|
|
市場調査レポート
商品コード
1556458
抗体薬物複合体 (ADC):各種技術と世界の市場Antibody-Drug Conjugates: Technologies and Global Markets |
||||||
|
|||||||
| 抗体薬物複合体 (ADC):各種技術と世界の市場 |
|
出版日: 2024年09月13日
発行: BCC Research
ページ情報: 英文 89 Pages
納期: 即納可能
|
全表示
- 概要
- 図表
- 目次
世界の抗体薬物複合体 (ADC) の市場規模は、2023年の108億米ドルから、予測期間中はCAGR 28.4%で推移し、2029年には470億米ドルの規模に達すると予測されています。
微小管阻害剤の部門は、2023年の67億米ドルから、CAGR 25.6%で推移し、2029年には257億米ドルに達すると予測されています。また、DNAターゲティング剤の部門は、2023年の41億米ドルから、CAGR 32.2%で推移し、2029年には211億米ドルに達すると予測されています。
当レポートでは、世界の抗体薬物複合体 (ADC) の市場を調査し、市場概要、市場影響因子および市場機会の分析、法規制環境、新興技術および技術開発の動向、市場規模の推移・予測、各種区分・地域別の詳細分析、競合情勢、主要企業のプロファイルなどをまとめています。
目次
第1章 エグゼクティブサマリー
- 市場見通し
- 調査範囲
- 市場概要
第2章 市場概要
- 概要
- ADCのコンポーネント
- 薬物抗体比(DAR)
- ADCの仕組み
- ADCの利点
- コンジュゲーション
- 従来のコンジュゲーション
- 部位特異的結合
- 主要なADC標的
- 承認されたADC
第3章 市場力学
- 市場力学スナップショット
- 市場促進要因
- 癌罹患率の上昇と腫瘍学におけるアンメットニーズ
- リスクの少ない開発
- ADCの複雑さ
- ADC治療モダリティに対する投資家の信頼
- 規制当局からの支援
- 制限事項
- 技術と製造の課題
- ADC抵抗
第4章 新興技術と開発
- 二重特異性または二重標的ADC
- 二重ペイロードADC
- 免疫チェックポイント阻害剤と組み合わせたADC
- B7-H3抗体標的
- 部位特異的結合
- パイプライン分析
- ペイロードタイプ
- リンカータイプ
- 抗体の種類
- 表示タイプ
- 標的タイプ
- コンジュゲーションの種類
- 開発段階別の新規ADC
第5章 市場セグメンテーション分析
- セグメンテーションの内訳
- 市場分析:ペイロードタイプ別
- 市場概要
- 市場収益・予測
- リンカータイプ別市場分析
- 市場概要
- 市場収益・予測
- 市場分析:抗体タイプ別
- 市場概要
- 市場収益・予測
- 市場分析:適応症別
- 市場概要
- 市場収益・予測
- 地理的内訳
- 市場分析:地域別
- 北米
- 欧州
- その他の地域
第6章 競合情報
- ADC販売
- 市場シェア分析
第7章 付録
- 調査手法
- 出典
- 略語
- 企業プロファイル
- ABBVIE INC.
- ADC THERAPEUTICS SA
- DAIICHI SANKYO CO. LTD.
- F. HOFFMANN-LA ROCHE LTD.
- GILEAD SCIENCES INC.
- PFIZER INC.
List of Tables
- Summary Table : Global Market for ADCs, by Type of Payload, Through 2029
- Table 1 : Key Antigen Targets for ADCs
- Table 2 : Commercially Available ADCs: Key Components and Target Antigens
- Table 3 : Discontinued ADCs: Key Components and Target Antigens
- Table 4 : Acquisitions in the ADC Market, 2023 and 2024
- Table 5 : Recent Strategic Partnerships/Deals in the ADC Market
- Table 6 : ADCs Approved Under FDA's Accelerated Approval Program
- Table 7 : Bispecific ADCs in Clinical Development
- Table 8 : ADCs in Combination with Immune Checkpoint Inhibitors
- Table 9 : ADC Candidates Targeted Against B7-H3
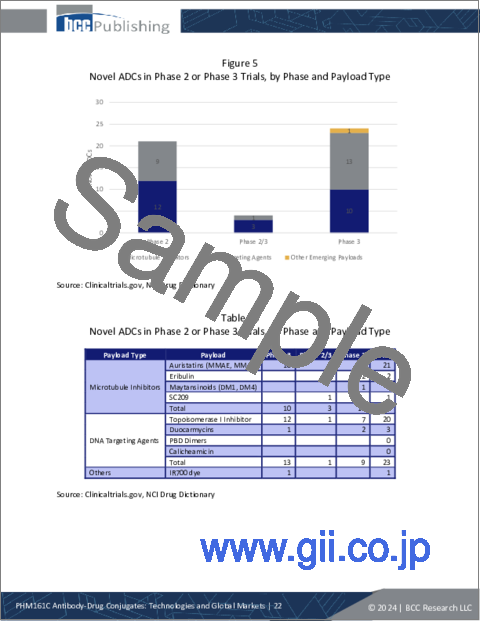
- Table 10 : Novel ADCs in Phase 2 or Phase 3 Trials, by Phase and Payload Type
- Table 11 : Novel ADCs in Phase 2 or Phase 3 Trials, by Phase and Linker Type
- Table 12 : Novel ADCs in Phase 2 or Phase 3 Trials, by Phase and Antibody Type
- Table 13 : Novel ADCs in Phase 2 or Phase 3 Trials for Selected Tumor Types
- Table 14 : Novel ADCs in Phase 2 or Phase 3 Trials, by Target Types
- Table 15 : ADCs in Phase 3 Clinical Trials
- Table 16 : ADCs in Phase 2 and Phase 3 Clinical Trials
- Table 17 : ADCs in Phase 2 Clinical Trials
- Table 18 : Commonly Used ADC Payloads
- Table 19 : Global Market for ADCs, by Type of Payload, Through 2029
- Table 20 : Cleavable Linkers in FDA-approved ADCs
- Table 21 : Non-cleavable Linkers in FDA-Approved ADCs
- Table 22 : Global Market for ADCs, by Type of Linker, Through 2029
- Table 23 : Global Market for ADCs, by Type of Antibody, Through 2029
- Table 24 : ADCs Approved for Breast Cancer Treatment
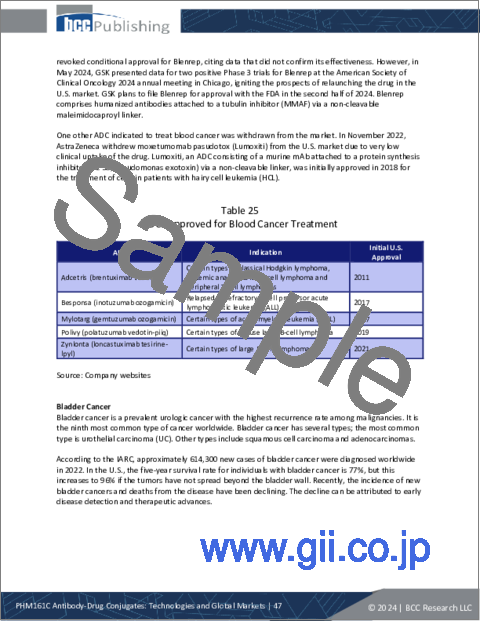
- Table 25 : ADCs Approved for Blood Cancer Treatment
- Table 26 : ADCs Approved for Bladder Cancer Treatment
- Table 27 : ADCs Approved for Cancers Other than Breast, Blood and Bladder Cancer
- Table 28 : Global Market for ADCs, by Indication, Through 2029
- Table 29 : Global Market for ADCs, by Region, Through 2029
- Table 30 : North American Market for ADCs, by Type of Payload, Through 2029
- Table 31 : North American Market for ADCs, by Type of Linker, Through 2029
- Table 32 : North American Market for ADCs, by Type of Antibody, Through 2029
- Table 33 : North American Market for ADCs, by Indication, Through 2029
- Table 34 : European Market for ADCs, by Type of Payload, Through 2029
- Table 35 : European Market for ADCs, by Type of Linker, Through 2029
- Table 36 : European Market for ADCs, by Type of Antibody, Through 2029
- Table 37 : European Market for ADCs, by Application, Through 2029
- Table 38 : Other Regions Market for ADCs, by Type of Payload, Through 2029
- Table 39 : Other Regions Market for ADCs, by Type of Linker, Through 2029
- Table 40 : Other Regions Market for ADCs, by Type of Antibody, Through 2029
- Table 41 : Other Regions Market for ADCs, by Application, Through 2029
- Table 42 : Sales of ADCs, 2021-2023
- Table 43 : Global Market Shares of Manufacturers/Marketers of ADCs, 2023
- Table 44 : Report Sources
- Table 45 : Abbreviations Used in This Report
- Table 46 : AbbVie Inc.: Company Snapshot
- Table 47 : AbbVie Inc.: Financial Performance, FY 2022 and 2023
- Table 48 : AbbVie Inc.: Product Portfolio
- Table 49 : AbbVie Inc.: News/Key Developments, 2023 and 2024
- Table 50 : ADC Therapeutics SA: Company Snapshot
- Table 51 : ADC Therapeutics SA: Financial Performance, FY 2022 and 2023
- Table 52 : ADC Therapeutics SA: Product Portfolio
- Table 53 : ADC Therapeutics SA: News/Key Developments, 2022
- Table 54 : Daiichi Sankyo Co. Ltd.: Company Snapshot
- Table 55 : Daiichi Sankyo Co. Ltd.: Financial Performance, FY 2022 and 2023
- Table 56 : Daiichi Sankyo Co. Ltd.: Product Portfolio
- Table 57 : Daiichi Sankyo Co. Ltd.: News/Key Developments, 2022-2024
- Table 58 : F. Hoffmann-La Roche Ltd.: Company Snapshot
- Table 59 : F. Hoffmann-La Roche Ltd.: Financial Performance, FY 2022 and 2023
- Table 60 : F. Hoffmann-La Roche Ltd.: Product Portfolio
- Table 61 : F. Hoffmann-La Roche Ltd.: News/Key Developments, 2022-2024
- Table 62 : Gilead Sciences Inc.: Company Snapshot
- Table 63 : Gilead Sciences Inc.: Financial Performance, FY 2022 and 2023
- Table 64 : Gilead Sciences Inc.: Product Portfolio
- Table 65 : Gilead Sciences Inc.: News/Key Developments, 2023 and 2024
- Table 66 : Pfizer Inc.: Company Snapshot
- Table 67 : Pfizer Inc.: Financial Performance, FY 2022 and 2023
- Table 68 : Pfizer Inc: Product Portfolio
- Table 69 : Pfizer Inc.: News/Key Developments, 2022-2024
List of Figures
- Summary Figure : Global Market Shares of ADCs, by Type of Payload, 2023
- Figure 1 : Market Dynamics of Antibody-Drug Conjugates
- Figure 2 : Estimated New Cancer Cases Globally, 2022, 2045 and 2050
- Figure 3 : Key Considerations for Next-Generation ADCs
- Figure 4 : Novel ADCs in Clinical Development, by Clinical Trial Phase
- Figure 5 : Novel ADCs in Phase 2 or Phase 3 Trials, by Phase and Payload Type
- Figure 6 : Distribution Share of ADC Pipeline, by Conjugation Method
- Figure 7 : Global Market Shares of ADCs, by Type of Payload, 2023
- Figure 8 : Global Market Shares of ADCs, by Type of Linker, 2023
- Figure 9 : Global Market Shares of ADCs, by Type of Antibody, 2023
- Figure 10 : Global Blood Cancer Incidence, by Type, 2022 vs. 2045
- Figure 11 : Global Market Shares of ADCs, by Indication Type, 2023
- Figure 12 : Global Market Shares of ADCs, by Region, 2023
- Figure 13 : AbbVie Inc.: Revenue Share, by Business Unit, FY 2023
- Figure 14 : AbbVie Inc.: Revenue Share, by Country/Region, FY 2023
- Figure 15 : ADC Therapeutics SA: Revenue Share, by Business Unit, FY 2023
- Figure 16 : ADC Therapeutics SA: Revenue Share, by Region, FY 2023
- Figure 17 : Daiichi Sankyo Co. Ltd.: Revenue Share, by Business Unit, FY 2023
- Figure 18 : Daiichi Sankyo Co. Ltd.: Revenue Share, by Region, FY 2023
- Figure 19 : F. Hoffmann-La Roche Ltd.: Revenue Share, by Business Unit, FY 2023
- Figure 20 : F. Hoffmann-La Roche Ltd.: Revenue Share, by Country/Region, FY 2023
- Figure 21 : Gilead Sciences Inc.: Revenue Share, by Business Unit, FY 2023
- Figure 22 : Gilead Sciences Inc.: Revenue Share, by Country/Region, FY 2023
- Figure 23 : Pfizer Inc.: Revenue Share, by Business Unit, FY 2023
- Figure 24 : Pfizer Inc.: Revenue Share, by Region, FY 2023
The global market for antibody-drug conjugates is estimated to increase from $10.8 billion in 2023 to reach $47.0 billion by 2029, at a compound annual growth rate (CAGR) of 28.4% from 2024 through 2029.
The microtubule inhibitors market for antibody-drug conjugates is estimated to increase from $6.7 billion in 2023 to reach $25.7 billion by 2029, at a CAGR of 25.6% from 2024 through 2029.
The DNA targeting agents market for antibody-drug conjugates is estimated to increase from $4.1 billion in 2023 to reach $21.1 billion by 2029, at a CAGR of 32.2% from 2024 through 2029.
Report Scope
This report focuses on the global market for antibody-drug conjugates (ADCs), offering an updated review that covers their fundamental design and use across different oncology fields. In this report, market segmentation is based on payload, linker, antibody, indications, and region. The report also discusses current and developing technologies, market projections and market shares. An analysis of clinical trials, innovations, opportunities, and the latest trends in the ADC market are also discussed in the report.
The report only covers antibody-drug conjugates in which an antibody is conjugated with small-molecule cytotoxic agents (also called payload) through a linker. Alternative antibody conjugates, such as those involving antibodies linked to radioisotopes, are not covered in this report.
Report Includes
- 25 data tables and 45 additional tables
- An updated review of the global market for antibody drug conjugates
- Analyses of the global market trends, with data from 2021-2023, estimates for 2024, forecasts for 2025, and projections of compound annual growth rates (CAGRs) through 2029
- Evaluation of the overall market for antibody drug conjugates, and corresponding market share analysis by payload type, linker type, antibody type, indication and region
- Coverage of the latest approvals, recalls, safety alerts, and clinical trials; the technical issues related to human anti-mouse antibody (HAMA); and the factors affecting mAB drugs
- Information about major and emerging technologies for the formulation of antibody drug conjugates and assessment of their relation to biotechnology, immunology, pharmaceuticals, and biodefense companies
- Discussions of the market dynamics, opportunities and challenges
- Overview of the sustainability trends and ESG developments in the industry, with emphasis on the ESG practices followed by leading companies, their ESG ratings, and consumer attitudes
- Competitive intelligence, including companies' market shares, recent M&A activity and venture funding
- Company profiles of major players within the industry, including F. Hoffmann-La Roche Ltd., Daiichi Sankyo, Pfizer Inc., and Gilead Sciences
Table of Contents
Chapter 1 Executive Summary
- Market Outlook
- Scope of Report
- Market Summary
Chapter 2 Market Overview
- Overview
- Components of ADCs
- Drug-to-antibody Ratio (DAR)
- How ADCs Work
- Advantages of ADCs
- Conjugation Methods
- Conventional Conjugation Method
- Site-Specific Conjugation
- Key ADC Targets
- Approved ADCs
Chapter 3 Market Dynamics
- Market Dynamics Snapshot
- Market Drivers
- Rising Prevalence of Cancer and Unmet Need in Oncology
- De-risked Development
- Complexity of ADCs
- Investor Confidence in ADC Therapeutic Modality
- Support from Regulatory Agencies
- Limitations
- Technical and Manufacturing Challenges
- ADC Resistance
Chapter 4 Emerging Technologies and Developments
- Bispecific or Dual-Targeted ADCs
- Dual Payload ADCs
- ADCs in Combination with Immune Checkpoint Inhibitors
- B7-H3 Antibody Target
- Site-specific Conjugation
- Pipeline Analysis
- Payload Type
- Linker Type
- Antibody Type
- Indication Type
- Target Type
- Conjugation Method Type
- Novel ADCs in Development, by Phase
Chapter 5 Market Segmentation Analysis
- Segmentation Breakdown
- Market Analysis, by Payload Type
- Market Overview
- Market Revenue and Forecast
- Market Analysis, by Linker Type
- Market Overview
- Market Revenue and Forecast
- Market Analysis, by Type of Antibody
- Market Overview
- Market Revenue and Forecast
- Market Analysis, by Indication
- Market Overview
- Market Revenue and Forecast
- Geographic Breakdown
- Market Analysis, by Region
- North America
- Europe
- Other Regions
Chapter 6 Competitive Intelligence
- ADC Sales
- Market Share Analysis
Chapter 7 Appendix
- Methodology
- Sources
- Abbreviations
- Company Profiles
- ABBVIE INC.
- ADC THERAPEUTICS SA
- DAIICHI SANKYO CO. LTD.
- F. HOFFMANN-LA ROCHE LTD.
- GILEAD SCIENCES INC.
- PFIZER INC.