|
|
市場調査レポート
商品コード
1619082
体外診断市場規模、シェア、成長分析、製品別、技術別、検体別、検査タイプ別、用途別、エンドユーザー別、地域別 - 産業予測、2024~2031年In-Vitro Diagnostics Market Size, Share, Growth Analysis, By Product (Reagents & Kits, Instruments), By Technology (Immunoassays, Clinical Chemistry), By Specimen, By Test Type, By Application, By End User, By Region - Industry Forecast 2024-2031 |
||||||
|
|||||||
| 体外診断市場規模、シェア、成長分析、製品別、技術別、検体別、検査タイプ別、用途別、エンドユーザー別、地域別 - 産業予測、2024~2031年 |
|
出版日: 2024年12月18日
発行: SkyQuest
ページ情報: 英文 157 Pages
納期: 3~5営業日
|
全表示
- 概要
- 目次
体外診断の世界市場規模は2022年に835億米ドルと評価され、予測期間(2024~2031年)のCAGRは5.3%で、2023年の879億3,000万米ドルから2031年には1,329億米ドルに成長する見通しです。
体外診断市場は、慢性疾患の有病率の上昇と、早期かつ正確な診断ソリューションに対する消費者の需要の高まりにより、成長する見込みです。医療研究開発への投資と技術の進歩は、体外診断企業の能力をさらに高めると思われます。加えて、高齢化、疾病検査要件の急増、個別化医療に向けた動向の高まりは、市場プレーヤーに大きなビジネスチャンスをもたらします。しかし、高い開発コスト、厳しい規制要件、限られた償還オプションといった課題は、長期的な需要を阻害する可能性があります。全体として、利害関係者は、2023年以降を通じて体外診断分野の有望な成長展望を活用するために、これらのダイナミクスをうまく操る必要があります。
目次
イントロダクション
- 調査の目的
- 調査範囲
- 定義
調査手法
- 情報調達
- 二次データと一次データの方法
- 市場規模予測
- 市場の前提条件と制限
エグゼクティブサマリー
- 世界市場の見通し
- 供給と需要の動向分析
- セグメント別機会分析
市場力学と見通し
- 市場概要
- 市場規模
- 市場力学
- 促進要因と機会
- 抑制要因と課題
- ポーター分析と影響
- 競争企業間の敵対関係
- 代替品の脅威
- 買い手の交渉力
- 新規参入業者の脅威
- 供給企業の交渉力
主な市場の考察
- 重要成功要因
- 競合の程度
- 主な投資機会
- 市場エコシステム
- 市場の魅力指数(2023年)
- PESTEL分析
- マクロ経済指標
- バリューチェーン分析
- 価格分析
- 技術の進歩
- 規制情勢
- ケーススタディ
- 顧客と購買基準の分析
体外診断市場規模:製品別およびCAGR(2024~2031年)
- 市場概要
- 試薬とキット
- 機器
- データ管理ソフトウェアとサービス
体外診断市場規模:技術別およびCAGR(2024~2031年)
- 市場概要
- 免疫測定
- 酵素免疫測定法(ELISA)
- 化学発光免疫測定
- 免疫蛍光アッセイ
- 迅速検査
- 酵素結合免疫スポットアッセイ(Elispot)
- ウエスタンブロッティング
- その他
- 臨床化学
- 基礎代謝パネル
- 肝臓パネル
- 腎臓プロファイル
- 脂質プロファイル
- 甲状腺機能パネル
- 電解質パネル
- 特殊化学試験
- 分子診断
- ポリメラーゼ連鎖反応(PCR)
- 等温核酸増幅技術(INAAT)
- DNAシーケンシングと次世代シーケンシング(NGS)
- インサイチューハイブリダイゼーション(ISH)
- DNAマイクロアレイ
- その他
- 血液学
- 微生物学
- 凝固と止血
- 尿検査
- クロマトグラフィーと質量分析
体外診断市場規模:検体別およびCAGR(2024~2031年)
- 市場概要
- 血液
- 血清
- プラズマ
- 唾液
- 尿
体外診断市場規模:テストタイプ別およびCAGR(2024~2031年)
- 市場概要
- 臨床検査
- ポイントオブケア検査
体外診断市場規模:用途別およびCAGR(2024~2031年)
- 市場概要
- 感染症
- 腫瘍学
- 内分泌学
- 心臓病学
- 血液検査
- 遺伝子検査
- 自己免疫疾患
- アレルギー診断
- 薬物モニタリングと検査
- 骨およびミネラル障害
- 凝固検査
- 血液型分類
- その他の用途
体外診断市場規模:エンドユーザー別およびCAGR(2024~2031年)
- 市場概要
- 病院・クリニック
- 臨床検査室
- 血液銀行
- ホームケア設定
- 製薬・バイオテクノロジー企業
- 学術機関
- その他のエンドユーザー
体外診断市場規模およびCAGR(2024~2031年)
- 北米
- 米国
- カナダ
- 欧州
- 英国
- ドイツ
- スペイン
- フランス
- イタリア
- その他欧州地域
- アジア太平洋地域
- 中国
- インド
- 日本
- 韓国
- その他アジア太平洋地域
- ラテンアメリカ
- ブラジル
- その他ラテンアメリカ地域
- 中東・アフリカ
- GCC諸国
- 南アフリカ
- その他中東・アフリカ
競合情報
- 上位5社の比較
- 主要企業の市場ポジショニング(2023年)
- 主な市場企業が採用した戦略
- 市場の最近の動向
- 企業の市場シェア分析(2023年)
- 主要企業の企業プロファイル
- 会社概要
- 製品ポートフォリオ分析
- セグメント別シェア分析
- 収益の前年比比較(2021~2023年)
主要企業プロファイル
- Roche
- Siemens Healthineers
- Thermo Fisher Scientific
- Abbott Laboratories
- bioMerieux
- Bio-Rad Laboratories
- QIAGEN
- Agilent Technologies
- Sysmex Corporation
- Fujifilm Holdings
- Illumina
- Shimadzu Corporation
- DiaSorin
- Grifols
- Quest Diagnostics
- Transasia Bio-Medicals
- Tulip Diagnostics
- NeoDx
- Voxtur Bio
- Peerless Biotech
結論と推奨事項
Global In-Vitro Diagnostics Market size was valued at USD 83.50 Billion in 2022 and is poised to grow from USD 87.93 Billion in 2023 to USD 132.90 Billion by 2031, growing at a CAGR of 5.3% during the forecast period (2024-2031).
The in-vitro diagnostics market is poised for growth, driven by the rising prevalence of chronic diseases and an increasing consumer demand for early and precise diagnostic solutions. Investments in medical research and development, along with advancements in technologies, will further enhance the capabilities of in-vitro diagnostics firms. Additionally, an aging population, a surge in disease testing requirements, and the growing trend towards personalized medicine present substantial opportunities for market players. Nonetheless, challenges such as high development costs, stringent regulatory requirements, and limited reimbursement options may hinder long-term demand. Overall, stakeholders should navigate these dynamics to capitalize on the promising growth landscape within the in-vitro diagnostics sector through 2023 and beyond.
Top-down and bottom-up approaches were used to estimate and validate the size of the Global In-Vitro Diagnostics market and to estimate the size of various other dependent submarkets. The research methodology used to estimate the market size includes the following details: The key players in the market were identified through secondary research, and their market shares in the respective regions were determined through primary and secondary research. This entire procedure includes the study of the annual and financial reports of the top market players and extensive interviews for key insights from industry leaders such as CEOs, VPs, directors, and marketing executives. All percentage shares split, and breakdowns were determined using secondary sources and verified through Primary sources. All possible parameters that affect the markets covered in this research study have been accounted for, viewed in extensive detail, verified through primary research, and analyzed to get the final quantitative and qualitative data.
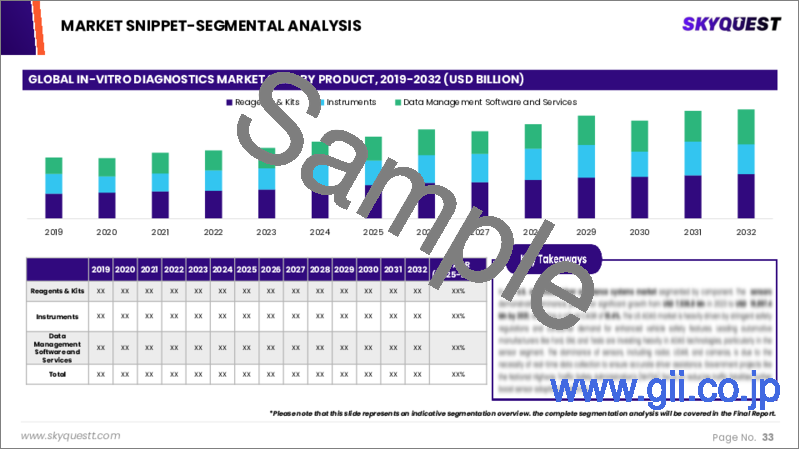
Global In-Vitro Diagnostics Market Segmental Analysis
Global In-Vitro Diagnostics Market is segmented by Product, Technology, Specimen, Test Type, Application, End User and region. Based on Product, the market is segmented into Reagents & Kits, Instruments, Data Management Software & Services. Based on Technology, the market is segmented into Immunoassays, Clinical Chemistry, Molecular Diagnostics, Hematology, Microbiology, Coagulation & Hemostasis, Urinalysis, Chromatography & Mass Spectrometry. Based on Specimen, the market is segmented into Blood, Serum, Plasma, Saliva, Urine. Based on Test Type, the market is segmented into Laboratory Tests, Point-Of-Care Tests. Based on Application, the market is segmented into Infectious Diseases, Oncology, Endocrinology, Cardiology, Blood Screening, Genetic Testing, Autoimmune Diseases, Autoimmune Diseases, Allergy Diagnostics, Drug Monitoring & Testing, Bone & Mineral Disorders, Coagulation Testing, Blood Group Typing, Other Applications. Based on End User, the market is segmented into Hospitals & Clinics, Clinical Laboratories, Blood Banks, Home Care Settings, Pharmaceutical & Biotechnology Companies, Academic Institutes, Other End Users. Based on region, the market is segmented into North America, Europe, Asia Pacific, Latin America and Middle East & Africa.
Driver of the Global In-Vitro Diagnostics Market
The Global In-Vitro Diagnostics market is significantly influenced by several key drivers. Rapid advancements in technology and the growing demand for early and accurate disease detection are paramount in propelling market growth. Additionally, the increasing prevalence of chronic and infectious diseases, along with the rise in patient awareness regarding health monitoring, further fuels the market. Government initiatives promoting healthcare access and investments in research and development also contribute to the expansion of this sector. Furthermore, the aging population, which demands advanced diagnostic solutions, is another critical factor driving the growth of the In-Vitro Diagnostics market globally.
Restraints in the Global In-Vitro Diagnostics Market
The Global In-Vitro Diagnostics market faces several constraints that may hinder its growth. Factors such as stringent regulatory requirements, which vary significantly by region, can complicate the approval process for new diagnostic products. Furthermore, the high costs associated with research and development pose a significant barrier, particularly for smaller firms lacking substantial financial resources. Additionally, the increasing competition among established players and emerging startups can lead to market saturation, driving prices down and affecting profitability. Lastly, the ongoing challenges related to data privacy and cybersecurity in healthcare can create further obstacles for innovation and market expansion in the in-vitro diagnostics sector.
Market Trends of the Global In-Vitro Diagnostics Market
The Global In-Vitro Diagnostics market is experiencing a significant trend toward home care solutions, driven by increasing consumer demand for convenience and accessibility. This shift is prompting in-vitro diagnostics companies to innovate and develop user-friendly test kits designed for at-home use, negating the need for medical supervision. As healthcare systems evolve and patients seek personalized and immediate testing options, these developments represent a lucrative opportunity for manufacturers. By capitalizing on the growing popularity of homecare, the in-vitro diagnostics sector is poised for robust revenue growth, while also improving patient engagement and outcomes on a global scale.
Table of Contents
Introduction
- Objectives of the Study
- Scope of the Report
- Definitions
Research Methodology
- Information Procurement
- Secondary & Primary Data Methods
- Market Size Estimation
- Market Assumptions & Limitations
Executive Summary
- Global Market Outlook
- Supply & Demand Trend Analysis
- Segmental Opportunity Analysis
Market Dynamics & Outlook
- Market Overview
- Market Size
- Market Dynamics
- Driver & Opportunities
- Restraints & Challenges
- Porters Analysis & Impact
- Competitive rivalry
- Threat of substitute
- Bargaining power of buyers
- Threat of new entrants
- Bargaining power of suppliers
Key Market Insights
- Key Success Factors
- Degree of Competition
- Top Investment Pockets
- Market Ecosystem
- Market Attractiveness Index, 2023
- PESTEL Analysis
- Macro-Economic Indicators
- Value Chain Analysis
- Pricing Analysis
- Technological Advancement
- Regulatory Landscape
- Case Studies
- Customer & Buying Criteria Analysis
Global In-Vitro Diagnostics Market Size by Product & CAGR (2024-2031)
- Market Overview
- Reagents & Kits
- Instruments
- Data Management Software & Services
Global In-Vitro Diagnostics Market Size by Technology & CAGR (2024-2031)
- Market Overview
- Immunoassays
- Enzyme-Linked Immunosorbent Assays (ELISA)
- Chemiluminescence Immunoassays
- Immunofluorescence Assays
- Rapid Tests
- Enzyme-Linked Immunospot Assays (Elispot)
- Western Blotting
- Other
- Clinical Chemistry
- Basic Metabolic Panels
- Liver Panels
- Renal Profiles
- Lipid Profiles
- Thyroid Function Panels
- Electrolyte Panels
- Specialty Chemical Tests
- Molecular Diagnostics
- Polymerase Chain Reaction (PCR)
- Isothermal Nucleic Acid Amplification Technology (INAAT)
- DNA Sequencing & Next-Generation Sequencing (NGS)
- In Situ Hybridization (ISH)
- DNA Microarrays
- Other
- Hematology
- Microbiology
- Coagulation & Hemostasis
- Urinalysis
- Chromatography & Mass Spectrometry
Global In-Vitro Diagnostics Market Size by Specimen & CAGR (2024-2031)
- Market Overview
- Blood
- Serum
- Plasma
- Saliva
- Urine
Global In-Vitro Diagnostics Market Size by Test Type & CAGR (2024-2031)
- Market Overview
- Laboratory Tests
- Point-Of-Care Tests
Global In-Vitro Diagnostics Market Size by Application & CAGR (2024-2031)
- Market Overview
- Infectious Diseases
- Oncology
- Endocrinology
- Cardiology
- Blood Screening
- Genetic Testing
- Autoimmune Diseases
- Autoimmune Diseases
- Allergy Diagnostics
- Drug Monitoring & Testing
- Bone & Mineral Disorders
- Coagulation Testing
- Blood Group Typing
- Other Applications
Global In-Vitro Diagnostics Market Size by End User & CAGR (2024-2031)
- Market Overview
- Hospitals & Clinics
- Clinical Laboratories
- Blood Banks
- Home Care Settings
- Pharmaceutical & Biotechnology Companies
- Academic Institutes
- Other End Users
Global In-Vitro Diagnostics Market Size & CAGR (2024-2031)
- North America, (Product, Technology, Specimen, Test Type, Application, End User)
- US
- Canada
- Europe, (Product, Technology, Specimen, Test Type, Application, End User)
- UK
- Germany
- Spain
- France
- Italy
- Rest of Europe
- Asia-Pacific, (Product, Technology, Specimen, Test Type, Application, End User)
- China
- India
- Japan
- South Korea
- Rest of Asia Pacific
- Latin America, (Product, Technology, Specimen, Test Type, Application, End User)
- Brazil
- Rest of Latin America
- Middle East & Africa, (Product, Technology, Specimen, Test Type, Application, End User)
- GCC Countries
- South Africa
- Rest of Middle East & Africa
Competitive Intelligence
- Top 5 Player Comparison
- Market Positioning of Key Players, 2023
- Strategies Adopted by Key Market Players
- Recent Developments in the Market
- Company Market Share Analysis, 2023
- Company Profiles of All Key Players
- Company Details
- Product Portfolio Analysis
- Company's Segmental Share Analysis
- Revenue Y-O-Y Comparison (2021-2023)
Key Company Profiles
- Roche
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- Siemens Healthineers
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- Thermo Fisher Scientific
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- Abbott Laboratories
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- bioMerieux
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- Bio-Rad Laboratories
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- QIAGEN
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- Agilent Technologies
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- Sysmex Corporation
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- Fujifilm Holdings
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- Illumina
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- Shimadzu Corporation
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- DiaSorin
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- Grifols
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- Quest Diagnostics
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- Transasia Bio-Medicals
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- Tulip Diagnostics
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- NeoDx
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- Voxtur Bio
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments
- Peerless Biotech
- Company Overview
- Business Segment Overview
- Financial Updates
- Key Developments