|
|
市場調査レポート
商品コード
1716267
医療美容デバイス:各種技術と世界市場Medical Aesthetic Devices: Technologies and Global Markets |
||||||
|
|||||||
| 医療美容デバイス:各種技術と世界市場 |
|
出版日: 2025年04月21日
発行: BCC Research
ページ情報: 英文 144 Pages
納期: 即納可能
|
全表示
- 概要
- 図表
- 目次
世界の医療美容デバイスの世界市場規模は、2023年の201億米ドル、2024年の222億米ドルから、予測期間中はCAGR 11.3%で推移し、2029年末には379億米ドルに成長すると予測されています。
北米市場は、2024年の81億米ドルから、予測期間中はCAGR 10.0%で推移し、2029年末には130億米ドルに成長すると予測されています。アジア太平洋市場は、2024年の51億米ドルから、予測期間中はCAGR 12.8%で推移し2029年末には93億米ドルに成長すると予測されています。
当レポートでは、世界の医療美容デバイスの市場を調査し、市場概要、市場影響因子および市場機会の分析、法規制環境、新興技術および技術開発の動向、市場規模の推移・予測、各種区分・地域別の詳細分析、競合情勢、主要企業のプロファイルなどをまとめています。
目次
第1章 エグゼクティブサマリー
- 市場見通し
- 調査範囲
- 市場サマリー
第2章 市場概要
- 医療美容:概要
- 美容処置
- 健康と美をつなぐ
- 成長と市場の可能性
- 人口統計と消費者の嗜好
- 美容治療の概要
- レーザー治療における技術革新
- 医療美容の専門団体
- 医療ツーリズム
- 医療ツーリズムにおける美容整形の役割
- 医療ツーリズムのメリット
- 将来の見通し
- 医療美容に関する統計
第3章 市場力学
- 市場力学
- 市場促進要因
- 低侵襲デバイスの採用
- 医療美容処置の増加
- レーザー技術を用いた美容整形手術の発展
- 肥満率の上昇
- 豊胸手術の需要増加
- 医療ツーリズムの増加
- 市場の課題と制約
- 医療美容の費用
- 低い償還ポリシーと厳しい政府規制
- 医療美容に関連する倫理的および社会的偏見
- 美容整形手術による副作用
- 市場機会
- 新興経済国
- 医療美容企業向けマーケティング戦略
第4章 業界展望:規制の枠組み
- 米国の規制の枠組み
- 規制当局と歴史的背景
- FDAの監督プロセス
- カナダの規制の枠組み
- EUにおける規制の枠組み
- 管轄当局の主な責任
- EU域外製造業者に対する規制要件
- EUと米国間の相互承認協定 (MRA)
- 承認および品質システム評価
- 品質基準と検査
- 日本の規制の枠組み
- 医療美容デバイスの承認プロセス
- 臨床試験
- 外国の臨床データの受け入れ
- 医療美容デバイス製造業者への影響
- 中国の規制の枠組み
- 医療美容デバイスの分類
- 登録プロセス
- ラベルおよび文書化の要件
- 追加の規制措置
- 課題とタイムライン
- インドの規制の枠組み
- 医療美容デバイスの輸入・承認
- 規制監督における課題
- リスクベースの枠組みへの移行
- 国内製造業者への影響
- 今後の道のり
- ラテンアメリカにおける規制の枠組み
- サウジアラビアの規制の枠組み
第5章 新興技術と開発
- 新興技術
- 脂肪減少および皮膚引き締め機器の開発
- 美容レーザー機器の進歩
- ロボット手術の導入
- AIの導入
- RFおよび超音波技術の進歩
- 再生医療と幹細胞療法の統合
第6章 市場セグメンテーション分析
- 市場動向
- セグメンテーションの内訳
- 医療美容デバイスの世界市場
- 市場分析:デバイスタイプ別
- 非エネルギーベース
- エネルギーベース
- 市場分析:用途別
- フェイシャルエステ
- 皮膚の再生と引き締め
- ボディコントゥアリング・セルライト除去
- 豊胸手術
- 脱毛
- 市場分析:エンドユーザー別
- 病院
- 痩身・美容クリニック
- 皮膚科クリニック
- 地理的内訳
- 市場分析:地域別
- 北米
- 欧州
- アジア太平洋
- その他の地域
第7章 競合情報
- 競合情勢
- 企業ランキング
- 開発と戦略
- パートナーシップとコラボレーション
- 製品の発売と承認
第8章 特許レビュー
- 一般的な特許出願手続き
第9章 医療美容デバイス業界における持続可能性
- ESG:イントロダクション
- 医療美容デバイス業界における持続可能性
- 医療美容デバイスにおける持続可能性の促進要因
- ESGリスク評価
- BCCによる総論
第10章 付録
- 調査手法
- 出典
- 略語
- 企業プロファイル
- ABBVIE INC.
- ALMA LASERS
- BAUSCH HEALTH COMPANIES INC.
- CANDELA CORP.
- CUTERA INC.
- CYNOSURE LUTRONIC
- EL.EN. SPA
- IMPLANTECH
- JOHNSON & JOHNSON SERVICES INC.
- LUMENIS BE LTD.
- MEDYTOX
- MERZ PHARMA
- PHOTOMEDEX
- TIGER BIOSCIENCES
- VENUS CONCEPT
List of Tables
- Summary Table : Global Market for Medical Aesthetic Devices, by Region, Through 2029
- Table 1 : Number of Surgical Procedures Performed by Plastic Surgeons Worldwide, 2022 vs. 2023
- Table 2 : Number of Nonsurgical Procedures Performed by Plastic Surgeons Worldwide, 2022 vs. 2023
- Table 3 : Botulinum Toxin Procedures Performed Worldwide, by Age Group, 2023
- Table 4 : Total Number of Cosmetic Procedures Performed, by Country, 2023
- Table 5 : Top 30 Countries for Estimated Number of Plastic Surgeons, 2023
- Table 6 : Number and Percentage of Overweight or Obese People (Over the Age of 5), 2020-2035
- Table 7 : Global Obesity Trends for Children and Adolescents Age 5 to 19, by Gender, 2020-2035
- Table 8 : Global Obesity Trends for Adults (Age 20 and Above), by Gender, 2020-2035
- Table 9 : Global Market for Medical Aesthetic Devices, Through 2029
- Table 10 : Global Market for Medical Aesthetic Devices, by Device Type, Through 2029
- Table 11 : Global Market for Non-Energy-based Aesthetic Devices, by Region, Through 2029
- Table 12 : Global Market for Energy-based Aesthetic Devices, by Region, Through 2029
- Table 13 : Global Market for Medical Aesthetic Devices, by Application, Through 2029
- Table 14 : Global Market for Medical Aesthetic Devices Used in Facial Aesthetic Procedures, by Region, Through 2029
- Table 15 : Global Market for Medical Aesthetic Devices Used in Skin Resurfacing and Tightening, by Region, Through 2029
- Table 16 : Global Market for Medical Aesthetic Devices Used in Body Contouring and Cellulite Reduction, by Region, Through 2029
- Table 17 : Global Market for Medical Aesthetic Devices Used in Breast Augmentation, by Region, Through 2029
- Table 18 : Global Market for Medical Aesthetic Devices Used in Hair Removal, by Region, Through 2029
- Table 19 : Global Market for Medical Aesthetic Devices, by End User, Through 2029
- Table 20 : Global Market for Medical Aesthetic Devices Used in Hospitals, by Region, Through 2029
- Table 21 : Global Market for Medical Aesthetic Devices Used in Slimming and Beauty Clinics, by Region, Through 2029
- Table 22 : Global Market for Medical Aesthetic Devices Used in Dermatology Clinics, by Region, Through 2029
- Table 23 : Global Market for Medical Aesthetic Devices, by Region, Through 2029
- Table 24 : North American Market for Medical Aesthetic Devices, by Country, Through 2029
- Table 25 : North American Market for Medical Aesthetic Devices, by Device Type, Through 2029
- Table 26 : North American Market for Medical Aesthetic Devices, by Application, Through 2029
- Table 27 : North American Market for Medical Aesthetic Devices, by End User, Through 2029
- Table 28 : Surgical Cosmetic Procedures Performed in the U.S., 2023
- Table 29 : Most Common Surgical Cosmetic Procedures Performed in the U.S., 2023
- Table 30 : Number of Nonsurgical Cosmetic Procedures Performed in the U.S., 2023
- Table 31 : Most Common Nonsurgical Cosmetic Procedures Performed in the U.S., 2023
- Table 32 : Surgical Cosmetic Procedures Performed in Mexico, 2023
- Table 33 : Most Common Surgical Cosmetic Procedures Performed in Mexico, 2023
- Table 34 : Nonsurgical Cosmetic Procedures Performed in Mexico, 2023
- Table 35 : Most Common Nonsurgical Cosmetic Procedures Performed in Mexico, 2023
- Table 36 : European Market for Medical Aesthetic Devices, by Country, Through 2029
- Table 37 : European Market for Medical Aesthetic Devices, by Device Type, Through 2029
- Table 38 : European Market for Medical Aesthetic Devices, by Application, Through 2029
- Table 39 : European Market for Medical Aesthetic Devices, by End User, Through 2029
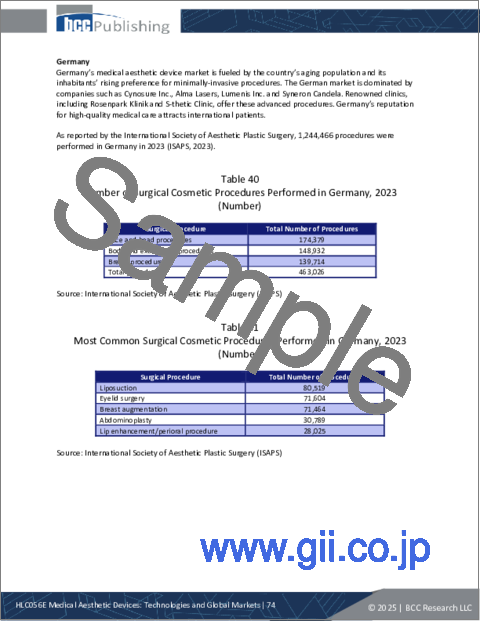
- Table 40 : Number of Surgical Cosmetic Procedures Performed in Germany, 2023
- Table 41 : Most Common Surgical Cosmetic Procedures Performed in Germany, 2023
- Table 42 : Nonsurgical Cosmetic Procedures Performed in Germany, 2023
- Table 43 : Most Common Nonsurgical Cosmetic Procedures Performed in Germany, 2023
- Table 44 : Surgical Cosmetic Procedures Performed in France, 2023
- Table 45 : Most Common Surgical Cosmetic Procedures Performed in France, 2023
- Table 46 : Number of Nonsurgical Cosmetic Procedures Performed in France, 2023
- Table 47 : Most Common Nonsurgical Cosmetic Procedures Performed in France, 2023
- Table 48 : Asia-Pacific Market for Medical Aesthetic Devices, by Country, Through 2029
- Table 49 : Asia-Pacific Market for Medical Aesthetic Devices, by Device Type, Through 2029
- Table 50 : Asia-Pacific Market for Medical Aesthetic Devices, by Application, Through 2029
- Table 51 : Asia-Pacific Market for Medical Aesthetic Devices, by End User, Through 2029
- Table 52 : Surgical Cosmetic Procedures Performed in India, 2023
- Table 53 : Most Common Surgical Cosmetic Procedures Performed in India, 2023
- Table 54 : Nonsurgical Cosmetic Procedures Performed in India, 2023
- Table 55 : Most Common Nonsurgical Cosmetic Procedures Performed in India, 2023
- Table 56 : RoW Market for Medical Aesthetic Devices, by Device Type, Through 2029
- Table 57 : RoW Market for Medical Aesthetic Devices, by Application, Through 2029
- Table 58 : RoW Market for Medical Aesthetic Devices, by End User, Through 2029
- Table 59 : Rank of Medical Aesthetics Device Companies, 2023
- Table 60 : Recent Mergers and Partnerships in the Medical Aesthetic Device Industry, 2024
- Table 61 : Recent Product Launches and Approvals in the Medical Aesthetic Devices Market, 2023-2025
- Table 62 : Patents on Medical Aesthetic Devices, 2023-2024
- Table 63 : Sustainability Initiatives of Leading Companies
- Table 64 : ESG Accomplishments of Major Medical Aesthetic Companies
- Table 65 : ESG Rankings for Major Companies, 2024
- Table 66 : Information Sources for this Report
- Table 67 : Abbreviations Used in the Report
- Table 68 : AbbVie Inc.: Company Snapshot
- Table 69 : AbbVie Inc.: Financial Performance, FY 2022 and 2023
- Table 70 : AbbVie Inc.: Product Portfolio
- Table 71 : AbbVie Inc.: News/Key Developments, 2023 and 2024
- Table 72 : Alma Lasers: Company Snapshot
- Table 73 : Alma Lasers: Product Portfolio
- Table 74 : Bausch Health Companies Inc.: Company Snapshot
- Table 75 : Bausch Health Companies Inc.: Financial Performance, FY 2022 and 2023
- Table 76 : Bausch Health Companies Inc.: Product Portfolio
- Table 77 : Bausch Health Companies Inc.: News/Key Developments, 2024
- Table 78 : Candela Corp.: Company Snapshot
- Table 79 : Candela Corp.: Product Portfolio
- Table 80 : Candela Corp.: News/Key Developments, 2023 and 2024
- Table 81 : Cutera Inc.: Company Snapshot
- Table 82 : Cutera Inc.: Financial Performance, FY 2022 and 2023
- Table 83 : Cutera Inc.: Product Portfolio
- Table 84 : Cutera Inc.: News/Key Developments, 2023 and 2024
- Table 85 : Cynosure Lutronic: Company Snapshot
- Table 86 : Cynosure Lutronic: Product Portfolio
- Table 87 : El.En. SpA: Company Snapshot
- Table 88 : El.En. SpA: Financial Performance, FY 2022 and 2023
- Table 89 : El.En. SpA: Product Portfolio
- Table 90 : El.En. SpA: News/Key Developments, 2023
- Table 91 : Implantech: Company Snapshot
- Table 92 : Implantech: Product Portfolio
- Table 93 : Johnson & Johnson Services Inc.: Company Snapshot
- Table 94 : Johnson & Johnson Services Inc.: Financial Performance, FY 2022 and 2023
- Table 95 : Johnson & Johnson Services Inc.: Product Portfolio
- Table 96 : Johnson & Johnson Services Inc.: News/Key Developments, 2024
- Table 97 : Lumines Be Ltd.: Company Snapshot
- Table 98 : Lumines Be Ltd.: Product Portfolio
- Table 99 : Medytox: Company Snapshot
- Table 100 : Medytox: Product Portfolio
- Table 101 : Medytox: News/Key Developments, 2024
- Table 102 : Merz Pharma: Company Snapshot
- Table 103 : Merz Pharma: Product Portfolio
- Table 104 : Merz Pharma: News/Key Developments, 2024
- Table 105 : Photomedex: Company Snapshot
- Table 106 : Photomedex: Product Portfolio
- Table 107 : Tiger BioSciences: Company Snapshot
- Table 108 : Tiger BioSciences: Product Portfolio
- Table 109 : Venus Concept: Company Snapshot
- Table 110 : Venus Concept: Financial Performance, FY 2022 and 2023
- Table 111 : Venus Concept: Product Portfolio
List of Figures
- Summary Figure : Global Market Shares of Medical Aesthetic Devices, by Region, 2023
- Figure 1 : Insights and Statistics on Medical Aesthetics, 2023
- Figure 2 : Most Popular Surgical Cosmetic Procedures Performed Worldwide, 2023
- Figure 3 : Most Popular Nonsurgical Cosmetic Procedures Performed Worldwide, 2023
- Figure 4 : Global Market Dynamics of Medical Aesthetic Devices
- Figure 5 : Increase in Minimally-Invasive Cosmetic Procedures, 2022-2023
- Figure 6 : Factors Influencing the Cost of a Cosmetic Procedure
- Figure 7 : Emerging Technologies in the Medical Aesthetic Devices Market
- Figure 8 : Global Market Shares of Medical Aesthetic Devices, by Device Type, 2023
- Figure 9 : Global Market Shares of Medical Aesthetic Devices, by Application, 2023
- Figure 10 : Global Market Shares of Medical Aesthetic Devices, by End User, 2023
- Figure 11 : Global Market Shares of Medical Aesthetic Devices, by Region, 2023
- Figure 12 : North American Market Shares of Medical Aesthetic Devices, by Country, 2023
- Figure 13 : European Market Shares of Medical Aesthetic Devices, by Country, 2023
- Figure 14 : Asia-Pacific Market Shares of Medical Aesthetic Devices, by Country, 2023
- Figure 15 : Product Approvals, Launches and Expansions, January 2023-December 2024
- Figure 16 : Snapshot of the Three ESG Pillars
- Figure 17 : AbbVie Inc.: Revenue Share, by Business Unit, FY 2023
- Figure 18 : AbbVie Inc.: Revenue Share, by Country/Region, FY 2023
- Figure 19 : Bausch Health Companies Inc.: Revenue Share, by Business Unit, FY 2023
- Figure 20 : Bausch Health Companies Inc.: Revenue Share, by Country/Region, FY 2023
- Figure 21 : Cutera Inc.: Revenue Share, by Business Unit, FY 2023
- Figure 22 : Cutera Inc.: Revenue Share, by Country/Region, FY 2023
- Figure 23 : El.En. SpA: Revenue Share, by Business Unit, FY 2023
- Figure 24 : El.En. SpA: Revenue Share, by Country/Region, FY 2023
- Figure 25 : Johnson & Johnson Services Inc.: Revenue Share, by Business Unit, FY 2023
- Figure 26 : Johnson & Johnson Services Inc.: Revenue Share, by Country/Region, FY 2023
- Figure 27 : Venus Concept: Revenue Share, by Business Unit, FY 2023
- Figure 28 : Venus Concept: Revenue Share, by Country/Region, FY 2023
The global market for medical aesthetic devices was valued at $20.1 billion in 2023. It is projected to grow from $22.2 billion in 2024 to $37.9 billion by the end of 2029, at a compound annual growth rate (CAGR) of 11.3% from 2024 through 2029.
The North American market for medical aesthetic devices is projected to grow from $8.1 billion in 2024 to $13.0 billion by the end of 2029, at a CAGR of 10.0% from 2024 through 2029.
The Asia-Pacific market for medical aesthetic devices is projected to grow from $5.1 billion in 2024 to $9.3 billion by the end of 2029, at a CAGR of 12.8% from 2024 through 2029.
Report Scope:
This report analyzes the market for medical aesthetic devices by market segment, examining trends, challenges and opportunities, evaluating applications, and providing forecasts through 2029. It also looks at emerging technologies and recent technological advances. The report also evaluates the financial performance, product portfolios and recent initiatives of leading companies.
This report segments the global market by device type, application, end user and region. Regions are North America, Europe, Asia-Pacific and the Rest of the World (RoW). Regional analysis is further segmented by country as follows:
- North America: U.S., Canada and Mexico.
- Europe: Germany, U.K., France, Italy, Spain and the Rest of Europe.
- Asia-Pacific: China, Japan, India, South Korea, Australia and the Rest of Asia-Pacific.
Data for market estimates have been provided for 2021 and 2022 as the historical years, 2023 as the base year and forecast through the end of 2029.
The report also features profiles of leading product manufacturers in the industry, including Alma Lasers, Johnson & Johnson Services Inc., El. En. S.p.A., Bausch Health Companies Inc. and Allergan Aesthetics, an AbbVie company.
Report Includes
- 65 data tables and 47 additional tables
- An analysis of the global markets for medical aesthetic devices
- Analyses of the global market trends, with data from 2021-2023, estimates for 2024, and projections of compound annual growth rates (CAGRs) through 2029
- Evaluation of and forecast for the overall market size for medical aesthetic devices, and quantification of the market potential by device type, application, and region
- Discussion of the COVID-19 implications for the medical aesthetic devices market and statistics for the number of surgical procedures performed
- Coverage of technological breakthroughs in laser treatments, and discussion on the role of cosmetic surgery in medical tourism
- Insights into the regulatory environment for medical aesthetic devices in the U.S. and Europe
- Review of the patent filings and research publications for innovations in medical aesthetic devices
- A discussion of the industry's ESG challenges and practices
- Identification of the companies that are best positioned to meet this demand because of their proprietary technologies, strategic alliances, or other advantages
- Insights into the industry structure, competitive landscape, clinical trials and ongoing research activity
- Profiles of the leading companies, including Alma Lasers, Johnson & Johnson Services Inc., AbbVie Inc., Bausch Health Companies Inc., and El.En. S.p.A.
Table of Contents
Chapter 1 Executive Summary
- Market Outlook
- Scope of Report
- Market Summary
Chapter 2 Market Overview
- Aesthetic Medicine: An Overview
- Aesthetic Procedures
- Connecting Health and Beauty
- Growth and Market Potential
- Demographics and Consumer Preferences
- Overview of Aesthetic Treatments
- Technological Breakthroughs in Laser Treatments
- Professional Societies in Medical Aesthetics
- Medical Tourism
- Role of Cosmetic Surgery in Medical Tourism
- Benefits of Medical Tourism
- Future Outlook
- Statistics for Medical Aesthetics
Chapter 3 Market Dynamics
- Market Dynamics
- Market Drivers
- Adoption of Minimally-Invasive Devices
- Increase in the Number of Medical Aesthetic Procedures
- Developments in Cosmetic Surgery Using Laser Technology
- Rise in the Prevalence of Obesity
- Increase in Demand for Breast Augmentation Surgeries
- Rise in Medical Tourism
- Market Challenges and Restraints
- Cost of Medical Aesthetic Procedures
- Low Reimbursement Policies and Stringent Government Regulations
- Ethical and Social Stigmas Related to Medical Aesthetics
- Adverse Effects from Cosmetic Surgeries
- Market Opportunities
- Emerging Economies
- Marketing Strategies for Medical Aesthetic Companies
Chapter 4 Industry Outlook: Regulatory Framework
- Regulatory Framework in the U.S.
- Regulatory Authority and Historical Context
- FDA Oversight Process
- Regulatory Framework in Canada
- Regulatory Framework in the EU
- Key Responsibilities of Competent Authorities
- Regulatory Requirements for Non-EU Manufacturers
- Mutual Recognition Agreement (MRA) Between the EU and U.S.
- Approval and Quality System Evaluations
- Quality Standards and Inspections
- Regulatory Framework in Japan
- Approval Process for Medical Aesthetic Devices
- Clinical Trials
- Acceptance of Foreign Clinical Data
- Implications for Medical Aesthetic Device Manufacturers
- Regulatory Framework in China
- Classification of Medical Aesthetic Devices
- Registration Process
- Labeling and Documentation Requirements
- Additional Regulatory Steps
- Challenges and Timelines
- Regulatory Framework in India
- Import and Approval of Medical Aesthetic Devices
- Challenges in Regulatory Oversight
- Transition Toward a Risk-based Framework
- Impact on Domestic Manufacturers
- The Road Ahead
- Regulatory Framework in Latin America
- Regulatory Framework in Saudi Arabia
Chapter 5 Emerging Technologies and Developments
- Emerging Technologies
- Developments in Fat-Reducing and Skin-Tightening Devices
- Advances in Aesthetic Laser Devices
- Adoption of Robot-based Surgery
- Adoption of AI
- Advances in Radiofrequency and Ultrasound Technology
- Integrating Regenerative Medicine and Stem Cell Therapy
Chapter 6 Market Segmentation Analysis
- Market Trends
- Segmentation Breakdown
- Global Market for Medical Aesthetic Devices
- Market Analysis by Device Type
- Non-Energy-based Aesthetic Devices
- Energy-based Aesthetic Devices
- Market Analysis by Application
- Facial Aesthetic Procedures
- Skin Resurfacing and Tightening
- Body Contouring and Cellulite Reduction
- Breast Augmentation
- Hair Removal
- Market Analysis by End User
- Hospitals
- Slimming and Beauty Clinics
- Dermatology Clinics
- Geographic Breakdown
- Market Analysis by Region
- North America
- Europe
- Asia-Pacific
- Rest of the World
Chapter 7 Competitive Intelligence
- Competitive Landscape
- Market Rank of Companies
- Developments and Strategies
- Partnerships and Collaborations
- Product Launches and Approvals
Chapter 8 Patent Review
- General Patent Application Process
Chapter 9 Sustainability in the Medical Aesthetic Device Industry
- Introduction to ESG
- Sustainability in the Medical Aesthetic Device Industry
- Drivers for Sustainability in Medical Aesthetic Devices
- ESG Risk Ratings
- Concluding Remarks from BCC
Chapter 10 Appendix
- Methodology
- Sources
- Abbreviations
- Company Profiles
- ABBVIE INC.
- ALMA LASERS
- BAUSCH HEALTH COMPANIES INC.
- CANDELA CORP.
- CUTERA INC.
- CYNOSURE LUTRONIC
- EL.EN. SPA
- IMPLANTECH
- JOHNSON & JOHNSON SERVICES INC.
- LUMENIS BE LTD.
- MEDYTOX
- MERZ PHARMA
- PHOTOMEDEX
- TIGER BIOSCIENCES
- VENUS CONCEPT