|
|
市場調査レポート
商品コード
1268027
肥満症-パイプラインアナリティクス:2023年Obesity-Pipeline Analytics -2023 |
||||||
|
|||||||
| 肥満症-パイプラインアナリティクス:2023年 |
|
出版日: 2023年05月08日
発行: Mellalta Meets LLP
ページ情報: 英文 240 Pages
納期: 即納可能
|
- 全表示
- 概要
- 目次
肥満症は公衆衛生上の懸念事項であり、世界中で有病率が増加しています。現在では、米国医師会(2013年)および欧州委員会(2021年)により慢性疾患に分類されるほど、その蔓延が進んでいます。肥満症は生活の質に大きな影響を与え、医療費の増加につながり、肥満症のある人は、心血管疾患、2型糖尿病、特定の癌、筋骨格系障害など、数多くの健康リスクを発症するリスクが高まると言われています。
当レポートでは、肥満症について調査し、肥満症の概要とパイプライン動向、競合情勢、相別のパイプライン薬剤などをまとめています。
目次
概要
肥満症の標的
- イントロダクション
- 疫学と原因
- 肥満症の病態生理学
- 肥満症の発症機序
- 肥満症におけるグルカゴン様ペプチド-1(GLP-1)の役割
- 2022年肥満症治療ガイドライン
- 患者にとって最も適切な減量薬を選択するための証拠に基づいた推奨事項
- 肥満症の潜在的な転帰を伴うT2D(2型糖尿病)の市販薬
肥満症パイプライン分析、相別
- 肥満症 - 第III相および第II相パイプライン分析の概要
- パイプライン製品、開発段階別
- 肥満症の競合情勢、相別、経路別
- 臨床パイプライン(第3相~第1相)製品、企業別
- 肥満症の単剤療法と併用療法の臨床試験
- パイプライン製品、作用機序別
- 初期および臨床段階における新しいメカニズム
- 肥満症の臨床および規制のタイムライン
- 後期段階のプロファイルの比較の概要
肥満症のライセンシング、買収、コラボレーション
肥満症パイプラインの情勢
- 肥満症の臨床パイプラインの情勢
第Ⅲ相
- カグリセマ(Novo Nordisk A/S)
- DOR/3TC/TDF(Merck & Co)
- ティルゼパチド(Eli Lilly and Company)
- IBI362(Innovent Biologics (Suzhou) Co. Ltd.)
肥満症パイプラインの情勢
第II相
- DCCR(Soleno Therapeutics, Inc)
- MBL949(Novartis)
- BI 456906(Zealand Pharma/Boehringer Ingelheim)
- LY3502970(Eli Lilly and Company)
- XW-003(Sciwind Biosciences)
- ARD-101(Aardvark Therapeutics)
- ダヌグリプロン(Pfizer)
- PF-07081532(Sosei Heptares)
- ペムビドゥチド(Altimmune, Inc.)
- レタトルチド(Eli Lilly and Company)
- TG103(Genexine/I-Mab Biopharma/CSPC Pharmaceutical)
- APHD-012(Aphaia Pharma)
- ビマグルマブ(Versanis Bio, Inc./Novartis)
- HSG4112(Glaceum)
- SHR20004(Jiangsu HengRui Medicine Co.)
- AMG-133(Amgen)
- PYY 1875(Novo Nordisk A/S)
- カグリリンチド(Novo Nordisk A/S)
- EMP-16-02(Empros Pharma)
第I相
- EMP-16-02(Empros Pharma)
- AMG-786(Amgen)
- BMS-963272(Bristol-Myers Squibb)
- DD01(D&D Pharmatech)
- ERX1000(ERX Pharmaceuticals)
- GMA106(Gmax Biopharm)
- LY3457263(Eli Lilly and Company)
- NNC0247-0829(Novo Nordisk)
- アミクレチン(Novo Nordisk)
- NO-13065(Otsuka Pharmaceutical Factory, Inc)
- S-309309(Shionogi)
- HM15136(Hanmi Pharmaceutical Company Limited)
- DR10624(Zhejiang Doer Biologics)
- LY3841136(Eli Lilly and Company)
- DACRA QW II(Eli Lilly and Company)
- AZD6234(AstraZeneca)
- CT-388(Carmot Therapeutics, Inc)
- INV-202(Inversago Pharma Inc.)
- BI 1820237(Boehringer Ingelheim)
- ZP 8396(Zealand Pharma)
- MVD1(Eolo pharma)
- VK2735(Viking Therapeutics)
- GSBR-1290(Structure Therapeutics)
- XW014(Sciwind Biosciences)
- CIN-109(CinFina Pharma)
- CB4211(CohBar)
- DWP306001(Daewoong Pharmaceutical)
- LR19021(LG Life Sciences)
- ECC5004(Eccogene)
- SCO267(SCOHIA PHARMA, Inc)
- SCO094(SCOHIA PHARMA, Inc./Huadong Medicine)
- BC LisPram(Adocia)
- GUB002496(Gubra Therapeutics/Boehringer Ingelheim)
- MT961(Medytox)
肥満症の前臨床パイプライン情勢
肥満症のSWOT分析
付録
Obesity report provides a comprehensive analysis of the obesity market, with an in-depth analysis of key pipeline products, licensing, acquisition, and collaboration deals. The report covers around 120+ products with 50 companies and their 65 products is clinical stages, providing valuable insights to companies seeking to expand their research in this therapy area. In addition, our report provides in-depth profiles of obesity candidates, including preclinical and clinical studies, details of partnerships and business deal values, targeted technologies, investments, and acquisition trends. We also provide licensing opportunities, acquisition trends, and product analysis by phases, company & MOA. Furthermore, the report covers descriptive information on the competitive pipeline landscape.
Obesity is a growing public health concern, with increasing prevalence worldwide. It has become so prevalent that it is now classified by the American Medical Association (2013) and the European Commission (2021) as a chronic disease. Obesity has a significant impact on quality of life and results in increased healthcare costs, with individuals with obesity at increased risk of developing numerous health risks, including cardiovascular disease, type 2 diabetes, certain cancers, and musculoskeletal disorders.
In this report, Mellalta Meets provides an in-depth analysis of Obesity pipeline covering Phase III & Phase II Pipeline Analysis Overview, Pipeline Products by Stage of Development, Obesity Competitive Landscape Distinguished by Phase & Route, Clinical Pipeline Products by Company, Obesity Monotherapy & Combinations Clinical Trials, Pipeline Products by MOA, Novel Mechanisms in Early and Clinical Stages , Obesity Clinical & Regulatory Timelines, Late-Stage Profiles Comparisons At-a-glance, details of partnerships and business deal values and investments. Currently, there are more than 65 candidates under evaluation in clinical and preclinical studies. The major key players operating in the market are Novo Nordisk, Merck & Co, Eli Lilly and Company, and Innovent Biologics (Suzhou) Co. Ltd and many more which have robust clinical pipelines of OBESITY inhibitor candidates. As per analysis, despite the challenges in the pipeline development of obesity, the obesity market looks set to become the next blockbuster pharma category.
Key Highlights of Obesity Report:
- The Obesity segment is dominated by the pre-clinical assets (XX) which represent XX% of the total development followed by Phase 1 assets (XX) representing XX%, Phase 2 assets (XX) with XX%, discovery (XX) with 13% and Phase 3 (4) representing 3% of the pipeline development in Obesity.
- The obesity treatment pipeline is marked by a strong presence of US-based biotech companies, accounting for an impressive XX% of all clinical-stage obesity therapies, followed by China and Denmark with XX%. With Eli Lilly leading the pack in product development, closely trailed by Novo Nordisk and Zealand Pharma, the obesity pipeline showcases a robust and diverse range of innovative solutions to address the global obesity epidemic. These industry giants are set to transform the landscape with ground-breaking treatments, tapping into new mechanisms of action and combination therapies for enhanced efficacy and improved patient adherence.
- Obesity pipeline landscape includes 85% of Monotherapy trials, 3% combination therapy and 12% of Combination/monotherapy trials.
- The Obesity segment is dominated by the pre-clinical assets (46), phase 1 assets (37), phase 2 assets (24) and phase 3 (4).
Report Coverage:
- Indication Prioritisation: Obesity market potential based on Indications.
- Business Transactions & Strategies: Key collaborations and deal values
- Obesity Pipeline Development: Product Profiles, Clinical Trials & Results
- Obesity Acquisition Targets
- Obesity Competitive Intelligence
- Recent & Upcoming events
TABLE OF CONTENTS
OVERVIEW
The Obesity Target BACKGROUND
- Introduction
- Epidemiology & Causes
- Pathophysiology of Obesity
- Obesity pathomechanisms
- Role of Glucagon-like peptide-1 (GLP-1) in obesity
- 2022 Obesity Treatment Guidelines
- Evidence-based recommendations for selecting the most appropriate weight loss medications for their patients
- Marketed Drugs in T2D (Type 2 diabetes) with potential outcomes in Obesity
Obesity PIPELINE ANALYSIS by Phases
- Obesity - Phase III & Phase II Pipeline Analysis Overview
- Pipeline Products by Stage of Development
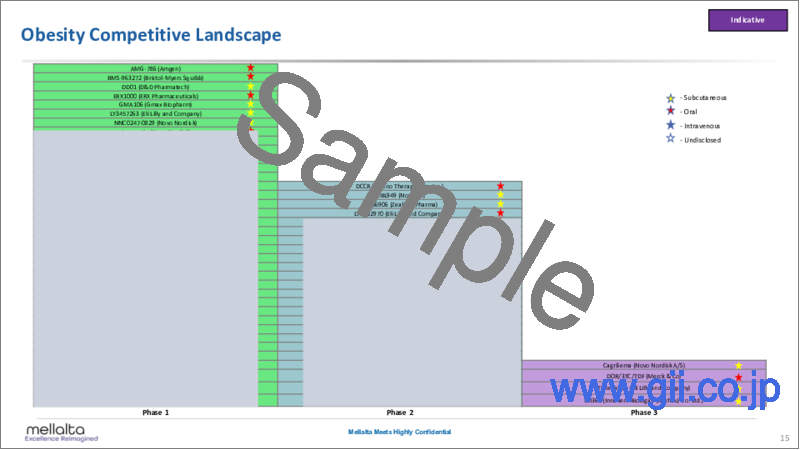
- Obesity Competitive Landscape Distinguished by Phase & Route
- Clinical Pipeline (Phase 3 - Phase 1) Products by Company
- Obesity Monotherapy & Combinations Clinical Trials
- Pipeline Products by MOA
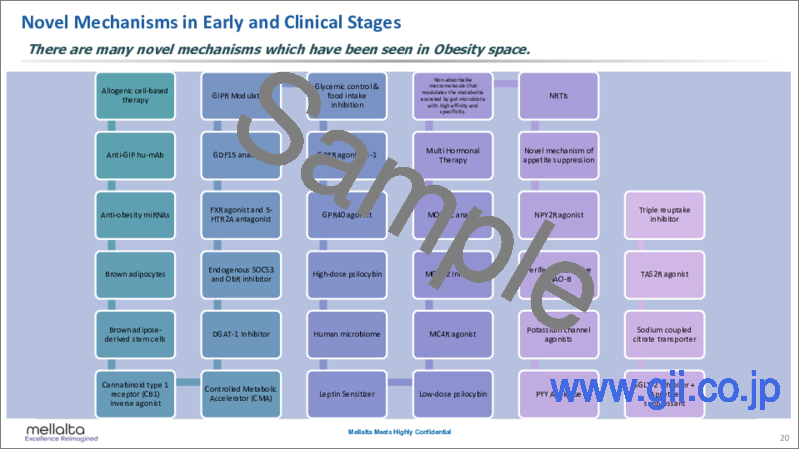
- Novel Mechanisms in Early and Clinical Stages
- Obesity Clinical & Regulatory Timelines
- Late-Stage Profiles Comparisons At-a-glance
Obesity Licensing, Acquisition And Collaboration
- Obesity Licensing, Acquisition, and Deal values
- Obesity Licensing by Transaction type and total amount size by Phases
Obesity Pipeline Landscape
- Obesity Clinical Pipeline Landscape
Phase III
- CagriSema (Novo Nordisk A/S)
- Product Profile
- Clinical Trials
- Collaborations
- Other Developments
- DOR/3TC/TDF (Merck & Co)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- Tirzepatide (Eli Lilly and Company)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- IBI362 (Innovent Biologics (Suzhou) Co. Ltd.)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
Obesity Pipeline Landscape
Phase II
- DCCR (Soleno Therapeutics, Inc.)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- MBL949 (Novartis)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- BI 456906 (Zealand Pharma/Boehringer Ingelheim)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- LY3502970 (Eli Lilly and Company)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- XW-003 (Sciwind Biosciences)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- ARD-101 (Aardvark Therapeutics)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- Danuglipron (Pfizer)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- PF-07081532 (Sosei Heptares)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- Pemvidutide (Altimmune, Inc.)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- Retatrutide (Eli Lilly and Company)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- TG103 (Genexine/I-Mab Biopharma/CSPC Pharmaceutical)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- APHD-012 (Aphaia Pharma)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- Bimagrumab (Versanis Bio, Inc./ Novartis)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- HSG4112 (Glaceum)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- SHR20004 (Jiangsu HengRui Medicine Co.)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- AMG-133 (Amgen)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- PYY 1875 (Novo Nordisk A/S)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- Cagrilintide (Novo Nordisk A/S)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- EMP-16-02 (Empros Pharma)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
Phase I
- EMP-16-02 (Empros Pharma)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- AMG-786 (Amgen)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- BMS-963272 (Bristol-Myers Squibb)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- DD01 (D&D Pharmatech)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- ERX1000 (ERX Pharmaceuticals)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- GMA106 (Gmax Biopharm)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- LY3457263 (Eli Lilly and Company)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- NNC0247-0829 (Novo Nordisk)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- Amycretin (Novo Nordisk)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- NO-13065 (Otsuka Pharmaceutical Factory, Inc.)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- S-309309 (Shionogi)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- HM15136 (Hanmi Pharmaceutical Company Limited)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- DR10624 (Zhejiang Doer Biologics)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- LY3841136 (Eli Lilly and Company)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- DACRA QW II (Eli Lilly and Company)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- AZD6234 (AstraZeneca)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- CT-388 (Carmot Therapeutics, Inc.)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- INV-202 (Inversago Pharma Inc.)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- BI 1820237 (Boehringer Ingelheim)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- ZP 8396 (Zealand Pharma)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- MVD1 (Eolo pharma)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- VK2735 (Viking Therapeutics)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- GSBR-1290 (Structure Therapeutics)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- XW014 (Sciwind Biosciences)
- Product Profile & Description
- Collaborations
- Other Developments
- CIN-109 (CinFina Pharma)
- Product Profile & Description
- Collaborations
- Other Developments
- CB4211 (CohBar)
- Product Profile & Description
- Collaborations
- Other Developments
- DWP306001 (Daewoong Pharmaceutical)
- Product Profile & Description
- Collaborations
- Other Developments
- LR19021 (LG Life Sciences)
- Product Profile & Description
- Collaborations
- Other Developments
- ECC5004 (Eccogene)
- Product Profile & Description
- Collaborations
- Other Developments
- SCO267 (SCOHIA PHARMA, Inc.)
- Product Profile & Description
- Collaborations
- Other Developments
- SCO094 (SCOHIA PHARMA, Inc./Huadong Medicine)
- Product Profile & Description
- Collaborations
- Other Developments
- BC LisPram (Adocia)
- Product Profile & Description
- Collaborations
- Other Developments
- GUB002496 (Gubra Therapeutics/Boehringer Ingelheim)
- Product Profile & Description
- Collaborations
- Other Developments
- MT961 (Medytox)
- Product Profile & Description
- Collaborations
- Other Developments
Obesity Pre-clinical Pipeline Landscape
Obesity SWOT Analysis
Appendix
- About us