|
|
市場調査レポート
商品コード
1362428
細胞・遺伝子治療製造サービス市場規模・予測、世界・地域シェア、動向、成長機会分析レポート対象範囲:タイプ別、適応症別、用途別、エンドユーザー別、地域別Cell and Gene Therapy Manufacturing Services Market Size and Forecasts, Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Type, Indication, Application, End User, and Geography |
||||||
|
|||||||
| 細胞・遺伝子治療製造サービス市場規模・予測、世界・地域シェア、動向、成長機会分析レポート対象範囲:タイプ別、適応症別、用途別、エンドユーザー別、地域別 |
|
出版日: 2023年09月14日
発行: The Insight Partners
ページ情報: 英文 216 Pages
納期: 即納可能
|
- 全表示
- 概要
- 図表
- 目次
細胞・遺伝子治療製造サービス市場規模は、2022年の75億8,197万米ドルから2030年には267億2,490万米ドルに成長すると予測され、2022年から2030年のCAGRは17.1%と推定されます。
当レポートでは、市場の動向と細胞・遺伝子治療製造サービス市場の成長を促進・阻害する要因を明らかにしています。市場の成長は、細胞・遺伝子治療の承認件数の増加と、細胞・遺伝子治療製造のアウトソーシング人気の高まりに起因しています。しかし、細胞・遺伝子治療製造のコストが高いことが市場成長の妨げとなっています。
企業の戦略的取り組み
細胞・遺伝子治療製造サービス市場で事業を展開する企業は、提携、拡大、合意、パートナーシップ、新製品発売などの戦略的開拓に注力し、売上の向上、地理的リーチの拡大、既存顧客ベースよりも多くの顧客に対応するための能力強化に役立てています。細胞・遺伝子治療製造サービス市場における注目すべき発展を以下にいくつか紹介します。
2023年5月、ロンザ社は、CAR T細胞製造の最適化と合理化のために考案された、化学的に定義された新しい製剤を用いたTheraPEAK T-VIVO細胞培養液を発売しました。TheraPEAK T-VIVO細胞培養培地は、他の無血清培地とは異なり、ヒト血清やその成分を添加することなく高い性能を発揮することができます。
2022年10月、ファイザー社は、成人におけるエピソード性片頭痛の急性期治療と予防の両方に承認された革新的な片頭痛治療薬であるNURTEC ODT(リメゲパント)の製造元であるバイオヘイブン・ファーマシューティカル・ホールディング社の買収を完了しました。
2022年3月、セルボルブ・バイオはシアトル・チルドレンズ・セラピューティクスと提携し、小児がんを対象とした新たなマルチプレックスCARの開発と商業化を目指します。この提携のもと、両社は小児の中枢神経系(CNS)悪性腫瘍を治療するため、5種類のマルチプレックスCARからなるBrainChild研究プログラムに注力します。提携により、両社はシアトル・チルドレンズ・キュア・ファクトリーの施設を活用し、新規CARの早期臨床GMP調査を実施します。
2022年3月、Twist Bioscience CorporationとKriya Therapeutics, Inc.は、がん治療用途でアデノ随伴ウイルス(AAV)遺伝子治療を用いて送達される抗体に関する抗体探索契約を締結しました。両社の提携により、ツイストの抗体ライブラリーとクリヤ独自のベクターエンジニアリングプラットフォームを組み合わせ、クリヤの遺伝子治療技術で送達される特定の標的に対する新規抗体を発見する予定。
2022年2月、エディジーン社はボストン小児病院(BCH)と、主要遺伝子を破壊することによる胎児ヘモグロビンのアップレギュレーションに関する知的財産権の世界ライセンス契約を締結しました。胎児ヘモグロビンのアップレギュレーションは、異常なヘモグロビン構造に起因する多くの遺伝性疾患(ヘモグロビン異常症)に対する治療法の可能性があり、欠陥のあるヘモグロビン分子を胎児版に置き換えます。
2022年1月、富士フイルムはAtara Biotherapeutics, Inc.から細胞治療製造施設を買収することに合意しました。この施設は拡張が容易で、同種T細胞やCAR T免疫療法を含む臨床・商業両方の細胞療法を製造できる柔軟性があります。
2021年1月、サーモフィッシャーサイエンティフィック社は、ベルギーを拠点とするグループ・ノヴァセップSASのウイルスベクター製造事業であるHenogen S.A.を買収しました。ヘノジェン社はバイオテクノロジー企業や大手バイオ医薬品企業にワクチンや治療薬の製造サービスを提供しています。この買収により、サーモフィッシャーサイエンティフィックは細胞・遺伝子ワクチンおよび治療薬カテゴリーにおける能力を拡大しました。
したがって、ウイルスベクター、細胞治療製造用培地、同種T細胞などの製品の導入、様々な健康問題をターゲットとした革新的な製品の開発による新製品や改良品の創出、市場競争力を維持するための新規事業の立ち上げなど、提携やパートナーシップを通じて、細胞・遺伝子治療製造サービスの新たなプラットフォームの開発を加速させることができます。したがって、こうした戦略的イニシアティブは、細胞・遺伝子治療製造サービス市場に大きな成長機会をもたらします。
細胞・遺伝子治療製造の高コスト
細胞・遺伝子治療は複雑な製造工程を伴い、生物学的成分を使用するため、その価格は法外です。例えば、Kymriahの価格は47万5,000米ドル、Yescartaの価格は37万3,000米ドルです。Institute for Clinical and Economic Review(ICER)の分析によると、遺伝子治療の平均コストは1回あたり100万~200万米ドルです。細胞治療や遺伝子治療の製造には、ブラックボックス効果、失敗の増幅、柔軟性の低下など様々なリスクが伴う。ブラックボックス効果とは、自動化されたシステムで実行される作業やプロセスのことで、プロセスが見えず、進捗状況も見えないです。そのため、製造工程が見えないことで失敗が増加し、製造工程に適応する柔軟性が低下します。さらに、製造業者にとって最も重要な課題は、FDAやEMAといった規制機関から製品の承認を得ること、そして治療による副作用から患者の安全性を確保することです。
さらに、消耗品や機器のコストははるかに高く、これが細胞治療の割高なコストにつながっています。例えば、Miltenyi Biotec社が提供する試薬キットのコストは米国内で500~5,000米ドルです。同様に、装置導入の資本コストは200万米ドル近くになります。このように、治療価格に関わる課題が細胞・遺伝子治療製造サービス市場の成長を制限しています。
細胞・遺伝子治療製造サービス市場、タイプ別インサイト
細胞・遺伝子治療製造サービス市場は、タイプ別に細胞治療と遺伝子治療に区分されます。細胞療法は自己由来と同種異系に区分されます。さらに、遺伝子治療はウイルスベクターと非ウイルスベクターに二分されます。2022年の市場シェアは、細胞療法分野が大きいです。しかし、遺伝子治療セグメントは予測期間中(2022-2030年)に16.6%と最も高いCAGRを記録すると予測されています。遺伝子治療は、遺伝子を用いて医学的疾患や疾病を予防、治療、治癒する技術です。遺伝子治療は多くの場合、患者の細胞内で欠損している遺伝子や壊れている遺伝子のコピーを健康な遺伝子と置き換えることによって行われます。遺伝子治療は、体内だけでなく体外の細胞の改変にも用いることができます。体内の細胞を改変するために遺伝子治療を行う場合、遺伝子を運ぶベクターが患者に直接注入されます。一方、体外の細胞を改変するために遺伝子治療を行う場合は、血液、骨髄、その他の組織を取り出し、研究室で特定の細胞タイプを分離します。
細胞・遺伝子治療製造サービス市場、適応症別インサイト
適応症に基づき、細胞・遺伝子治療製造サービス市場はがん、整形外科、その他に分けられます。がん分野は2022年に市場で最大のシェアを占め、同分野は予測期間中に最も高いCAGRで成長すると予測されています。細胞・遺伝子治療の幅広い分野は、がんによる死亡を予防する可能性を秘めた多くの革新的な治療を約束しています。Alliance for Cancer Gene Therapy, Inc.の2022年4月のニュースレターによると、6つのCAR T細胞療法が骨髄腫、白血病、リンパ腫の治療薬として米国食品医薬品局(FDA)から承認されています。2022年12月、FDAは、高リスクのバシルス・カルメット・ゲリン(BCG)非応答性の非筋肉浸潤性膀胱がん(NMIBC)で、乳頭状腫瘍を伴うか伴わないCIS(carcinoma in situ)を有する成人患者の治療薬として、非複製アデノウイルスベクターをベースとする遺伝子治療薬Adstiladrinを承認しました。2022年、FDAは眼に発生するまれなタイプの黒色腫-ブドウ膜黒色腫-に対するT細胞受容体療法であるキムトラックを承認しました。がん治療のための細胞治療や遺伝子治療のFDA承認数が増加するにつれて、これらの治療法の生産もここ数年で増加しています。そのため、多数のCDMOががん細胞・遺伝子治療薬の製造サービスの提供に注力しています。
用途別では、細胞・遺伝子治療製造サービス市場は臨床製造と商業製造に分類されます。商業用製造セグメントは2022年に市場で最大のシェアを占め、予測期間(2020~2030年)には市場で最も高いCAGR 17.1%を記録すると予測されています。CGTは、臨床的有用性、耐久性、総合的な奏効率の組み合わせによって、複数の治療領域にわたって大きな価値を提供しています。しかし、これらの治療法はまだ大きな市場規模を獲得するには至っていないです。CGTの治療効果は、適切な患者に適切な治療法を提供することに伴う難しさのために過小評価されており、そのため、過去10年間に市場に出たCGTベースの市販製品はわずかです。しかし、第3相臨床試験中のCGTベースの臨床パイプラインは、近い将来、承認数が飛躍的に増加する可能性を示しています。著名なCDMOでありWuXi AppTecの完全子会社であるWuXi Advanced Therapies Inc.は、2021年10月に上海に新しいプロセス開発と商業生産施設を開設すると発表しました。この新しい製造施設は、GMP商業製造と統合試験サービスを通じて世界の能力を拡大するのに役立ち、今後数年間のCGT製品の商業化をサポートすると思われます。
細胞・遺伝子治療製造サービス市場は、エンドユーザー別に製薬・バイオテクノロジー企業とCRO(医薬品開発業務受託機関)に分類されます。製薬・バイオテクノロジー分野は2022年に市場で最大のシェアを占め、予測期間(2020-2030年)には市場で最も高いCAGR17.1%を記録すると予測されています。米国を拠点とする主な製薬・バイオテクノロジー企業は、従来のアプローチでは対応できない急性疾患や希少疾患の治療を目的としたCGTの提供に取り組んでおり、ElevateBio社やDiscovery Labs社などが含まれます。これらの企業は、医薬品開発・製造受託機関(CDMO)という全く新しい分野の創設に注力しています。彼らはCGTに投資することで、互いに、また一握りの巨大サービス企業と競争することになります。
目次
第1章 イントロダクション
第2章 エグゼクティブサマリー
- 主要な洞察
第3章 調査手法
- 調査範囲
- 2次調査
- 1次調査
第4章 細胞・遺伝子治療の市場情勢
- PEST分析
第5章 細胞・遺伝子治療市場- 主要産業力学
- 主な市場促進要因
- 細胞・遺伝子治療の承認件数の増加
- 細胞・遺伝子治療製造のアウトソーシング人気の高まり
- 市場抑制要因
- 細胞・遺伝子治療製造の高コスト
- 市場機会
- 企業による戦略的取り組み
- 今後の動向
- 細胞・遺伝子治療製造サービスの自動化
- インパクト分析
第6章 細胞・遺伝子治療市場:世界市場分析
- 細胞・遺伝子治療市場の収益、2022年~2030年
第7章 細胞・遺伝子治療製造サービス市場-2030年までの収益と予測- タイプ別
- 細胞・遺伝子治療製造サービス市場2022年・2030年タイプ別売上高シェア(%)
- 細胞療法
- 遺伝子治療
第8章 細胞・遺伝子治療製造サービス市場:2030年までの分析・予測:適応症別
- 細胞・遺伝子治療製造サービス市場:適応症別2022年および2030年(%)
- がん
- 整形外科
- その他
第9章 細胞・遺伝子治療製造サービス市場:2030年までの収益と予測:用途別
- 細胞・遺伝子治療製造サービス市場2022年・2030年用途別売上高シェア(%)
- 臨床製造
- 商業用製造
第10章 細胞・遺伝子治療製造サービス市場-2030年までの収益と予測- エンドユーザー別
- 細胞・遺伝子治療製造サービス市場エンドユーザー別売上高シェア 2022年・2030年(%)
- 製薬企業およびバイオテクノロジー企業
- CRO(医薬品開発業務受託機関)
第11章 細胞・遺伝子治療製造サービス市場-2030年までの収益と予測- 地域別分析
- 北米:細胞・遺伝子治療製造サービス市場
- 欧州細胞・遺伝子治療製造サービス市場
- アジア太平洋:細胞・遺伝子治療製造サービス市場
.
- アジア太平洋:細胞・遺伝子治療製造サービス市場:タイプ別、2020年~2030年
- アジア太平洋:細胞療法・遺伝子治療製造サービス市場:細胞療法別、2020-2030年
- アジア太平洋:細胞・遺伝子治療製造サービス市場:遺伝子治療別、2020-2030年
- アジア太平洋:細胞・遺伝子治療製造サービス市場:適応症別、2020年~2030年
- アジア太平洋:細胞・遺伝子治療製造サービス市場:用途別、2020年~2030年
- アジア太平洋:細胞・遺伝子治療製造サービス市場:エンドユーザー別、2020年~2030年
- アジア太平洋:細胞・遺伝子治療製造サービス市場:地域別、2022年・2030年(%)
- 中東・アフリカ:細胞・遺伝子治療製造サービス市場
- 中東&アフリカ:細胞・遺伝子治療製造サービス市場:タイプ別、2020年~2030年
- 中東&アフリカ:細胞療法・遺伝子治療製造サービス市場:細胞療法別、2020-2030年
- 中東&アフリカ:細胞・遺伝子治療製造サービス市場:遺伝子治療別、2020-2030年
- 中東&アフリカ:細胞・遺伝子治療製造サービス市場:適応症別、2020-2030年
- 中東&アフリカ:細胞・遺伝子治療製造サービス市場:用途別、2020-2030年
- 中東&アフリカ:細胞・遺伝子治療製造サービス市場:エンドユーザー別、2020年~2030年
- 中東・アフリカ:細胞・遺伝子治療製造サービス市場細胞・遺伝子治療製造サービス市場:地域別、2022年・2030年(%)
- 中東&アフリカ:細胞・遺伝子治療製造サービス市場:タイプ別、2020年~2030年
- 中南米:細胞・遺伝子治療製造サービス市場
- 中南米:細胞・遺伝子治療製造サービス市場:タイプ別、2020年~2030年
- 中南米:細胞療法・遺伝子治療製造サービス市場:細胞療法別、2020年~2030年
- 中南米:細胞・遺伝子治療製造サービス市場:遺伝子治療別、2020-2030年
- 中南米:細胞・遺伝子治療製造サービス市場:適応症別、2020-2030年
- 中南米:細胞・遺伝子治療製造サービス市場:用途別、2020-2030年
- 中南米:細胞・遺伝子治療製造サービス市場:エンドユーザー別、2020年~2030年
- 中南米:細胞・遺伝子治療製造サービス市場:地域別、2022年・2030年(%)
- 中南米:細胞・遺伝子治療製造サービス市場:タイプ別、2020年~2030年
第12章 細胞・遺伝子治療製造サービス市場:業界情勢
- 細胞・遺伝子治療製造サービス市場における成長戦略
- 有機的成長戦略
- 無機的成長戦略
第13章 企業プロファイル
- Thermo Fisher Scientific Inc
- Merck KGaA
- Charles River Laboratories International Inc
- Lonza Group AG
- WuXi AppTec Co Ltd
- Catalent Inc
- Takara Bio Inc
- Nikon Corp
- FUJIFILM Holdings Corp
- National Resilience Inc
- Oxford BioMedica Plc
List Of Tables
- Table 1. Cell and Gene Therapy Market Segmentation
- Table 2. Recent Organic Growth Strategies in Cell and Gene Therapy Manufacturing Services Market
- Table 3. Recent Inorganic Growth Strategies in the Cell and Gene Therapy Manufacturing Services Market
List Of Figures
- Figure 1. Cell and Gene Therapy Market Segmentation, By Geography
- Figure 2. PEST Analysis
- Figure 3. Cell and Gene Therapy Market - Key Industry Dynamics
- Figure 4. Impact Analysis of Drivers and Restraints
- Figure 5. Cell and Gene Therapy Market Revenue (US$ Mn), 2022 - 2030
- Figure 6. Cell and Gene Therapy Manufacturing Services Market Revenue Share, by Type 2022 & 2030 (%)
- Figure 7. Cell Therapy: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 8. Autologous: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 9. Allogenic: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 10. Gene Therapy: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 11. Viral Vector: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 12. Non-Viral Vector: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 13. Cell and Gene Therapy Manufacturing Services Market, by Indication 2022 & 2030 (%)
- Figure 14. Cancer: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 15. Orthopedics: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 16. Others: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 17. Cell and Gene Therapy Manufacturing Services Market Revenue Share, by Application 2022 & 2030 (%)
- Figure 18. Clinical Manufacturing: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 19. Commercial Manufacturing: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 20. Cell and Gene Therapy Manufacturing Services Market Revenue Share, by End User 2022 & 2030 (%)
- Figure 21. Pharmaceutical & Biotechnology Companies: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 22. Contract Research Organizations (CROs): Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 23. North America: Cell and Gene Therapy Manufacturing Services Market, by Key Region - Revenue (2022) (US$ Million)
- Figure 24. North America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 25. North America: Cell and Gene Therapy Manufacturing Services Market, by Region, 2022 & 2030 (%)
- Figure 26. US: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 27. Canada: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 28. Mexico: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 29. Europe: Cell and Gene Therapy Manufacturing Services Market, by Key Region - Revenue (2022) (US$ Million)
- Figure 30. Europe Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 31. Europe: Cell and Gene Therapy Manufacturing Services Market, by Region, 2022 & 2030 (%)
- Figure 32. Germany: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 33. UK: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 34. France: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 35. Italy: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 36. Spain: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 37. Rest of Europe: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 38. Asia Pacific: Cell and Gene Therapy Manufacturing Services Market, by Key Region - Revenue (2022) (US$ Million)
- Figure 39. Asia Pacific Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 40. Asia Pacific: Cell and Gene Therapy Manufacturing Services Market, by Region, 2022 & 2030 (%)
- Figure 41. China: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 42. Japan: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 43. India: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 44. Australia: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 45. South Korea: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 46. Rest of Asia Pacific: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 47. Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market, by Key Region - Revenue (2022) (US$ Million)
- Figure 48. Middle East & Africa Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 49. Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market, by Region, 2022 & 2030 (%)
- Figure 50. South Africa: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 51. Saudi Arabia: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 52. UAE: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 53. Rest of Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 54. South & Central America: Cell and Gene Therapy Manufacturing Services Market, by Key Region - Revenue (2022) (US$ Million)
- Figure 55. South & Central America Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 56. Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market, by Region, 2022 & 2030 (%)
- Figure 57. Brazil: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 58. Argentina: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 59. Rest of South & Central America: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 60. Growth Strategies in Cell and Gene Therapy Manufacturing Services Market
The cell and gene therapy manufacturing services market size is expected to grow from US$ 7,581.97 million in 2022 to US$ 26,724.90 million by 2030; it is estimated to register a CAGR of 17.1% during 2022-2030.
The report highlights trends prevailing in the market and the factors driving and hindering the cell and gene therapy manufacturing services market growth. The growth of the market is attributed increase in number of approvals of cell and gene therapies and increasing popularity of outsourcing cell and gene therapy manufacturing. However, high cost of cell and gene therapy manufacturing hinders the market growth.
Strategic Initiatives by Companies
Companies operating in the cell and gene therapy manufacturing services market focus on strategic developments such as collaborations, expansions, agreements, partnerships, and new product launches, which help them improve their sales, expand their geographic reach, and enhance their capacities to cater to a larger than existing customer base. A few of the noteworthy developments in the cell and gene therapy manufacturing services market are mentioned below.
In May 2023, Lonza launched the TheraPEAK T-VIVO Cell Culture Medium with a novel chemically defined formulation devised to optimize and streamline CAR T-cell manufacturing. The TheraPEAK T-VIVO Cell Culture Medium can exhibit a high performance without the need to add human serum or its components, unlike other serum-free media.
In October 2022, Pfizer Inc. completed the acquisition of Biohaven Pharmaceutical Holding Company Ltd., the manufacturer of NURTEC ODT (rimegepant), an innovative migraine therapy approved for both acute treatment and prevention of episodic migraine in adults.
In March 2022, Cellevolve Bio partnered with Seattle Children's Therapeutics to develop and commercialize new multiplex CARs for paediatric cancers. Under the collaboration, the partners will focus on the BrainChild research program, a suite of five multiplex CARs, to treat pediatric central nervous system (CNS) malignancies. In partnership, they would leverage the Seattle Children's Cure Factory facility to conduct early clinical GMP research on new CARs.
In March 2022, Twist Bioscience Corporation and Kriya Therapeutics, Inc. entered an antibody discovery agreement for antibodies delivered using adeno-associated viral (AAV) gene therapy in therapeutic oncology applications. Upon collaboration, the companies had plans of combining Twist's antibody libraries with Kriya's proprietary vector engineering platform to discover novel antibodies against specific targets of interest to be delivered with Kriya's gene therapy technology.
In February 2022, Edigene Inc. entered into a global license agreement with Boston Children's Hospital (BCH) for intellectual property rights covering the upregulation of foetal haemoglobin by disrupting a key gene. Upregulation of foetal haemoglobin is a potential treatment for many genetic diseases resulting from abnormal haemoglobin structures (hemoglobinopathies), replacing the defective haemoglobin molecule with its foetal version.
In January 2022, FUJIFILM Corporation agreed to acquire a cell therapy manufacturing facility from Atara Biotherapeutics, Inc. The facility is readily expandable with the flexibility to produce both clinical and commercial cell therapies, including allogeneic T-cell and CAR T immunotherapies.
In January 2021, Thermo Fisher Scientific Inc acquired Henogen S.A., the Belgium-based viral vector manufacturing business of Groupe Novasep SAS. Henogen provides vaccine and therapeutics manufacturing services to biotechnology companies and large biopharmaceutical customers. With this acquisition, Thermo Fisher Scientific expanded its capabilities in the cell and gene vaccines and therapies category.
Therefore, introducing products such as viral vectors, media for cell therapy manufacturing, and allogeneic T-cells; the development of innovative products targeting various health issues to create new or improved products; and the initiation of new businesses to remain competitive in the market, all, through collaborations and partnerships can help speed up the development of new platforms for cell and gene therapy manufacturing services. Thus, these strategic initiatives create significant growth opportunities in the cell and gene therapy manufacturing services market.
High Cost of Cell and Gene Therapy Manufacturing
As cell and gene therapies involve a complex manufacturing process and use biological components, these therapies are priced exorbitantly. For instance, the cost of Kymriah is ~US$ 475,000, and Yescarta is ~US$ 373,000, respectively. According to the analysis by the Institute for Clinical and Economic Review (ICER), the average cost of a gene therapy ranges US$ 1-2 million per dose. The manufacturing of cell and gene therapies involves various risks, such as the black-box effect, amplified failure, and reduced flexibility. The black-box effect refers to the tasks and processes performed in an automated system; there is no visibility to the process, and the progress status is hidden. Thus, the lack of visibility to the manufacturing processes increases failure and reduces the flexibility to adapt to the manufacturing processes. In addition, the most critical challenge for the manufacturers is to gain product approval from the regulatory bodies-such as FDA and EMA-and ensure patient's safety from the side effects of the therapy.
Furthermore, the cost of consumables and equipment is much higher, which leads to the premium cost of cell therapies. For instance, the cost of the reagent kit offered by Miltenyi Biotec ranges from US$ 500 to US$ 5,000. Similarly, the capital cost of installing the equipment is nearly US$ 2 million. Thus, the challenges pertaining to therapy prices limit the cell and gene therapy manufacturing services market growth.
Cell and Gene Therapy Manufacturing Services Market, By Type Insights
Based on type, the cell and gene therapy manufacturing services market is segmented into cell therapy and gene therapy. Cell therapy is segmented as autologous and allogenic. Further, gene therapy is bifurcated into viral vector and non-viral vector. The cell therapy segment held a larger market share in 2022. However, gene therapy segment is anticipated to register the highest CAGR of 16.6% during the forecast period (2022-2030). Gene therapy is a technique that uses a gene(s) to prevent, treat or cure a medical disorder or disease. Gene therapies often works by replacing a defective, or by adding new copies of a gene that is broken or missing gene in a patient's cells with a healthy version of that gene. Gene therapy can be used to modify cells outside as well as inside the body. A vector is injected carrying the gene directly into the patient when a gene therapy is used to modify cells inside the body. Whereas blood, bone marrow, or another tissue are taken out when gene therapy is used to modify cells outside the body and separate the specific cell types in the lab.
Cell and Gene Therapy Manufacturing Services Market, By Indication-Based Insights
Based on indication, the cell and gene therapy manufacturing services market is divided into cancer, orthopedics, and others. The cancer segment held the largest share of the market in 2022 and same segment is expected to grow at the highest CAGR during the forecast period. The broad field of cell and gene therapy promises many innovative treatments that have the potential to prevent deaths from cancer. According to the April 2022 newsletter by Alliance for Cancer Gene Therapy, Inc., 6 CAR T-cell therapies have been approved by the US Food and Drug Administration (FDA) for the treatment of myeloma, leukemia, and lymphoma. In December 2022, the FDA approved Adstiladrin, a non-replicating adenoviral vector-based gene therapy, for the treatment of adult patients with high-risk Bacillus Calmette-Guerin (BCG)-unresponsive non-muscle-invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors. In 2022, FDA approved Kimmtrak, a T-cell receptor therapy for a rare type of melanoma in the eye-uveal melanoma. As there is an increase in the number of FDA approvals of cell and gene therapy for the treatment of cancer, the production of these therapies has increased in the last several years. Thus, a large number of CDMOs are focusing on providing manufacturing services for cancer cell and gene therapies.
Based on Application, the cell and gene therapy manufacturing services market is categorized into clinical manufacturing and commercial manufacturing. The commercial manufacturing segment held the largest share of the market in 2022 and is anticipated to register the highest CAGR of 17.1% in the market during the forecast period (2020-2030). CGTs provide significant value across multiple therapeutic areas, driven by a combination of clinical benefit, durability, and overall response rate. However, these therapies are yet to gain a significant market reach. The therapeutic gain of CGTs is underestimated due to the difficulty associated with delivering the right therapy to the right patients, and thus, only a few commercial CGTs-based products have reached the market over the past decade. However, the clinical pipeline of CGT-based products in Phase 3 clinical trials indicates the possibility of a dramatic rise in the number of approvals in the near future. WuXi Advanced Therapies Inc., a prominent CDMO and a wholly subsidiary of WuXi AppTec, announced the opening of new process development and commercial manufacturing facility in Shanghai in October 2021. This new manufacturing facility would help it expand its global capacity through GMP commercial manufacturing and integrated testing services that would support the commercialization of CGT products in the coming years.
The cell and gene therapy manufacturing services market, by end user, is categorized into pharmaceutical & biotechnology companies and contract research organizations (CROs). The pharmaceutical and biotechnology segment held the largest share of the market in 2022 and is anticipated to register the highest CAGR of 17.1% in the market during the forecast period (2020-2030). Major US-based pharmaceutical and biotechnology companies committed to offering CGTs intended to treat acute and rare conditions, which are unresponsive to traditional approaches, include ElevateBio and Discovery Labs. These companies are focused on creating a completely new branch of pharmaceutical contract development and manufacturing organizations (CDMOs). They would compete with one another and with a handful of giant services firms by investing in CGTs.
Reasons to Buy:
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players, and segments in the cell and gene therapy manufacturing services market.
- Highlights key business priorities in order to assist companies to realign their business strategies.
- The key findings and recommendations highlight crucial progressive industry trends in the cell and gene therapy manufacturing services market, thereby allowing players across the value chain to develop effective long-term strategies.
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets.
- Scrutinize in-depth market trends and outlook coupled with the factors driving the cell and gene therapy manufacturing services market, as well as those hindering it.
- Enhance the decision-making process by understanding the strategies that underpin security interest with respect to client products, segmentation, pricing, and distribution.
Table Of Contents
1. Introduction
- 1.1 The Insight Partners Research Report Guidance
- 1.2 Market Segmentation
2. Executive Summary
- 2.1 Key Insights
3. Research Methodology
- 3.1 Coverage
- 3.2 Secondary Research
- 3.3 Primary Research
4. Cell and Gene Therapy Market Landscape
- 4.1 Overview
- 4.2 PEST Analysis
5. Cell and Gene Therapy Market - Key Industry Dynamics
- 5.1 Key Market Drivers:
- 5.1.1 Increase in Number of Approval of Cell and Gene Therapies
- 5.1.2 Increasing Popularity of Outsourcing Cell and Gene Therapy Manufacturing
- 5.2 Market Restraints
- 5.2.1 High Cost of Cell and Gene Therapy Manufacturing
- 5.3 Market Opportunities
- 5.3.1 Strategic Initiatives by Companies
- 5.4 Future Trends
- 5.4.1 Automation of Cell and Gene Therapy Manufacturing Services
- 5.5 Impact Analysis:
6. Cell and Gene Therapy Market - Global Market Analysis
- 6.1 Cell and Gene Therapy Market Revenue (US$ Mn), 2022 - 2030
7. Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 - by Type
- 7.1 Overview
- 7.2 Cell and Gene Therapy Manufacturing Services Market Revenue Share, by Type 2022 & 2030 (%)
- 7.3 Cell Therapy
- 7.3.1 Overview
- 7.3.2 Cell Therapy: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 7.3.2.1 Autologous
- 7.3.2.1.1 Overview
- 7.3.2.1.2 Autologous: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 7.3.2.2 Allogenic
- 7.3.2.2.1 Overview
- 7.3.2.2.2 Allogenic: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 7.3.2.1 Autologous
- 7.4 Gene Therapy
- 7.4.1 Overview
- 7.4.2 Gene Therapy: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 7.4.2.1 Viral Vector
- 7.4.2.1.1 Overview
- 7.4.2.1.2 Viral Vector: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 7.4.2.2 Non-Viral Vector
- 7.4.2.2.1 Overview
- 7.4.2.2.2 Non-Viral Vector: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 7.4.2.1 Viral Vector
8. Cell and Gene Therapy Manufacturing Services Market Analysis and Forecasts to 2030 - by Indication
- 8.1 Overview
- 8.2 Cell and Gene Therapy Manufacturing Services Market, by Indication 2022 & 2030 (%)
- 8.3 Cancer
- 8.3.1 Overview
- 8.3.2 Cancer: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 8.4 Orthopedics
- 8.4.1 Overview
- 8.4.2 Orthopedics: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 8.5 Others
- 8.5.1 Overview
- 8.5.2 Others: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
9. Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 - by Application
- 9.1 Overview
- 9.2 Cell and Gene Therapy Manufacturing Services Market Revenue Share, by Application 2022 & 2030 (%)
- 9.4 Clinical Manufacturing
- 9.4.1 Overview
- 9.4.2 Clinical Manufacturing: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 9.5 Commercial Manufacturing
- 9.5.1 Overview
- 9.5.2 Commercial Manufacturing: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
10. Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 - by End User
- 10.1 Overview
- 10.2 Cell and Gene Therapy Manufacturing Services Market Revenue Share, by End User 2022 & 2030 (%)
- 10.4 Pharmaceutical and Biotechnology Companies
- 10.4.1 Overview
- 10.4.2 Pharmaceutical & Biotechnology Companies: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
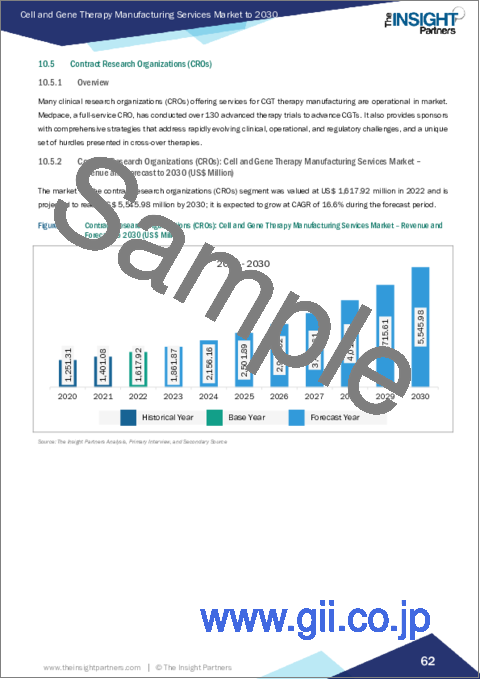
- 10.5 Contract Research Organizations (CROs)
- 10.5.1 Overview
- 10.5.2 Contract Research Organizations (CROs): Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
11. Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 - Regional Analysis
- 11.1 North America: Cell and Gene Therapy Manufacturing Services Market
- 11.1.1 Overview
- 11.1.2 North America: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.1.3 North America: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.1.3.1 North America: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.1.3.2 North America: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.1.4 North America: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.1.5 North America: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.1.6 North America: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.1.7 North America: Cell and Gene Therapy Manufacturing Services Market, by Region, 2022 & 2030 (%)
- 11.1.7.1 US: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.1.7.1.1 US: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.1.7.1.1.1 US: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.1.7.1.1.2 US: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.1.7.1.2 US: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.1.7.1.3 US: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.1.7.1.4 US: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.1.7.2 Canada: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.1.7.2.1 Canada: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.1.7.2.1.1 Canada: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.1.7.2.1.2 Canada: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.1.7.2.2 Canada: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.1.7.2.3 Canada: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.1.7.2.4 Canada: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.1.7.3 Mexico: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.1.7.3.1 Mexico: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.1.7.3.1.1 Mexico: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.1.7.3.1.2 Mexico: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.1.7.3.2 Mexico: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.1.7.3.3 Mexico: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.1.7.3.4 Mexico: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.1.7.1 US: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.2 Europe: Cell and Gene Therapy Manufacturing Services Market
- 11.2.1 Overview
- 11.2.2 Europe: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.2.3 Europe: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.2.3.1 Europe: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.2.3.2 Europe: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.2.4 Europe: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.2.5 Europe: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.2.6 Europe: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.2.7 Europe: Cell and Gene Therapy Manufacturing Services Market, by Region, 2022 & 2030 (%)
- 11.2.7.1 Germany: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.2.7.1.1 Germany: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.2.7.1.1.1 Germany: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.2.7.1.1.2 Germany: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.2.7.1.2 Germany: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.2.7.1.3 Germany: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.2.7.1.4 Germany: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.2.7.2 UK: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.2.7.2.1 UK: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.2.7.2.1.1 UK: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.2.7.2.1.2 UK: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.2.7.2.2 UK: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.2.7.2.3 UK: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.2.7.2.4 UK: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.2.7.3 France: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.2.7.3.1 France: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.2.7.3.1.1 France: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.2.7.3.1.2 France: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.2.7.3.2 France: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.2.7.3.3 France: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.2.7.3.4 France: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.2.7.4 Italy: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.2.7.4.1 Italy: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.2.7.4.1.1 Italy: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.2.7.4.1.2 Italy: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.2.7.4.2 Italy: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.2.7.4.3 Italy: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.2.7.4.4 Italy: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.2.7.5 Spain: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.2.7.5.1 Spain: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.2.7.5.1.1 Spain: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.2.7.5.1.2 Spain: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.2.7.5.2 Spain: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.2.7.5.3 Spain: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.2.7.5.4 Spain: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.2.7.6 Rest of Europe: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.2.7.6.1 Rest of Europe: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.2.7.6.1.1 Rest of Europe: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.2.7.6.1.2 Rest of Europe: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.2.7.6.2 Rest of Europe: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.2.7.6.3 Rest of Europe: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.2.7.6.4 Rest of Europe: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.2.7.1 Germany: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.3 Asia Pacific: Cell and Gene Therapy Manufacturing Services Market
- 11.3.1 Overview
.
- 11.3.2 Asia Pacific: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.3.3 Asia Pacific: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.3.3.1 Asia Pacific: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.3.3.2 Asia Pacific: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.3.4 Asia Pacific: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.3.5 Asia Pacific: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.3.6 Asia Pacific: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.3.7 Asia Pacific: Cell and Gene Therapy Manufacturing Services Market, by Region, 2022 & 2030 (%)
- 11.3.7.1 China: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.3.7.1.1 China: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.3.7.1.1.1 China: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.3.7.1.1.2 China: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.3.7.1.2 China: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.3.7.1.3 China: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.3.7.1.4 China: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.3.7.2 Japan: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.3.7.2.1 Japan: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.3.7.2.1.1 Japan: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.3.7.2.1.2 Japan: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.3.7.2.2 Japan: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.3.7.2.3 Japan: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.3.7.2.4 Japan: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.3.7.3 India: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.3.7.3.1 India: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.3.7.3.1.1 India: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.3.7.3.1.2 India: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.3.7.3.2 India: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.3.7.3.3 India: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.3.7.3.4 India: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.3.7.4 Australia: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.3.7.4.1 Australia: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.3.7.4.1.1 Australia: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.3.7.4.1.2 Australia: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.3.7.4.2 Australia: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.3.7.4.3 Australia: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.3.7.4.4 Australia: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.3.7.5 South Korea: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.3.7.5.1 South Korea: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.3.7.5.1.1 South Korea: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.3.7.5.1.2 South Korea: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.3.7.5.2 South Korea: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.3.7.5.3 South Korea: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.3.7.5.4 South Korea: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.3.7.6 Rest of Asia Pacific: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.3.7.6.1 Rest of Asia Pacific: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.3.7.6.1.1 Rest of Asia Pacific: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.3.7.6.1.2 Rest of Asia Pacific: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.3.7.6.2 Rest of Asia Pacific: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.3.7.6.3 Rest of Asia Pacific: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.3.7.6.4 Rest of Asia Pacific: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.3.7.1 China: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.4 Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market
- 11.4.1 Overview
- 11.4.2 Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.4.3 Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.4.3.1 Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.4.3.2 Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.4.4 Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.4.5 Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.4.6 Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.4.7 Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market, by Region, 2022 & 2030 (%)
- 11.4.7.1 South Africa: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.4.7.1.1 South Africa: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.4.7.1.1.1 South Africa: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.4.7.1.1.2 South Africa: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.4.7.1.2 South Africa: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.4.7.1.3 South Africa: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.4.7.1.4 South Africa: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.4.7.2 Saudi Arabia: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.4.7.2.1 Saudi Arabia: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.4.7.2.1.1 Saudi Arabia: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.4.7.2.1.2 Saudi Arabia: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.4.7.2.2 Saudi Arabia: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.4.7.2.3 Saudi Arabia: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.4.7.2.4 Saudi Arabia: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.4.7.3 UAE: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.4.7.3.1 UAE: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.4.7.3.1.1 UAE: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.4.7.3.1.2 UAE: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.4.7.3.2 UAE: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.4.7.3.3 UAE: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.4.7.3.4 UAE: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.4.7.4 Rest of Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.4.7.4.1 Rest of Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.4.7.4.1.1 Rest of Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.4.7.4.1.2 Rest of Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.4.7.4.2 Rest of Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.4.7.4.3 Rest of Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.4.7.4.4 Rest of Middle East & Africa: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.4.7.1 South Africa: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.5 South & Central America: Cell and Gene Therapy Manufacturing Services Market
- 11.5.1 Overview
- 11.5.2 South & Central America: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.5.3 South & Central America: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.5.3.1 South & Central America: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.5.3.2 South & Central America: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.5.4 South & Central America: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.5.5 South & Central America: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.5.6 South & Central America: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.5.7 South & Central America: Cell and Gene Therapy Manufacturing Services Market, by Region, 2022 & 2030 (%)
- 11.5.7.1 Brazil: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.5.7.1.1 Brazil: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.5.7.1.1.1 Brazil: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.5.7.1.1.2 Brazil: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.5.7.1.2 Brazil: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.5.7.1.3 Brazil: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.5.7.1.4 Brazil: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.5.7.2 Argentina: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.5.7.2.1 Argentina: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.5.7.2.1.1 Argentina: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.5.7.2.1.2 Argentina: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.5.7.2.2 Argentina: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.5.7.2.3 Argentina: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.5.7.2.4 Argentina: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.5.7.3 Rest of South & Central America: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
- 11.5.7.3.1 Rest of South & Central America: Cell and Gene Therapy Manufacturing Services Market, by Type, 2020-2030 (US$ Million)
- 11.5.7.3.1.1 Rest of South & Central America: Cell and Gene Therapy Manufacturing Services Market, by Cell Therapy, 2020-2030 (US$ Million)
- 11.5.7.3.1.2 Rest of South & Central America: Cell and Gene Therapy Manufacturing Services Market, by Gene Therapy, 2020-2030 (US$ Million)
- 11.5.7.3.2 Rest of South & Central America: Cell and Gene Therapy Manufacturing Services Market, by Indication, 2020-2030 (US$ Million)
- 11.5.7.3.3 Rest of South & Central America: Cell and Gene Therapy Manufacturing Services Market, by Application, 2020-2030 (US$ Million)
- 11.5.7.3.4 Rest of South & Central America: Cell and Gene Therapy Manufacturing Services Market, by End User, 2020-2030 (US$ Million)
- 11.5.7.1 Brazil: Cell and Gene Therapy Manufacturing Services Market - Revenue and Forecast to 2030 (US$ Million)
12. Cell and Gene Therapy Manufacturing Services Market - Industry Landscape
- 12.1 Overview
- 12.2 Growth Strategies in Cell and Gene Therapy Manufacturing Services Market
- 12.3 Organic Growth Strategies
- 12.3.1 Overview
- 12.4 Inorganic Growth Strategies
- 12.4.1 Overview
13. Company Profiles
- 13.1 Thermo Fisher Scientific Inc
- 13.1.1 Key Facts
- 13.1.2 Business Description
- 13.1.3 Products and Services
- 13.1.4 Financial Overview
- 13.1.5 SWOT Analysis
- 13.1.6 Key Developments
- 13.2 Merck KGaA
- 13.2.1 Key Facts
- 13.2.2 Business Description
- 13.2.3 Products and Services
- 13.2.4 Financial Overview
- 13.2.5 SWOT Analysis
- 13.2.6 Key Developments
- 13.3 Charles River Laboratories International Inc
- 13.3.1 Key Facts
- 13.3.2 Business Description
- 13.3.3 Products and Services
- 13.3.4 Financial Overview
- 13.3.5 SWOT Analysis
- 13.3.6 Key Developments
- 13.4 Lonza Group AG
- 13.4.1 Key Facts
- 13.4.2 Business Description
- 13.4.3 Products and Services
- 13.4.4 Financial Overview
- 13.4.5 SWOT Analysis
- 13.4.6 Key Developments
- 13.5 WuXi AppTec Co Ltd?
- 13.5.1 Key Facts
- 13.5.2 Business Description
- 13.5.3 Products and Services
- 13.5.4 Financial Overview
- 13.5.5 SWOT Analysis
- 13.5.6 Key Developments
- 13.6 Catalent Inc
- 13.6.1 Key Facts
- 13.6.2 Business Description
- 13.6.3 Products and Services
- 13.6.4 Financial Overview
- 13.6.5 SWOT Analysis
- 13.6.6 Key Developments
- 13.7 Takara Bio Inc
- 13.7.1 Key Facts
- 13.7.2 Business Description
- 13.7.3 Products and Services
- 13.7.4 Financial Overview
- 13.7.5 SWOT Analysis
- 13.7.6 Key Developments
- 13.8 Nikon Corp
- 13.8.1 Key Facts
- 13.8.2 Business Description
- 13.8.3 Products and Services
- 13.8.4 Financial Overview
- 13.8.5 SWOT Analysis
- 13.8.6 Key Developments
- 13.9 FUJIFILM Holdings Corp
- 13.9.1 Key Facts
- 13.9.2 Business Description
- 13.9.3 Products and Services
- 13.9.4 Financial Overview
- 13.9.5 SWOT Analysis
- 13.9.6 Key Developments
- 13.10 National Resilience Inc
- 13.10.1 Key Facts
- 13.10.2 Business Description
- 13.10.3 Products and Services
- 13.10.4 Financial Overview
- 13.10.5 SWOT Analysis
- 13.10.6 Key Developments
- 13.11 Oxford BioMedica Plc
- 13.11.1 Key Facts
- 13.11.2 Business Description
- 13.11.3 Products and Services
- 13.11.4 Financial Overview
- 13.11.5 SWOT Analysis
- 13.11.6 Key Developments