|
|
市場調査レポート
商品コード
1452624
欧州の細胞・遺伝子治療製造サービス市場の2030年までの予測-地域別分析-タイプ別、適応症別、用途別、エンドユーザー別Europe Cell and Gene Therapy Manufacturing Services Market Forecast to 2030 - Regional Analysis - by Type, Indication (Cancer, Orthopedics, and Others), Application (Clinical Manufacturing and Commercial Manufacturing), and End User |
||||||
|
|||||||
| 欧州の細胞・遺伝子治療製造サービス市場の2030年までの予測-地域別分析-タイプ別、適応症別、用途別、エンドユーザー別 |
|
出版日: 2024年01月15日
発行: The Insight Partners
ページ情報: 英文 119 Pages
納期: 即納可能
|
- 全表示
- 概要
- 図表
- 目次
欧州の細胞・遺伝子治療製造サービス市場は、2022年の18億7,977万米ドルから2030年には65億6,385万米ドルに成長すると予測されています。2022年から2030年までのCAGRは16.9%と推定されます。
企業による戦略的取り組みが欧州の細胞・遺伝子治療製造サービス市場を牽引
細胞・遺伝子治療製造サービス市場で事業を展開する企業は、提携、拡大、合意、パートナーシップ、新製品発売などの戦略的開拓に注力しており、これらの戦略的開拓は、売上の向上、地理的範囲の拡大、既存顧客ベースよりも多くの顧客に対応するための能力強化に役立っています。細胞・遺伝子治療製造サービス市場における注目すべき発展を以下にいくつか紹介します。
2023年5月、Lonzaは、CAR T細胞製造の最適化と合理化のために考案された、化学的に定義された新しい製剤を用いたTheraPEAK T-VIVO細胞培養液を発売しました。TheraPEAK T-VIVO細胞培養培地は、他の無血清培地とは異なり、ヒト血清やその成分を添加することなく高い性能を発揮することができます。
2022年10月、Pfizer Inc.は、成人におけるエピソード性片頭痛の急性期治療と予防の両方に承認された革新的な片頭痛治療薬であるNURTEC ODT(リメゲパント)の製造元であるBiohaven Pharmaceutical Holding Company Ltd.の買収を完了しました。
2022年3月、Cellevolve BioはSeattle Children's Therapeuticsと提携し、小児がんを対象とした新たなマルチプレックスCARの開発と商業化を目指します。この提携のもと、両社は小児の中枢神経系(CNS)悪性腫瘍を治療するため、5種類のマルチプレックスCARからなるBrainChild研究プログラムに注力します。提携により、両社はSeattle Children's Cure施設を活用し、新規CARの早期臨床GMP調査を実施します。
このように、これらの戦略的イニシアチブは、細胞・遺伝子治療製造サービス市場に大きな成長機会をもたらします。
欧州の細胞・遺伝子治療製造サービス市場概要
欧州の細胞・遺伝子治療製造サービス市場は、ドイツ、英国、フランス、イタリア、スペイン、その他の欧州に区分されます。この地域は今後数年間、大きな市場シェアを占めると思われます。米国と欧州の細胞・遺伝子治療製造サービス市場は、生産能力のセットアップのための投資、高い政府支援、同地域への関与などの要因により、予測期間中に大きな成長が見込まれます。また、競合上位企業の存在も、予測期間2022~2030年の同地域市場の成長をさらに高めると思われます。
ドイツは強力な製薬産業であり、研究開発への注力は顕著です。同国には660社のバイオテクノロジー企業があり、5万人の従業員を雇用しています。このうち660社がCGTに注力しています。NecstGenの報告書によると、2021年にはCAR修飾免疫細胞を評価する29以上の活発な臨床試験が進行中であり、生産の大部分はCAR-T細胞に関するものでした。また、ドイツではこれまでに50以上の遺伝子治療の臨床試験が実施されています。ドイツのCGT企業の技術革新への取り組みは、国際的なプレゼンスがないため、主に地域または国レベルに集中しています。
英国は、先端治療薬の研究、開発、製造、臨床採用、償還において世界最高のエコシステムのひとつです。現在、英国では85以上の臨床試験が進行中で、70のCGTs企業が治癒の可能性のある治療法の開発のために活動しています。2018年、国民保健サービス(NHS)は、がん患者に対するCAR-T細胞治療の提供を発表しました。
カタパルトは、英国のCGTエコシステムに大きく貢献しているもう1つのトップ企業です。同社は、国内の製造プロセス開発でトップ5に入る企業です。同社は、最小限の介入で変化する環境やプロセス要件に自動的に適応できる、よりスマートな自動CGT製造プロセスを開発しています。したがって、生産性向上と最終コスト削減のためにこれらの企業が行っている努力が、英国における欧州細胞・遺伝子治療製造サービス市場の成長を促進する主要要因となっています。さらに、研究への持続的かつ的を絞った投資により、英国は欧州の細胞・遺伝子治療製造サービス市場で強い地位を占めています。
欧州の細胞・遺伝子治療製造サービス市場の収益と2030年までの予測(金額)
欧州の細胞・遺伝子治療製造サービス市場のセグメンテーション
欧州の細胞・遺伝子治療製造サービス市場は、タイプ、適応症、用途、エンドユーザー、国に区分されます。
タイプ別では、欧州の細胞・遺伝子治療製造サービス市場は細胞治療と遺伝子治療に二分されます。2022年、欧州の細胞・遺伝子治療製造サービス市場では、細胞治療セグメントがより大きなシェアを記録しました。細胞療法セグメントはさらに自己由来と同種異系に区分されます。遺伝子治療セグメントはさらに、ウイルスベクターと非ウイルスベクターに区分されます。
適応症に基づき、欧州の細胞・遺伝子治療製造サービス市場はがん、整形外科、その他に区分されます。2022年、欧州の細胞・遺伝子治療製造サービス市場では、がんセグメントが最大のシェアを記録しました。
用途別では、欧州の細胞・遺伝子治療製造サービス市場は臨床製造と商業製造に区分されます。2022年には、商業用製造セグメントが欧州細胞・遺伝子治療製造サービス市場で最大のシェアを記録しました。
エンドユーザーに基づくと、欧州の細胞・遺伝子治療製造サービス市場は製薬企業とバイオテクノロジー企業とCROに二分されます。2022年には、製薬企業とバイオテクノロジー企業セグメントが欧州細胞・遺伝子治療製造サービス市場でより大きなシェアを記録しました。
国別に見ると、欧州の細胞・遺伝子治療製造サービス市場は、英国、フランス、ドイツ、スペイン、イタリア、その他の欧州に区分されます。2022年、英国は欧州の細胞・遺伝子治療製造サービス市場で最大のシェアを記録しました。
Catalent Inc、Charles River Laboratories International Inc、FUJIFILM Holdings Corp、Lonza Group AG、Merck KgaA、Nikon Corp、Oxford BioMedica Plc、Takara Bio Inc、Thermo Fisher Scientific Inc、WuXi AppTec Co Ltdは、欧州の細胞・遺伝子治療製造サービス市場で事業を展開している大手企業です。
目次
第1章 イントロダクション
第2章 エグゼクティブサマリー
- 主要な洞察
第3章 調査手法
- 調査範囲
- 2次調査
- 1次調査
第4章 欧州の細胞・遺伝子治療製造サービス市場情勢
- 概観
- 欧州PEST分析
第5章 欧州の細胞・遺伝子治療製造サービス市場-主要産業力学
- 主要市場の促進要因
- 細胞・遺伝子治療の承認件数の増加
- 細胞・遺伝子治療製造アウトソーシングの人気の高まり
- 市場抑制要因
- 細胞・遺伝子治療製造の高コスト
- 市場機会
- 企業による戦略的取り組み
- 今後の動向
- 細胞・遺伝子治療製造サービスの自動化
- 影響分析
第6章 欧州の細胞・遺伝子治療製造サービス市場-欧州市場分析
- 欧州の細胞・遺伝子治療市場売上高、2022~2030年
第7章 欧州の細胞・遺伝子治療製造サービス市場:2030年までの収益と予測:タイプ別
- イントロダクション
- 欧州細胞・遺伝子治療製造サービス市場2022年・2030年売上高シェア(%):タイプ別
- 細胞療法
- 遺伝子治療
第8章 欧州の細胞・遺伝子治療製造サービス市場:2030年までの収益と予測:適応症別
- イントロダクション
- 欧州の細胞・遺伝子治療製造サービス市場2022年・2030年売上高シェア(%):適応症別
- がん
- 整形外科
- その他
第9章 欧州の細胞・遺伝子治療製造サービス市場:2030年までの収益と予測:用途別
- イントロダクション
- 欧州細胞・遺伝子治療製造サービス市場2022年・2030年売上高シェア(%):用途別
- 臨床製造
- 商業用製造
第10章 欧州の細胞・遺伝子治療製造サービス市場:2030年までの収益と予測:エンドユーザー別
- イントロダクション
- 欧州細胞・遺伝子治療製造サービス市場2022年・2030年売上高シェア(%):エンドユーザー別
- 製薬企業とバイオテクノロジー企業
- CRO
第11章 欧州の細胞・遺伝子治療製造サービス市場:2030年までの収益と予測:国別分析
- 欧州
- ドイツ
- 英国
- フランス
- イタリア
- スペイン
- その他の欧州
第12章 欧州の細胞・遺伝子治療製造サービス市場:業界情勢
- イントロダクション
- 欧州の細胞・遺伝子治療製造サービス市場における成長戦略
- 有機的成長戦略
- 概要
- 無機的成長戦略
- 概要
第13章 企業プロファイル
- Thermo Fisher Scientific Inc
- Merck KGaA
- Charles River Laboratories International Inc
- Lonza Group AG
- WuXi AppTec Co Ltd
- Catalent Inc
- Takara Bio Inc
- Nikon Corp
- FUJIFILM Holdings Corp
- Oxford BioMedica Plc
List Of Tables
- Table 1. Europe Cell and Gene Therapy Market Segmentation
- Table 2. Germany: Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- Table 3. Germany: Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- Table 4. Germany: Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- Table 5. Germany: Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- Table 6. Germany: Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- Table 7. Germany: Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- Table 8. UK: Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- Table 9. UK: Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- Table 10. UK: Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- Table 11. UK: Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- Table 12. UK: Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- Table 13. UK: Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- Table 14. France: Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- Table 15. France: Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- Table 16. France: Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- Table 17. France: Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- Table 18. France: Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- Table 19. France: Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- Table 20. Italy: Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- Table 21. Italy: Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- Table 22. Italy: Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- Table 23. Italy: Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- Table 24. Italy: Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- Table 25. Italy: Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- Table 26. Spain: Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- Table 27. Spain: Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- Table 28. Spain: Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- Table 29. Spain: Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- Table 30. Spain: Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- Table 31. Spain: Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- Table 32. Rest of Europe: Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- Table 33. Rest of Europe: Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- Table 34. Rest of Europe: Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- Table 35. Rest of Europe: Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- Table 36. Rest of Europe: Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- Table 37. Rest of Europe: Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- Table 38. Recent Organic Growth Strategies in Europe Cell and Gene Therapy Manufacturing Services Market
- Table 39. Recent Inorganic Growth Strategies in the Europe Cell and Gene Therapy Manufacturing Services Market
List Of Figures
- Figure 1. Europe Cell and Gene Therapy Market Segmentation, By Country
- Figure 2. Europe PEST Analysis
- Figure 3. Europe Cell and Gene Therapy Market - Key Industry Dynamics
- Figure 4. Impact Analysis of Drivers and Restraints
- Figure 5. Europe Cell and Gene Therapy Market Revenue (US$ Mn), 2022 - 2030
- Figure 6. Europe Cell and Gene Therapy Manufacturing Services Market Revenue Share, by Type 2022 & 2030 (%)
- Figure 7. Cell Therapy: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 8. Autologous: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 9. Allogenic: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 10. Gene Therapy: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
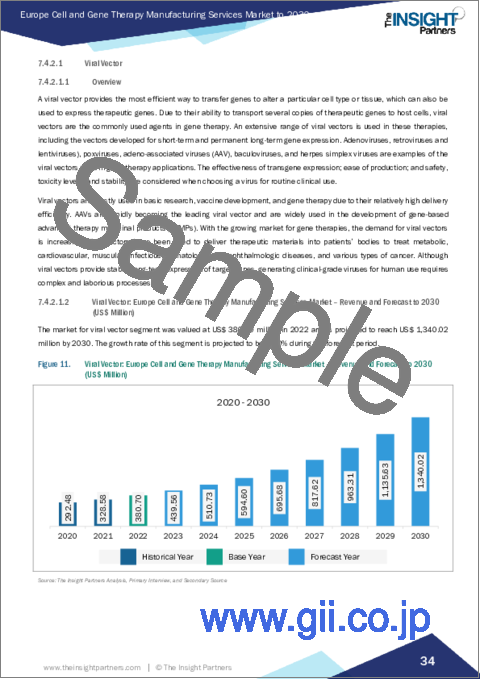
- Figure 11. Viral Vector: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 12. Non-Viral Vector: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 13. Europe Cell and Gene Therapy Manufacturing Services Market, by Indication 2022 & 2030 (%)
- Figure 14. Cancer: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 15. Orthopedics: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 16. Others: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 17. Europe Cell and Gene Therapy Manufacturing Services Market Revenue Share, by Application 2022 & 2030 (%)
- Figure 18. Clinical Manufacturing: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 19. Commercial Manufacturing: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 20. Europe Cell and Gene Therapy Manufacturing Services Market Revenue Share, by End User 2022 & 2030 (%)
- Figure 21. Pharmaceutical & Biotechnology Companies: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 22. Contract Research Organizations (CROs): Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 23. Europe: Cell and Gene Therapy Manufacturing Services Market, by Key Region - Revenue (2022) (US$ Million)
- Figure 24. Europe: Europe Cell and Gene Therapy Manufacturing Services Market, By Country, 2022 & 2030 (%)
- Figure 25. Germany: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 26. UK: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 27. France: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 28. Italy: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 29. Spain: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 30. Rest of Europe: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 31. Growth Strategies in Europe Cell and Gene Therapy Manufacturing Services Market
The Europe cell and gene therapy manufacturing services market is expected to grow from US$ 1,879.77 million in 2022 to US$ 6,563.85 million by 2030. It is estimated to grow at a CAGR of 16.9% from 2022 to 2030.
Strategic Initiatives by Companies Drive Europe Cell and Gene Therapy Manufacturing Services Market
Companies operating in the cell and gene therapy manufacturing services market focus on strategic developments such as collaborations, expansions, agreements, partnerships, and new product launches, which help them improve their sales, expand their geographic reach, and enhance their capacities to cater to a larger than existing customer base. A few of the noteworthy developments in the cell and gene therapy manufacturing services market are mentioned below.
In May 2023, Lonza launched the TheraPEAK T-VIVO Cell Culture Medium with a novel chemically defined formulation devised to optimize and streamline CAR T-cell manufacturing. The TheraPEAK T-VIVO Cell Culture Medium can exhibit a high performance without the need to add human serum or its components, unlike other serum-free media.
In October 2022, Pfizer Inc. completed the acquisition of Biohaven Pharmaceutical Holding Company Ltd., the manufacturer of NURTEC ODT (rimegepant), an innovative migraine therapy approved for both acute treatment and prevention of episodic migraine in adults.
In March 2022, Cellevolve Bio partnered with Seattle Children's Therapeutics to develop and commercialize new multiplex CARs for paediatric cancers. Under the collaboration, the partners will focus on the BrainChild research program, a suite of five multiplex CARs, to treat pediatric central nervous system (CNS) malignancies. In partnership, they would leverage the Seattle Children's Cure Factory facility to conduct early clinical GMP research on new CARs.
Thus, these strategic initiatives create significant growth opportunities in the cell and gene therapy manufacturing services market.
Europe Cell and Gene Therapy Manufacturing Services Market Overview
The Europe cell and gene therapy manufacturing services market is segmented into Germany, the UK, France, Italy, Spain, and the Rest of Europe. The region will hold a significant market share in the coming years. The European US and Europe cell and gene therapy manufacturing services market is expected to witness significant growth during the forecast period due to factors such as the investment for setup of production capacity, high government support and involvement in the region. Also, presence of top competitive players will further enhance the regional market growth during the forecast period 2022-2030.
Germany has a strong pharmaceutical industry with a notable focus on R&D. The country has 660 biotechnology companies that employ 50,000 employees. Among these, 660 companies are focused on CGT. As per the NecstGen report, more than 29 active clinical trials evaluating CAR-modified immune cells were ongoing in 2021, and the majority of the production involved CAR-T cells. Also, over 50 clinical studies have been conducted in gene therapy in Germany so far. The innovation efforts of German CGT companies are majorly focused on the local or country level, as they lack international presence.
The UK is one of the world's best ecosystems for research, development, manufacturing, clinical adoption, and reimbursement of advanced therapeutics. Currently, over 85 clinical trials are ongoing in the UK, and 70 CGTs companies are operating for the development of potentially curative therapies. In 2018, National Health Services (NHS) England announced the availability of CAR-T cell treatment for cancer patients, which was the first time when this therapy was made available to these patients.
Catapult is another top company that has been contributing significantly to the CGT ecosystem in the UK; it stands as one of the top five companies in developing manufacturing processes in the country. The company is developing smarter automated CGT manufacturing processes that can automatically adapt to changing environments and process requirements with minimal intervention. Therefore, efforts made by these companies for enhancing productivity and reducing the final costs are the major factors driving the growth of the Europe cell and gene therapy manufacturing services market in the UK. Moreover, the UK holds a strong position in the Europe cell and gene therapy manufacturing services market in Europe owing to sustained and targeted investment in research.
Europe Cell and Gene Therapy Manufacturing Services Market Revenue and Forecast to 2030 (US$ Million)
Europe Cell and Gene Therapy Manufacturing Services Market Segmentation
The Europe cell and gene therapy manufacturing services market is segmented into type, indication, application, end user, and country.
Based on type, the Europe cell and gene therapy manufacturing services market is bifurcated into cell therapy and gene therapy. In 2022, the cell therapy segment registered a larger share in the Europe cell and gene therapy manufacturing services market. The cell therapy segment is further segmented into autologous and allogenic. The gene therapy segment is further segmented into viral and non-viral vector.
Based on indication, the Europe cell and gene therapy manufacturing services market is segmented into cancer, orthopedics, and others. In 2022, the cancer segment registered the largest share in the Europe cell and gene therapy manufacturing services market.
Based on application, the Europe cell and gene therapy manufacturing services market is segmented into clinical manufacturing and commercial manufacturing. In 2022, the commercial manufacturing segment registered the largest share in the Europe cell and gene therapy manufacturing services market.
Based on end user, the Europe cell and gene therapy manufacturing services market is bifurcated into pharmaceutical and biotechnology companies and contract research organization (CROs). In 2022, the pharmaceutical and biotechnology companies segment registered a larger share in the Europe cell and gene therapy manufacturing services market.
Based on country, the Europe cell and gene therapy manufacturing services market is segmented into the UK, France, Germany, Spain, Italy, and the Rest of Europe. In 2022, the UK registered the largest share in the Europe cell and gene therapy manufacturing services market.
Catalent Inc, Charles River Laboratories International Inc, FUJIFILM Holdings Corp, Lonza Group AG, Merck KgaA, Nikon Corp, Oxford BioMedica Plc, Takara Bio Inc, Thermo Fisher Scientific Inc, and WuXi AppTec Co Ltd are some of the leading companies operating in the Europe cell and gene therapy manufacturing services market.
Table Of Contents
1. Introduction
- 1.1 The Insight Partners Research Report Guidance
- 1.2 Market Segmentation
2. Executive Summary
- 2.1 Key Insights
3. Research Methodology
- 3.1 Coverage
- 3.2 Secondary Research
- 3.3 Primary Research
4. Europe Cell and Gene Therapy Manufacturing Services Market Landscape
- 4.1 Overview
- 4.2 Europe PEST Analysis
5. Europe Cell and Gene Therapy Manufacturing Services Market - Key Industry Dynamics
- 5.1 Key Market Drivers:
- 5.1.1 Increase in Number of Approval of Cell and Gene Therapies
- 5.1.2 Increasing Popularity of Outsourcing Cell and Gene Therapy Manufacturing
- 5.2 Market Restraints
- 5.2.1 High Cost of Cell and Gene Therapy Manufacturing
- 5.3 Market Opportunities
- 5.3.1 Strategic Initiatives by Companies
- 5.4 Future Trends
- 5.4.1 Automation of Cell and Gene Therapy Manufacturing Services
- 5.5 Impact Analysis:
6. Europe Cell and Gene Therapy Manufacturing Services Market - Europe Market Analysis
- 6.1 Europe Cell and Gene Therapy Market Revenue (US$ Mn), 2022 - 2030
7. Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 - by Type
- 7.1 Overview
- 7.2 Europe Cell and Gene Therapy Manufacturing Services Market Revenue Share, by Type 2022 & 2030 (%)
- 7.3 Cell Therapy
- 7.3.1 Overview
- 7.3.2 Cell Therapy: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 7.3.2.1 Autologous
- 7.3.2.1.1 Overview
- 7.3.2.1.2 Autologous: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 7.3.2.2 Allogenic
- 7.3.2.2.1 Overview
- 7.3.2.2.2 Allogenic: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 7.3.2.1 Autologous
- 7.4 Gene Therapy
- 7.4.1 Overview
- 7.4.2 Gene Therapy: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 7.4.2.1 Viral Vector
- 7.4.2.1.1 Overview
- 7.4.2.1.2 Viral Vector: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 7.4.2.2 Non-Viral Vector
- 7.4.2.2.1 Overview
- 7.4.2.2.2 Non-Viral Vector: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 7.4.2.1 Viral Vector
8. Europe Cell and Gene Therapy Manufacturing Services Market Analysis and Forecasts to 2030 - by Indication
- 8.1 Overview
- 8.2 Europe Cell and Gene Therapy Manufacturing Services Market, by Indication 2022 & 2030 (%)
- 8.3 Cancer
- 8.3.1 Overview
- 8.3.2 Cancer: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 8.4 Orthopedics
- 8.4.1 Overview
- 8.4.2 Orthopedics: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 8.5 Others
- 8.5.1 Overview
- 8.5.2 Others: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
9. Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 - by Application
- 9.1 Overview
- 9.2 Europe Cell and Gene Therapy Manufacturing Services Market Revenue Share, by Application 2022 & 2030 (%)
- 9.4 Clinical Manufacturing
- 9.4.1 Overview
- 9.4.2 Clinical Manufacturing: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 9.5 Commercial Manufacturing
- 9.5.1 Overview
- 9.5.2 Commercial Manufacturing: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
10. Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 - by End User
- 10.1 Overview
- 10.2 Europe Cell and Gene Therapy Manufacturing Services Market Revenue Share, by End User 2022 & 2030 (%)
- 10.4 Pharmaceutical and Biotechnology Companies
- 10.4.1 Overview
- 10.4.2 Pharmaceutical & Biotechnology Companies: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 10.5 Contract Research Organizations (CROs)
- 10.5.1 Overview
- 10.5.2 Contract Research Organizations (CROs): Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
11. Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 - Country Analysis
- 11.1 Overview
- 11.1.1 Europe: Europe Cell and Gene Therapy Manufacturing Services Market, By Country, 2022 & 2030 (%)
- 11.1.1.1 Germany: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.1.1.1.1 Germany: Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.1.1.1.1.1 Germany: Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.1.1.1.1.2 Germany: Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.1.1.1.2 Germany: Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.1.1.1.3 Germany: Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.1.1.1.4 Germany: Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.1.1.2 UK: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.1.1.2.1 UK: Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.1.1.2.1.1 UK: Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.1.1.2.1.2 UK: Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.1.1.2.2 UK: Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.1.1.2.3 UK: Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.1.1.2.4 UK: Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.1.1.3 France: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.1.1.3.1 France: Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.1.1.3.1.1 France: Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.1.1.3.1.2 France: Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.1.1.3.2 France: Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.1.1.3.3 France: Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.1.1.3.4 France: Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.1.1.4 Italy: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.1.1.4.1 Italy: Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.1.1.4.1.1 Italy: Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.1.1.4.1.2 Italy: Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.1.1.4.2 Italy: Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.1.1.4.3 Italy: Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.1.1.4.4 Italy: Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.1.1.5 Spain: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.1.1.5.1 Spain: Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.1.1.5.1.1 Spain: Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.1.1.5.1.2 Spain: Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.1.1.5.2 Spain: Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.1.1.5.3 Spain: Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.1.1.5.4 Spain: Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.1.1.6 Rest of Europe: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.1.1.6.1 Rest of Europe: Europe Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.1.1.6.1.1 Rest of Europe: Europe Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.1.1.6.1.2 Rest of Europe: Europe Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.1.1.6.2 Rest of Europe: Europe Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.1.1.6.3 Rest of Europe: Europe Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.1.1.6.4 Rest of Europe: Europe Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.1.1.1 Germany: Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.1.1 Europe: Europe Cell and Gene Therapy Manufacturing Services Market, By Country, 2022 & 2030 (%)
12. Europe Cell and Gene Therapy Manufacturing Services Market - Industry Landscape
- 12.1 Overview
- 12.2 Growth Strategies in Europe Cell and Gene Therapy Manufacturing Services Market
- 12.3 Organic Growth Strategies
- 12.3.1 Overview
- 12.4 Inorganic Growth Strategies
- 12.4.1 Overview
13. Company Profiles
- 13.1 Thermo Fisher Scientific Inc
- 13.1.1 Key Facts
- 13.1.2 Business Description
- 13.1.3 Products and Services
- 13.1.4 Financial Overview
- 13.1.5 SWOT Analysis
- 13.1.6 Key Developments
- 13.2 Merck KGaA
- 13.2.1 Key Facts
- 13.2.2 Business Description
- 13.2.3 Products and Services
- 13.2.4 Financial Overview
- 13.2.5 SWOT Analysis
- 13.2.6 Key Developments
- 13.3 Charles River Laboratories International Inc
- 13.3.1 Key Facts
- 13.3.2 Business Description
- 13.3.3 Products and Services
- 13.3.4 Financial Overview
- 13.3.5 SWOT Analysis
- 13.3.6 Key Developments
- 13.4 Lonza Group AG
- 13.4.1 Key Facts
- 13.4.2 Business Description
- 13.4.3 Products and Services
- 13.4.4 Financial Overview
- 13.4.5 SWOT Analysis
- 13.4.6 Key Developments
- 13.5 WuXi AppTec Co Ltd?
- 13.5.1 Key Facts
- 13.5.2 Business Description
- 13.5.3 Products and Services
- 13.5.4 Financial Overview
- 13.5.5 SWOT Analysis
- 13.5.6 Key Developments
- 13.6 Catalent Inc
- 13.6.1 Key Facts
- 13.6.2 Business Description
- 13.6.3 Products and Services
- 13.6.4 Financial Overview
- 13.6.5 SWOT Analysis
- 13.6.6 Key Developments
- 13.7 Takara Bio Inc
- 13.7.1 Key Facts
- 13.7.2 Business Description
- 13.7.3 Products and Services
- 13.7.4 Financial Overview
- 13.7.5 SWOT Analysis
- 13.7.6 Key Developments
- 13.8 Nikon Corp
- 13.8.1 Key Facts
- 13.8.2 Business Description
- 13.8.3 Products and Services
- 13.8.4 Financial Overview
- 13.8.5 SWOT Analysis
- 13.8.6 Key Developments
- 13.9 FUJIFILM Holdings Corp
- 13.9.1 Key Facts
- 13.9.2 Business Description
- 13.9.3 Products and Services
- 13.9.4 Financial Overview
- 13.9.5 SWOT Analysis
- 13.9.6 Key Developments
- 13.10 Oxford BioMedica Plc
- 13.10.1 Key Facts
- 13.10.2 Business Description
- 13.10.3 Products and Services
- 13.10.4 Financial Overview
- 13.10.5 SWOT Analysis
- 13.10.6 Key Developments