|
|
市場調査レポート
商品コード
1604220
CD40標的療法の世界市場:臨床試験、治療アプローチ、市場機会の洞察(2025年)Global CD40 Targeted Therapies Clinical Trials, Therapeutic Approaches & Market Opportunity Insight 2025 |
||||||
|
|||||||
| CD40標的療法の世界市場:臨床試験、治療アプローチ、市場機会の洞察(2025年) |
|
出版日: 2024年11月01日
発行: KuicK Research
ページ情報: 英文 110 Pages
納期: 即日から翌営業日
|
全表示
- 概要
- 図表
- 目次
CD40標的療法は免疫療法研究の焦点となっており、CD40レセプターの潜在能力を利用してさまざまな疾患を治療することへの注目が高まっています。CD40標的療法はまだ臨床使用が承認されていませんが、いくつかの有望な候補が現在後期臨床開発段階にあります。さらに、来年中にはさらなる候補が後期臨床試験に入る予定で、臨床開発中のCD40標的療法のプールが拡大しています。これらの進行中の調査や臨床試験は、CD40標的療法が今後数年のうちに有病者にとって重要な治療選択肢となる可能性を示唆しています。
歴史的に、CD40標的療法の主な焦点は自己免疫疾患と炎症性疾患でした。しかし、がんやその他の疾患の治療に対するCD40標的療法の可能性を探ることに関心が高まっています。さらに最近の研究では、HIVやCOVID-19のような感染症、アルツハイマー病やパーキンソン病のような神経変性疾患にまでCD40標的化の範囲を広げています。また、CD40のシグナル伝達経路が疾患の発症には直接関与していないもの、疾患の進行や炎症に関与している心血管系疾患などにもCD40標的療法が有効である可能性を示唆する研究もあります。
CD40標的療法の開発は、独自の技術やプラットフォームの出現によって大いに促進されてきました。その顕著な例のひとつがGenMab社のDuoBodyプラットフォームで、これは2つの異なる抗原を同時に標的とする二特異性抗体の創出を可能にします。この技術は、悪性固形腫瘍を対象に現在第2相試験中のCD40標的療法GEN1042/BNT312を含む、いくつかの有望な免疫療法の開発に活用されています。
結論として、CD40標的療法はまだ臨床使用が承認されていないもの、幅広い疾患において有望かつ急速に発展している研究開発分野です。より多くの治療法が臨床試験に参入し、競合情勢が激化するにつれ、CD40標的療法が近い将来、がん、自己免疫疾患、感染症、そしてそれ以外の疾患の治療の礎となる可能性があることは明らかです。
当レポートは、世界のCD40標的療法市場について調査し、市場の概要とともに、薬剤動向、臨床試験動向、および市場に参入する企業の競合情勢などを提供しています。
目次
第1章 CD40標的療法のイントロダクション
第2章 CD40標的療法- 作用機序
第3章 CD40を標的とした治療法
- 抗体の形式
- 融合タンパク質
- ペプチド
- 核酸
第4章 CD40標的療法の研究開発動向、適応症別
- がん
- 神経疾患
- 自己免疫疾患および炎症性疾患
- 移植拒絶反応
- 微生物感染症
第5章 世界のCD40標的療法市場の見通し
- 現在の市場動向と動向
- 将来の市場機会
第6章 CD40標的療法市場動向分析、地域別
- 米国
- 欧州
- 中国
- 日本
- オーストラリア
第7章 CD40標的療法の臨床試験に関する企業、国、適応症、相別の考察
- 調査
- 前臨床
- 第I相
- 第I/II相
- 第II相
- 第III相
第8章 CD40標的療法- 企業別独自技術
第9章 世界のCD40標的療法市場力学
- 促進要因と機会
- 課題と抑制要因
第10章 競合情勢
- Alligator Bioscience
- Amgen
- Biogen
- BioNTech
- Eledon Pharmaceuticals
- EnnoDC
- Genmab
- Innovent Bio
- Novartis
- Sanofi
- Tonix Pharmaceuticals
- UCB
List of Figures
- Figure 1-1: CD40 - Salient Features
- Figure 1-2: CD40 Targeted Therapy Landscape - Major Milestones
- Figure 2-1: CD40 Agonists - Mechanism of Action
- Figure 3-1: Prominent Antibody Formats for Targeting CD40
- Figure 4-1: OPTIMIZE-1 Phase 1b/2 (NCT04888312) Study - Initiation & Completion Year
- Figure 4-2: Mitazalimab Phase 1 (NCT06205849) Study - Initiation & Completion Year
- Figure 4-3: 2141-V11 Phase 1/2 (NCT05126472) Study - Initiation & Completion Year
- Figure 4-4: 2141-V11 Phase 1 (NCT06455605) Study - Initiation & Completion Year
- Figure 4-5: 2141-V11 Phase 1/2 (NCT05734560) Study - Initiation & Completion Year
- Figure 4-6: ACT16877 Phase 2 (NCT04879628) Study - Initiation & Completion Year
- Figure 4-7: FREVIVA Phase 3 (NCT06141486) Study - Initiation & Completion Year
- Figure 4-8: FREXALT 3 (NCT06141473) Study - Initiation & Completion Year
- Figure 4-9: HZNP-DAZ-301 Phase 3 (NCT06104124) Study - Initiation & Completion Year
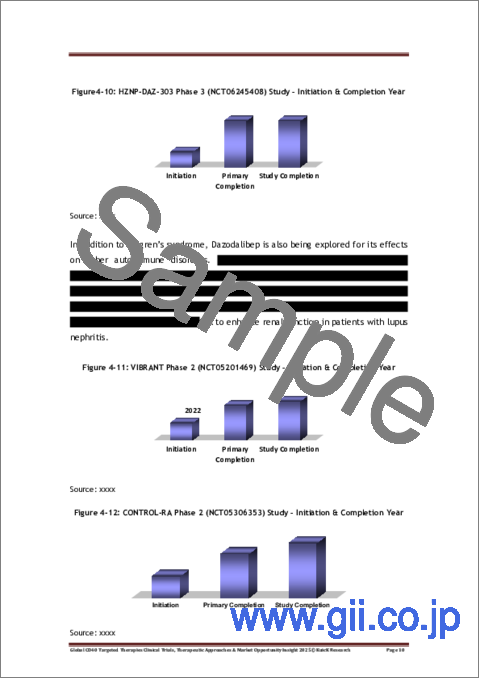
- Figure 4-10: HZNP-DAZ-303 Phase 3 (NCT06245408) Study - Initiation & Completion Year
- Figure 4-11: VIBRANT Phase 2 (NCT05201469) Study - Initiation & Completion Year
- Figure 4-12: CONTROL-RA Phase 2 (NCT05306353) Study - Initiation & Completion Year
- Figure 4-13: PHOENYCS FLY Phase 3 (NCT06617325) Study - Initiation & Completion Year
- Figure 4-14: SL0046 Phase 3 (NCT04976322) Study - Initiation & Completion Year
- Figure 4-15: APB-A1 Phase 1b (NCT06557850) Study - Initiation & Completion Year
- Figure 4-16: FABULINUS Phase 2 (NCT06111586) Study - Initiation & Completion Year
- Figure 4-17: IRB23-1367 Phase 1/2 (NCT06111586) Study - Initiation & Completion Year
- Figure 4-18: AT-1501-K102 Phase 1 (NCT05027906) Study - Initiation & Completion Year
- Figure 4-19: BESTOW/AT-1501-K207 Phase 2 (NCT05983770) Study - Initiation & Completion Year
- Figure 4-20: BESTOW-EXTENSION/AT-1501-K209 Phase 2 (NCT06126380) Study - Initiation & Completion Year
- Figure 4-21: ANRS VRI06 Phase 1 (NCT04842682) Study - Initiation & Completion Year
- Figure 4-22: HVTN 318 Phase 1 (NCT06665646) Study - Initiation & Completion Year
- Figure 4-23: ANRS0407s - LKV.Cov40 Phase 1/2 (NCT06255626) Study - Initiation & Completion Year
- Figure 8-1: Alligator Bioscience - FIND technology
- Figure 8-2: Alligator Bioscience - Neo-X-Prime
- Figure 8-3: Aprilbio - SAFA Antibody APB-A1
- Figure 8-4: Genmab - DuoBody
- Figure 8-5: Harbour BioMed - Harbour Mice
- Figure 8-6: Harbour BioMed - HCAb
- Figure 8-7: H2L2 & HCAb Platforms - Features
- Figure 8-8: Harbour BioMed - HBICE
- Figure 8-9: Kyowa Kirin - REGULGENT
- Figure 8-10: ADAC Technology Platform - Features
Figure 8-11 Strike Pharma - ADAC Technology Platform
- Figure 8-12: Strike Pharma - ADAC Molecule
- Figure 9-1: CD40 Targeted Therapy Market - Drivers & Opportunities
- Figure 9-2: CD40 Targeted Therapy Market - Challenges & Restraints
List of Tables
- Table 5-1: CD40 Targeted Therapies in Phase III Clinical Trials
- Table 7-1: CD40 Targeted Therapies in Research Stage, 2024
- Table 7-2: CD40 Targeted Therapies in Preclinical Stage, 2024
- Table 7-3: CD40 Targeted Therapies in Phase I, 2024
- Table 7-4: CD40 Targeted Therapies in Phase I/II, 2024
- Table 7-5: CD40 Targeted Therapies in Phase II, 2024
- Table 7-6: CD40 Targeted Therapies in Phase III, 2024
Global CD40 Targeted Therapies Clinical Trials, Therapeutic Approaches & Market Opportunity Insight 2025 Report Highlights:
- First CD40 Targeted Therapy Approval Expected By 2027
- CD40 Targeted Therapies In Clinical Trials: > 20 Therapies
- CD40 Inhibitors Clinical Trials Insight By Company, Country, Indication & Phase
- CD40 Targeted Therapy Research & Development Trends By Indication
- CD40 Targeted Therapy Market Trend Analysis By Region
- CD40 Targeted Therapies Proprietary Technologies By Company
CD40 targeted therapies have become a focal point in immunotherapy research, with growing attention on harnessing the potential of the CD40 receptor to treat a variety of diseases. While no CD40 targeted therapies have yet been approved for clinical use, some promising candidates are currently undergoing late stage clinical development. Moreover, additional candidates are slated to enter late-stage trials within the next year, expanding the pool of CD40 targeted therapies in clinical development. These ongoing research and clinical trials suggest that CD40 targeted therapies could become a vital treatment option for patients with prevalent diseases in the coming years.
Historically, the primary focus of CD40 targeted therapies has been on autoimmune and inflammatory diseases. However, there is an increasing interest in exploring their potential for treating cancer and other conditions. More recent studies have expanded the scope of CD40 targeting to include infectious diseases, such as HIV and COVID-19, as well as neurodegenerative diseases like Alzheimer's and Parkinson's disease. Some research has also suggested that CD40 targeted therapies might be beneficial for diseases like cardiovascular conditions, where CD40's signaling pathway, although not directly involved in disease initiation, plays a role in disease progression and inflammation.
Over the years, various strategies have been employed to target the CD40 receptor and its ligand. These include traditional monoclonal antibodies as well as next-generation molecular therapeutics such as small interfering RNA (siRNA). While antibodies are developed either as CD40 agonists or as antagonists, CD40 targeted siRNAs aim to reduce CD40 protein expression by degrading CD40 mRNA. Several other conventional and next-generation approaches are undergoing active research and clinical trials to assess their safety and efficacy.
Nonetheless, antibody therapies remain the dominant approach in CD40 targeted research. Among the most advanced CD40/CD40L targeted antibodies are those for autoimmune and inflammatory diseases, with some entering phase 3 clinical trials. For example, Sanofi's Frexalimab, a promising anti CD40 antibody, is currently in phase 3 trials for autoimmune conditions. In the cancer domain, CD40 targeted antibodies are also making significant strides, with companies like Alligator Bioscience preparing to initiate phase 3 clinical trials for its lead candidate, mitazalimab, an anti-CD40 antibody for pancreatic ductal adenocarcinoma (PDA) and pancreatic cancer.
The development of CD40 targeted therapies has been greatly facilitated by the advent of proprietary technologies and platforms. One notable example is GenMab's DuoBody platform, which enables the creation of bispecific antibodies that can target two different antigens simultaneously. This technology has been leveraged to develop several promising immunotherapies, including its CD40 targeted therapy, GEN1042/BNT312, currently in phase 2 trial for malignant solid tumors.
As the field of CD40 targeted therapies evolves, the competitive landscape is becoming increasingly crowded, with many major pharmaceutical companies entering the arena. Notable players include Amgen, Sanofi, GenMab, BioNTech, and Biogen, all of which are investing heavily in this space. Collaborations between pharmaceutical companies and academic institutions have been essential in advancing the scientific understanding of CD40 targeted therapies, combining cutting edge research with clinical expertise to bring these therapies closer to market.
In conclusion, while CD40 targeted therapies have not yet been approved for clinical use, they represent a promising and rapidly developing area of research across a wide range of diseases. As more therapies enter clinical trials and the competitive landscape intensifies, it is clear that CD40 targeted therapies could become a cornerstone of treatment for cancer, autoimmune diseases, infectious diseases, and beyond in the near future.
Table of Contents
1. Introduction To CD40 Targeted Therapies
- 1.1 Overview
- 1.2 Considering CD40 As Drug Target Over Other CD Markers
- 1.3 History & Evolution Of CD40 Targeting Therapies
2. CD40 Targeted Therapies - Mechanism Of Action
- 2.1 Agonist Mediated Activation Of CD40 Signaling
- 2.2 Antagonist-Mediated Inhibition Of CD40 Signaling
3. CD40 Targeted Therapeutic Approaches
- 3.1 Antibody Formats
- 3.2 Fusion Proteins
- 3.3 Peptides
- 3.4 Nucleic Acids
4. CD40 Targeted Therapy Research & Development Trends By Indication
- 4.1 Cancer
- 4.2 Neurological Diseases
- 4.3 Autoimmune & Inflammatory Disorders
- 4.4 Transplant Rejection
- 4.5 Microbial Infections
5. Global CD40 Targeted Therapy Market Outlook
- 5.1 Current Market Trends & Developments
- 5.2 Future Market Opportunities
6. CD40 Targeted Therapy Market Trend Analysis By Region
- 6.1 US
- 6.2 Europe
- 6.3 China
- 6.4 Japan
- 6.5 Australia
7. CD40 Targeted Therapies Clinical Trials Insight By Company, Country, Indication & Phase
- 7.1 Research
- 7.2 Preclinical
- 7.3 Phase I
- 7.4 Phase I/II
- 7.5 Phase II
- 7.6 Phase III
8. CD40 Targeted Therapies - Proprietary Technologies By Company
- 8.1 Alligator Bioscience - FIND Technology & Neo-X-Prime
- 8.2 Aprilbio - SAFA Platform
- 8.3 Biocytogen - RenMice
- 8.4 EnnoDC - Unnamed Platform
- 8.5 Genmab - DuoBody
- 8.6 Harbour BioMed - Harbour Mice & HBICE
- 8.7 Kyowa Kirin - REGULGENT
- 8.8 Strike Pharma - ADAC Technology Platform
9. Global CD40 Targeted Therapy Market Dynamics
- 9.1 Drivers & Opportunities
- 9.2 Challenges & Restraints
10. Competitive Landscape
- 10.1 Alligator Bioscience
- 10.2 Amgen
- 10.3 Biogen
- 10.4 BioNTech
- 10.5 Eledon Pharmaceuticals
- 10.6 EnnoDC
- 10.7 Genmab
- 10.8 Innovent Bio
- 10.9 Novartis
- 10.10 Sanofi
- 10.11 Tonix Pharmaceuticals
- 10.12 UCB