Status
- 8.3 By Indication
- 8.4 By Location
- 8.5 By Patient Segment
- 8.6 By Phase
9. Global DLL3 Targeted Drugs Clinical Trials By Company, Indication & Phase
- 9.1 Research
- 9.2 Preclinical
- 9.3 Phase-I
- 9.4 Phase-I/II
- 9.5 Phase-II
10. Marketed DLL3 Targeted Drugs Clinical Trials By Company, Indication & Phase
11. DLL3 Targeted Therapies Market Dynamics
- 11.1 Favorable Market & Clinical Development Parameters
- 11.2 Challenges & Restraints
12. Key Companies Involved In Research & Development Of DLL3 Targeted Therapies
- 12.1 Abdera Therapeutics
- 12.2 Allogene Therapeutics
- 12.3 Amgen
- 12.4 Boehringer Ingelheim
- 12.5 Chugai Pharmaceutical
- 12.6 Gensun Biopharma
- 12.7 Harpoon Therapeutics
- 12.8 ImaginAb Inc
- 12.9 Legend Biotech
- 12.10 Molecular Partners
- 12.11 Phanes Therapeutics
- 12.12 Qilu Pharmaceutical
- 12.13 Shanghai Affinity Biopharmaceutical
- 12.14 ZAI Lab