|
|
市場調査レポート
商品コード
1384882
リードレスペースメーカーの世界市場規模、シェア、産業動向分析レポート:タイプ別、最終用途別、地域別展望と予測、2023年~2030年Global Leadless Pacemakers Market Size, Share & Industry Trends Analysis Report By Type (Micra Transcatheter Pacing System, and Others), By End-use (Hospitals, and Outpatient Facilities), By Regional Outlook and Forecast, 2023 - 2030 |
||||||
|
|||||||
| リードレスペースメーカーの世界市場規模、シェア、産業動向分析レポート:タイプ別、最終用途別、地域別展望と予測、2023年~2030年 |
|
出版日: 2023年10月31日
発行: KBV Research
ページ情報: 英文 199 Pages
納期: 即納可能
|
全表示
- 概要
- 図表
- 目次
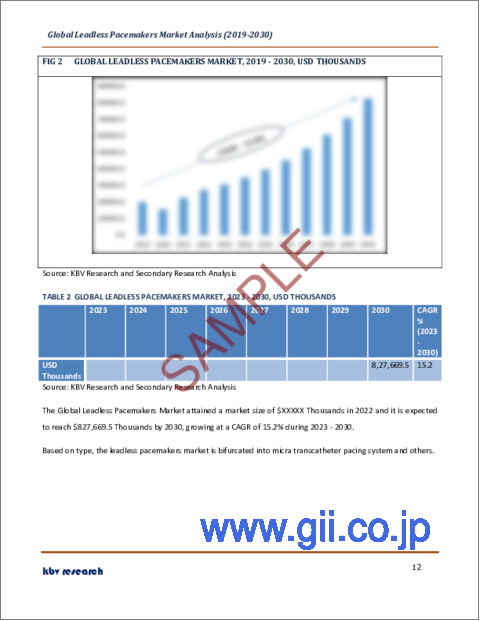
リードレスペースメーカー市場規模は2030年までに8億2,770万米ドルに達すると予測され、予測期間中のCAGRは15.2%の市場成長率で上昇する見込みです。
KBV Cardinalのマトリックスに掲載された分析によると、Medtronic PLCとAbbott Laboratoriesが同市場の先行企業です。ボストン・サイエンティフィック・コーポレーション、インテガー・ホールディングス・コーポレーション、テルモ・コーポレーションなどの企業は、同市場における主要なイノベーターです。2023年9月、ボストン・サイエンティフィック・コーポレーションは、脊椎性疼痛治療に特化した非上場の医療機器会社であるリリーバント・メドシステムズ社の買収を発表しました。この買収により、Relievant Medsystemsの臨床的裏付けのある技術とボストン・サイエンティフィックの戦略が統合され、個別化医療を必要とする個人の治療選択肢が拡大します。
COVID-19の影響
パンデミックの間、多くの病院やヘルスケア施設は、リソースを確保し、ウイルス感染のリスクを減らすために、選択的処置を延期または中止しました。このため、リードレスペースメーカーの植え込みは、多くの場合、選択的で緊急性のない処置とみなされるため、減少しました。リードレスペースメーカーを含む医療機器の世界サプライチェーンは、製造や輸送のロックダウンや制限により混乱に見舞われました。このため、医療機器の供給が遅れ、患者がこれらの先端技術を利用することができなくなる可能性があっています。ヘルスケア・プロバイダーは、COVID-19症例の急増に対応するため、リソースとスタッフを振り向けた。このようにCOVID以外の医療サービスから注目と資源が転用されたことは、リードレスペースメーカーの普及と植え込みに影響を与えました。
市場成長要因
低侵襲手術への需要の高まり
低侵襲手術の開発により、リードの留置や外科的切開の必要性が減少し、手術リスクが低下し、患者の快適性が向上しました。この侵襲性の低減は、患者の安全性の向上だけでなく、回復時間の短縮にもつながっています。リードレスペースメーカー植え込み術を受けた患者は、より短い入院期間と術後の不快感の軽減を経験しています。この迅速な回復により、患者は日常生活や活動に迅速に戻ることができます。これらの処置は、医療施設へのアクセスが制限されているような遠隔地や地方のヘルスケア環境に新たな可能性をもたらしています。その結果、低侵襲手技に対する需要が急速に高まっており、市場の拡大を後押ししています。
心臓不整脈の有病率の上昇
心不整脈の有病率の上昇は、人口動態の変化からライフスタイルの選択、ヘルスケアの進歩に至るまで、複数の要因が複雑に絡み合っています。高血圧は不整脈の確立された危険因子です。高血圧は心臓の構造や電気的シグナル伝達に変化をもたらし、不整脈の可能性を高める。同様に、糖尿病は不整脈、特に心房細動のリスクの増加と関連しています。さらに、不整脈の有病率は特定の年齢層に限定されるものではなく、さまざまな層の個人に影響を及ぼします。高齢者から若年者まで、不整脈を経験するすべての年齢層の患者がこれらの装置の恩恵を受けることができます。したがって、不整脈の有病率の増加がリードレスペースメーカーの需要を押し上げています。
市場抑制要因
規制と償還の課題
医療機器の複雑で長い承認プロセスなどの規制上の課題は、リードレスペースメーカーのイントロダクションを大幅に遅らせる可能性があります。規制機関は安全性と有効性の徹底的な評価を要求するため、製品認可までの期間が長期化します。規制当局の承認が遅れると、患者が先進的な医療ソリューションを利用できなくなるだけでなく、メーカーにとっては市場投入までの時間が長くなり、最先端機器を提供する能力に影響を与えることになります。これらのデバイスの経済的負担は、その潜在的な利点にもかかわらず、ヘルスケアプロバイダーと患者の両方がペーシングソリューションとしてリードレスペースメーカーを選択することを躊躇させる可能性があります。このような経済的負担は、特に、より費用対効果の高い代替医療が利用可能である場合には、これらの機器を選択することを躊躇させる可能性があります。これらすべての要因が市場の成長を妨げる可能性があります。
タイプ別展望
タイプ別に見ると、市場はマイクラ経カテーテルペーシングシステムとその他に二分されます。その他のセグメントは、2022年の市場でかなりの成長率を確保しました。ナノスティムやWiSE CRTシステムなどのデバイスを含むリードレスペースメーカーは、従来のリード付きペースメーカーと比較していくつかの利点があります。Nanostimは非常にコンパクトで、大きめのビタミン・カプセルに近いサイズです。この小ささにより、侵襲の少ない処置が可能となり、より控えめで快適な植え込みが実現します。WiSE CRTシステムは、心不全の治療法である心臓再同期療法用に設計されています。従来のペーシングリードを必要とせず、心臓再同期の利点を提供します。ナノスティムとWiSE CRTシステムはいずれも、低侵襲の植え込み、コンパクトな設計、感染リスクの低減、美容上の利点、長持ちするバッテリー寿命などの大きな利点を提供します。
最終用途の展望
最終用途別では、市場は病院と外来患者に分けられます。病院セグメントは2022年に市場で最大の収益シェアを獲得しました。病院では、ペースメーカー移植専用の手術室や手術チームが必要ないため、リソースを効率的に割り当てることができます。これはコスト削減につながり、他の重要な医療処置にリソースを振り向けることができます。体外に出るリード線がないため、リード線挿入部位に関連する感染症のリスクは事実上排除されます。病院は、機器に関連した感染率の低下から利益を得ることができます。
地域別展望
地域別に見ると、市場は北米、欧州、アジア太平洋、LAMEAで分析されます。北米セグメントが2022年の市場で最大の収益シェアを占めています。北米では、リードレスペースメーカーの需要が着実に増加しています。これらの要因は、心臓ペーシング技術の進化と、心臓不整脈治療のための、より患者中心の、低侵襲で技術的に先進的な選択肢へのシフトを強調しています。リードレスペースメーカーは、感染症にかかりやすいリードを必要としないです。北米のように感染対策が優先される地域では、この機能が高く評価されています。
目次
第1章 市場範囲と調査手法
- 市場の定義
- 目的
- 市場範囲
- セグメンテーション
- 調査手法
第2章 市場の概要
- 主なハイライト
第3章 市場概要
- イントロダクション
- 概要
- 市場構成とシナリオ
- 概要
- 市場に影響を与える主な要因
- 市場促進要因
- 市場抑制要因
第4章 競合分析- 世界
- KBV Cardinal Matrix
- 最近の業界全体の戦略的展開
- パートナーシップ、コラボレーション、および契約
- 製品の発売と製品の拡大
- 買収と合併
- 試験と承認
- 主要成功戦略
- 主な戦略
- 主要な戦略的動き
- ポーターのファイブフォース分析
第5章 世界のリードレスペースメーカー市場:タイプ別
- 世界のMicra経カカテーテルペーシングシステム市場:地域別
- 世界のその他の市場:地域別
第6章 世界の市場:、最終用途別
- 世界の病院市場:地域別
- 世界の外来施設市場:地域別
第7章 世界の市場:、地域別
- 北米市場
- 欧州市場
- アジア太平洋市場
- ラテンアメリカ・中東・アフリカ市場
第8章 企業プロファイル
- Medtronic PLC
- Abbott Laboratories
- Boston Scientific Corporation
- Biotronik SE & Co KG
- Lepu Medical Technology Co, Ltd
- Integer Holdings Corporation
- MicroPort Scientific Corporation
- OSYPKA AG
- Terumo Corporation
- EBR Systems, Inc
第9章 市場の成功必須条件
LIST OF TABLES
- TABLE 1 Global Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 2 Global Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 3 Partnerships, Collaborations and Agreements- Leadless Pacemakers Market
- TABLE 4 Product Launches And Product Expansions- Leadless Pacemakers Market
- TABLE 5 Acquisition and Mergers- Leadless Pacemakers Market
- TABLE 6 Trials & Approvals- Leadless Pacemakers Market
- TABLE 7 Global Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 8 Global Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 9 Global Micra Transcatheter Pacing System Market, By Region, 2019 - 2022, USD Thousands
- TABLE 10 Global Micra Transcatheter Pacing System Market, By Region, 2023 - 2030, USD Thousands
- TABLE 11 Global Others Market, By Region, 2019 - 2022, USD Thousands
- TABLE 12 Global Others Market, By Region, 2023 - 2030, USD Thousands
- TABLE 13 Global Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 14 Global Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 15 Global Hospitals Market, By Region, 2019 - 2022, USD Thousands
- TABLE 16 Global Hospitals Market, By Region, 2023 - 2030, USD Thousands
- TABLE 17 Global Outpatient Facilities Market, By Region, 2019 - 2022, USD Thousands
- TABLE 18 Global Outpatient Facilities Market, By Region, 2023 - 2030, USD Thousands
- TABLE 19 Global Leadless Pacemakers Market, By Region, 2019 - 2022, USD Thousands
- TABLE 20 Global Leadless Pacemakers Market, By Region, 2023 - 2030, USD Thousands
- TABLE 21 North America Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 22 North America Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 23 North America Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 24 North America Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 25 North America Micra Transcatheter Pacing System Market, By Country, 2019 - 2022, USD Thousands
- TABLE 26 North America Micra Transcatheter Pacing System Market, By Country, 2023 - 2030, USD Thousands
- TABLE 27 North America Others Market, By Country, 2019 - 2022, USD Thousands
- TABLE 28 North America Others Market, By Country, 2023 - 2030, USD Thousands
- TABLE 29 North America Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 30 North America Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 31 North America Hospitals Market, By Country, 2019 - 2022, USD Thousands
- TABLE 32 North America Hospitals Market, By Country, 2023 - 2030, USD Thousands
- TABLE 33 North America Outpatient Facilities Market, By Country, 2019 - 2022, USD Thousands
- TABLE 34 North America Outpatient Facilities Market, By Country, 2023 - 2030, USD Thousands
- TABLE 35 North America Leadless Pacemakers Market, By Country, 2019 - 2022, USD Thousands
- TABLE 36 North America Leadless Pacemakers Market, By Country, 2023 - 2030, USD Thousands
- TABLE 37 US Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 38 US Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 39 US Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 40 US Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 41 US Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 42 US Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 43 Canada Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 44 Canada Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 45 Canada Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 46 Canada Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 47 Canada Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 48 Canada Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 49 Mexico Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 50 Mexico Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 51 Mexico Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 52 Mexico Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 53 Mexico Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 54 Mexico Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 55 Rest of North America Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 56 Rest of North America Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 57 Rest of North America Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 58 Rest of North America Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 59 Rest of North America Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 60 Rest of North America Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 61 Europe Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 62 Europe Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 63 Europe Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 64 Europe Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 65 Europe Micra Transcatheter Pacing System Market, By Country, 2019 - 2022, USD Thousands
- TABLE 66 Europe Micra Transcatheter Pacing System Market, By Country, 2023 - 2030, USD Thousands
- TABLE 67 Europe Others Market, By Country, 2019 - 2022, USD Thousands
- TABLE 68 Europe Others Market, By Country, 2023 - 2030, USD Thousands
- TABLE 69 Europe Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 70 Europe Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 71 Europe Hospitals Market, By Country, 2019 - 2022, USD Thousands
- TABLE 72 Europe Hospitals Market, By Country, 2023 - 2030, USD Thousands
- TABLE 73 Europe Outpatient Facilities Market, By Country, 2019 - 2022, USD Thousands
- TABLE 74 Europe Outpatient Facilities Market, By Country, 2023 - 2030, USD Thousands
- TABLE 75 Europe Leadless Pacemakers Market, By Country, 2019 - 2022, USD Thousands
- TABLE 76 Europe Leadless Pacemakers Market, By Country, 2023 - 2030, USD Thousands
- TABLE 77 Germany Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 78 Germany Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 79 Germany Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 80 Germany Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 81 Germany Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 82 Germany Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 83 UK Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 84 UK Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 85 UK Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 86 UK Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 87 UK Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 88 UK Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 89 France Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 90 France Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 91 France Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 92 France Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 93 France Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 94 France Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 95 Russia Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 96 Russia Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 97 Russia Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 98 Russia Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 99 Russia Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 100 Russia Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 101 Spain Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 102 Spain Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 103 Spain Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 104 Spain Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 105 Spain Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 106 Spain Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 107 Italy Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 108 Italy Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 109 Italy Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 110 Italy Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 111 Italy Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 112 Italy Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 113 Rest of Europe Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 114 Rest of Europe Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 115 Rest of Europe Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 116 Rest of Europe Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 117 Rest of Europe Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 118 Rest of Europe Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 119 Asia Pacific Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 120 Asia Pacific Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 121 Asia Pacific Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 122 Asia Pacific Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 123 Asia Pacific Micra Transcatheter Pacing System Market, By Country, 2019 - 2022, USD Thousands
- TABLE 124 Asia Pacific Micra Transcatheter Pacing System Market, By Country, 2023 - 2030, USD Thousands
- TABLE 125 Asia Pacific Others Market, By Country, 2019 - 2022, USD Thousands
- TABLE 126 Asia Pacific Others Market, By Country, 2023 - 2030, USD Thousands
- TABLE 127 Asia Pacific Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 128 Asia Pacific Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 129 Asia Pacific Hospitals Market, By Country, 2019 - 2022, USD Thousands
- TABLE 130 Asia Pacific Hospitals Market, By Country, 2023 - 2030, USD Thousands
- TABLE 131 Asia Pacific Outpatient Facilities Market, By Country, 2019 - 2022, USD Thousands
- TABLE 132 Asia Pacific Outpatient Facilities Market, By Country, 2023 - 2030, USD Thousands
- TABLE 133 Asia Pacific Leadless Pacemakers Market, By Country, 2019 - 2022, USD Thousands
- TABLE 134 Asia Pacific Leadless Pacemakers Market, By Country, 2023 - 2030, USD Thousands
- TABLE 135 China Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 136 China Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 137 China Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 138 China Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 139 China Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 140 China Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 141 Japan Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 142 Japan Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 143 Japan Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 144 Japan Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 145 Japan Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 146 Japan Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 147 India Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 148 India Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 149 India Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 150 India Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 151 India Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 152 India Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 153 South Korea Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 154 South Korea Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 155 South Korea Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 156 South Korea Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 157 South Korea Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 158 South Korea Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 159 Singapore Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 160 Singapore Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 161 Singapore Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 162 Singapore Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 163 Singapore Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 164 Singapore Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 165 Malaysia Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 166 Malaysia Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 167 Malaysia Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 168 Malaysia Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 169 Malaysia Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 170 Malaysia Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 171 Rest of Asia Pacific Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 172 Rest of Asia Pacific Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 173 Rest of Asia Pacific Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 174 Rest of Asia Pacific Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 175 Rest of Asia Pacific Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 176 Rest of Asia Pacific Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 177 LAMEA Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 178 LAMEA Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 179 LAMEA Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 180 LAMEA Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 181 LAMEA Micra Transcatheter Pacing System Market, By Country, 2019 - 2022, USD Thousands
- TABLE 182 LAMEA Micra Transcatheter Pacing System Market, By Country, 2023 - 2030, USD Thousands
- TABLE 183 LAMEA Others Market, By Country, 2019 - 2022, USD Thousands
- TABLE 184 LAMEA Others Market, By Country, 2023 - 2030, USD Thousands
- TABLE 185 LAMEA Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 186 LAMEA Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 187 LAMEA Hospitals Market, By Country, 2019 - 2022, USD Thousands
- TABLE 188 LAMEA Hospitals Market, By Country, 2023 - 2030, USD Thousands
- TABLE 189 LAMEA Outpatient Facilities Market, By Country, 2019 - 2022, USD Thousands
- TABLE 190 LAMEA Outpatient Facilities Market, By Country, 2023 - 2030, USD Thousands
- TABLE 191 LAMEA Leadless Pacemakers Market, By Country, 2019 - 2022, USD Thousands
- TABLE 192 LAMEA Leadless Pacemakers Market, By Country, 2023 - 2030, USD Thousands
- TABLE 193 Brazil Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 194 Brazil Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 195 Brazil Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 196 Brazil Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 197 Brazil Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 198 Brazil Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 199 Argentina Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 200 Argentina Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 201 Argentina Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 202 Argentina Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 203 Argentina Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 204 Argentina Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 205 UAE Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 206 UAE Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 207 UAE Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 208 UAE Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 209 UAE Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 210 UAE Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 211 Saudi Arabia Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 212 Saudi Arabia Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 213 Saudi Arabia Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 214 Saudi Arabia Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 215 Saudi Arabia Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 216 Saudi Arabia Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 217 South Africa Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 218 South Africa Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 219 South Africa Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 220 South Africa Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 221 South Africa Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 222 South Africa Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 223 Nigeria Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 224 Nigeria Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 225 Nigeria Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 226 Nigeria Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 227 Nigeria Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 228 Nigeria Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 229 Rest of LAMEA Leadless Pacemakers Market, 2019 - 2022, USD Thousands
- TABLE 230 Rest of LAMEA Leadless Pacemakers Market, 2023 - 2030, USD Thousands
- TABLE 231 Rest of LAMEA Leadless Pacemakers Market, By Type, 2019 - 2022, USD Thousands
- TABLE 232 Rest of LAMEA Leadless Pacemakers Market, By Type, 2023 - 2030, USD Thousands
- TABLE 233 Rest of LAMEA Leadless Pacemakers Market, By End-use, 2019 - 2022, USD Thousands
- TABLE 234 Rest of LAMEA Leadless Pacemakers Market, By End-use, 2023 - 2030, USD Thousands
- TABLE 235 Key Information - Medtronic PLC
- TABLE 236 Key Information - Abbott Laboratories
- TABLE 237 Key Information - Boston Scientific Corporation
- TABLE 238 Key Information - Biotronik SE & Co. KG
- TABLE 239 Key Information - Lepu Medical Technology Co., Ltd
- TABLE 240 Key Information - Integer Holdings Corporation
- TABLE 241 key Information - MicroPort Scientific Corporation
- TABLE 242 Key Information - OSYPKA AG
- TABLE 243 Key Information - Terumo Corporation
- TABLE 244 Key Information - EBR Systems, Inc.
The Global Leadless Pacemakers Market size is expected to reach $827.7 million by 2030, rising at a market growth of 15.2% CAGR during the forecast period.
European patients and healthcare providers are increasingly valuing minimally invasive procedures. Thus, the European region would register nearly 28% share of the market by 2030. The catheter-based implantation of leadless pacemakers eliminates the need for surgical pockets and leads, reducing pain, scarring, and recovery time. Europe possesses a well-developed healthcare infrastructure with advanced medical facilities and resources. Many outpatient facilities as well as hospitals in this region are equipped to perform leadless pacemaker implantations and provide necessary follow-up care.
The major strategies followed by the market participants are Mergers & Acquisitionas the key developmental strategy to keep pace with the changing demands of end users. For instance, In August, 2022, Medtronic plc completed the acquisition of Affera, Inc., expanding its cardiac ablation portfolio with the addition of a groundbreaking cardiac mapping and navigation platform, featuring a fully integrated diagnostic, focal pulsed field, and radiofrequency ablation solution. Moreover, In April, 2023, Abbott Laboratories completed the acquisition of Cardiovascular Systems, Inc. (CSI), a medical device company known for its innovative atherectomy system in the treatment of peripheral and coronary artery disease. This acquisition enhances Abbott's offerings for vascular disease treatment, incorporating CSI's leading atherectomy system, which prepares vessels for angioplasty or stenting to restore blood flow.
Based on the Analysis presented in the KBV Cardinal matrix; Medtronic PLC and Abbott Laboratories are the forerunners in the Market. Companies such as Boston Scientific Corporation, Integer Holdings Corporation and Terumo Corporation are some of the key innovators in the Market. In September, 2023, Boston Scientific Corporation announced the acquisition of Relievant Medsystems, Inc., a privately held medical device company specializing in vertebrogenic pain treatment. This acquisition enables the integration of Relievant Medsystems' clinically backed technology with Boston Scientific's strategies, expanding treatment options for individuals in need of personalized care.
COVID-19 Impact
During the pandemic, many hospitals and healthcare facilities postponed or canceled elective procedures to free up resources and reduce the risk of virus transmission. This led to a decrease in leadless pacemaker implantations, as these procedures are often considered elective and non-urgent. The global supply chain for medical devices, including leadless pacemakers, experienced disruptions due to lockdowns and restrictions on manufacturing and transportation. This led to delays in the availability of devices and potentially impacted patients' access to these advanced technologies. Hospitals and healthcare providers redirected resources and staff to handle the surge of COVID-19 cases. This diversion of attention and resources from non-COVID medical services affected the promotion and implantation of leadless pacemakers.
Market Growth Factors
Growing demand for minimally invasive procedures
The development of minimally invasive procedures has decreased the need for lead placement and surgical incisions, lowering surgical risks and enhancing patient comfort. This reduction in invasiveness has not only translated into enhanced patient safety but also quicker recovery times. Patients undergoing leadless pacemaker implantation experience shorter hospital stays and reduced post-operative discomfort. This quick recovery benefits patients by allowing them to return to their daily routines and activities more swiftly. These procedures have opened up new possibilities for remote and rural healthcare settings, where access to healthcare facilities may be limited. As a result, the rapidly increasing demand for minimally invasive procedures will aid in the expansion of the market.
Rising prevalence of cardiac arrhythmias
The rising prevalence of cardiac arrhythmias is a complex interplay of multiple factors, from demographic changes to lifestyle choices and advances in healthcare. High blood pressure is a well-established risk factor for arrhythmias. It can lead to changes in the heart's structure and electrical signaling, increasing the likelihood of arrhythmias. Similarly, diabetes is associated with an increased risk of arrhythmias, particularly atrial fibrillation. Furthermore, the prevalence of arrhythmias is not limited to specific age groups; it affects individuals across various demographics. Patients of all ages who experience cardiac arrhythmias, from the elderly to younger individuals, can benefit from these devices. Therefore, the rising prevalence of arrhythmias is boosting the demand for leadless pacemakers.
Market Restraining Factors
Regulatory and reimbursement challenges
Regulatory challenges, such as the complex and lengthy approval process for medical devices, can significantly delay the introduction of leadless pacemakers. Regulatory agencies require thorough evaluations of safety and efficacy, leading to prolonged timeframes for product clearance. Delays in regulatory approval not only hinder patients' access to advanced medical solutions but also increase the time-to-market for manufacturers, impacting their ability to offer cutting-edge devices. The economic burden of these devices can deter both healthcare providers and patients from choosing leadless pacemakers as a pacing solution despite their potential benefits. Such financial burdens may discourage some individuals from opting for these devices, especially if more cost-effective alternatives are available. All these factors may hamper the growth of the market.
Type Outlook
Based on type, the market is bifurcated into micra transcatheter pacing system and others. The other segment procured a considerable growth rate in the market in 2022. Leadless pacemakers, including devices like Nanostim and WiSE CRT System, offer several benefits compared to traditional pacemakers with leads. Nanostim is remarkably compact, similar in size to a large vitamin capsule. This small size allows for a less invasive procedure and results in a more unobtrusive and comfortable implantation. The WiSE CRT System is designed for cardiac resynchronization therapy, a treatment for heart failure. It offers the benefits of cardiac resynchronization without the need for traditional pacing leads. Both Nanostim and the WiSE CRT System provide significant advantages, such as minimally invasive implantation, compact designs, reduced infection risk, cosmetic benefits, and long-lasting battery life.
End-use Outlook
On the basis of end-use, the market is divided into hospitals and outpatient facilities. The hospitals segment acquired the largest revenue share in the market in 2022. Hospitals can efficiently allocate resources, as there is no need for specialized surgical suites or surgical teams dedicated to pacemaker implantations. This can lead to cost savings and the reallocation of resources to other critical medical procedures. With no leads that exit the body, the risk of infections related to lead entry sites is virtually eliminated. Hospitals can benefit from reduced rates of device-related infections.
Regional Outlook
Region-wise, the market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America segment witnessed the maximum revenue share in the market in 2022. The demand for leadless pacemakers is steadily increasing in North America due to several factors contributing to their growing adoption in the region. These factors highlight the evolving landscape of cardiac pacing technology and the shift towards more patient-centric, minimally invasive, and technologically advanced options for treating cardiac arrhythmias. Leadless pacemakers eliminate the need for leads that can be susceptible to infection. In regions like North America, where infection control is a priority, this feature is highly valued.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Medtronic PLC, Abbott Laboratories, Boston Scientific Corporation, Biotronik SE & Co. KG, Lepu Medical Technology Co., Ltd, Integer Holdings Corporation, MicroPort Scientific Corporation, Osypka AG, Terumo Corporation, and EBR Systems, Inc.
Strategies Deployed in Leadless Pacemakers Market
Partnerships, Collaborations, and Agreements:
Jul-2022: Medtronic plc has formed a strategic partnership with CathWorks, a privately held company headquartered in Kefar Sava, Israel, investing up to $75 million and co-promoting the FFRangio System globally. The agreement included an option for Medtronic to acquire CathWorks based on milestones, with CathWorks having the right to compel the acquisition if Medtronic opts out.
Sep-2021: Terumo Cardiovascular partnered with Etiometry to provide enhanced monitoring insights for adult cardiac surgery patients. This collaboration expanded technologies and enabled the utilization of clinical-decision support tools throughout a patient's hospital stay, offering a more comprehensive view of critical data for improved and informed bedside decisions during the transition from the operating room to the ICU.
Product Launches and Product Expansions:
Feb-2022: Abbott Laboratories has revealed the world's first patient implants of a dual-chamber leadless pacemaker system in its AVEIR DR i2i™ pivotal clinical study. The implant of Abbott's investigational Aveir dual-chamber leadless pacemaker marks a notable technological milestone in leadless pacing, representing the first such occurrence globally within the pivotal trial.
Jan-2022: Medtronic received regulatory approval and launched the Micra AV Transcatheter Pacing System in Japan. This pacemaker was designed to treat patients with AV block, addressing impaired electrical signals between the heart's chambers. The Micra TPS is the world's first leadless pacemaker, with the initial version (Micra VR) approved in Japan in 2017 for patients requiring single-chamber pacing.
Jun-2021: Medtronic PLC has introduced the MICRA AV, a pacemaker smaller than conventional ones, offering a leadless and minimally invasive solution for patients with complete heart block. This innovation, following Medtronic's legacy of inventing and disrupting, provides a cosmetically invisible option for those in need.
Acquisition and Mergers:
Sep-2023: Boston Scientific Corporation has announced the acquisition of Relievant Medsystems, Inc., a privately held medical device company specializing in vertebrogenic pain treatment. This acquisition enables the integration of Relievant Medsystems' clinically backed technology with Boston Scientific's strategies, expanding treatment options for individuals in need of personalized care.
Apr-2023: Abbott Laboratories completed the acquisition of Cardiovascular Systems, Inc. (CSI), a medical device company known for its innovative atherectomy system in the treatment of peripheral and coronary artery disease. This acquisition enhances Abbott's offerings for vascular disease treatment, incorporating CSI's leading atherectomy system, which prepares vessels for angioplasty or stenting to restore blood flow.
Aug-2022: Medtronic plc completed the acquisition of Affera, Inc., expanding its cardiac ablation portfolio with the addition of a groundbreaking cardiac mapping and navigation platform, featuring a fully integrated diagnostic, focal pulsed field, and radiofrequency ablation solution.
May-2022: Medtronic PLC completed the acquisition of Intersect ENT, integrating PROPEL and SINUVA into its portfolio. PROPEL implants, maintaining sinus patency and delivering steroids, and SINUVA implants, treating nasal polyps in ethmoid sinus surgery patients, are now part of Medtronic's offerings.
Feb-2022: Boston Scientific has completed the acquisition of Baylis Medical Company Inc. This integration enables Boston Scientific to incorporate Baylis platforms into its existing electrophysiology and structural heart offerings, reinforcing its position in the rapidly growing cardiology markets.
Sep-2021: Boston Scientific Corporation completed the acquisition of Devoro Medical, Inc., a developer of a medical device intended to restore blood flow. Through this acquisition, the WOLF Thrombectomy Platform was integrated into Boston Scientific's products. Additionally, this acquisition provided the physicians with the necessary tools needed for the treatment.
Trials and Approval:
Dec-2022: Biotronik SE & Co. KG has initiated the first patient enrollment in BIO-CONDUCT, an FDA-approved investigational device exemption (IDE) trial. The study examines the use of the BIOTRONIK Solia S pacing lead when implanted in the left bundle branch (LBB) area, a location not currently approved for the Solia S lead.
Dec2021: Boston Scientific Corporation has started the MODULAR ATP clinical trial to assess the mCRM Modular Therapy System, which includes the EMBLEM™ MRI S-ICD System and the EMPOWER™ MPS, aiming to be the first leadless pacemaker capable of delivering bradycardia pacing support and antitachycardia pacing (ATP).
Scope of the Study
Market Segments covered in the Report:
By Type
- Micra Transcatheter Pacing System
- Others
By End-use
- Hospitals
- Outpatient Facilities
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Companies Profiled
- Medtronic PLC
- Abbott Laboratories
- Boston Scientific Corporation
- Biotronik SE & Co. KG
- Lepu Medical Technology Co., Ltd
- Integer Holdings Corporation
- MicroPort Scientific Corporation
- Osypka AG
- Terumo Corporation
- EBR Systems, Inc.
Unique Offerings from KBV Research
- Exhaustive coverage
- Highest number of market tables and figures
- Subscription based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Chapter 1. Market Scope & Methodology
- 1.1 Market Definition
- 1.2 Objectives
- 1.3 Market Scope
- 1.4 Segmentation
- 1.4.1 Global Leadless Pacemakers Market, by Type
- 1.4.2 Global Leadless Pacemakers Market, by End-use
- 1.4.3 Global Leadless Pacemakers Market, by Geography
- 1.5 Methodology for the research
Chapter 2. Market at a Glance
- 2.1 Key Highlights
Chapter 3. Market Overview
- 3.1 Introduction
- 3.1.1 Overview
- 3.1.1.1 Market Composition and Scenario
- 3.1.1 Overview
- 3.2 Key Factors Impacting the Market
- 3.2.1 Market Drivers
- 3.2.2 Market Restraints
Chapter 4. Competition Analysis - Global
- 4.1 KBV Cardinal Matrix
- 4.2 Recent Industry Wide Strategic Developments
- 4.2.1 Partnerships, Collaborations and Agreements
- 4.2.2 Product Launches and Product Expansions
- 4.2.3 Acquisition and Mergers
- 4.2.4 Trials & Approvals
- 4.3 Top Winning Strategies
- 4.3.1 Key Leading Strategies: Percentage Distribution (2019-2023)
- 4.3.2 Key Strategic Move: (Mergers & Acquisition: 2019, Nov - 2023, Sep) Leading Players
- 4.4 Porter's Five Forces Analysis
Chapter 5. Global Leadless Pacemakers Market, By Type
- 5.1 Global Micra Transcatheter Pacing System Market, By Region
- 5.2 Global Others Market, By Region
Chapter 6. Global Leadless Pacemakers Market, By End-use
- 6.1 Global Hospitals Market, By Region
- 6.2 Global Outpatient Facilities Market, By Region
Chapter 7. Global Leadless Pacemakers Market, By Region
- 7.1 North America Leadless Pacemakers Market
- 7.1.1 North America Leadless Pacemakers Market, By Type
- 7.1.1.1 North America Micra Transcatheter Pacing System Market, By Country
- 7.1.1.2 North America Others Market, By Country
- 7.1.2 North America Leadless Pacemakers Market, By End-use
- 7.1.2.1 North America Hospitals Market, By Country
- 7.1.2.2 North America Outpatient Facilities Market, By Country
- 7.1.3 North America Leadless Pacemakers Market, By Country
- 7.1.3.1 US Leadless Pacemakers Market
- 7.1.3.1.1 US Leadless Pacemakers Market, By Type
- 7.1.3.1.2 US Leadless Pacemakers Market, By End-use
- 7.1.3.2 Canada Leadless Pacemakers Market
- 7.1.3.2.1 Canada Leadless Pacemakers Market, By Type
- 7.1.3.2.2 Canada Leadless Pacemakers Market, By End-use
- 7.1.3.3 Mexico Leadless Pacemakers Market
- 7.1.3.3.1 Mexico Leadless Pacemakers Market, By Type
- 7.1.3.3.2 Mexico Leadless Pacemakers Market, By End-use
- 7.1.3.4 Rest of North America Leadless Pacemakers Market
- 7.1.3.4.1 Rest of North America Leadless Pacemakers Market, By Type
- 7.1.3.4.2 Rest of North America Leadless Pacemakers Market, By End-use
- 7.1.3.1 US Leadless Pacemakers Market
- 7.1.1 North America Leadless Pacemakers Market, By Type
- 7.2 Europe Leadless Pacemakers Market
- 7.2.1 Europe Leadless Pacemakers Market, By Type
- 7.2.1.1 Europe Micra Transcatheter Pacing System Market, By Country
- 7.2.1.2 Europe Others Market, By Country
- 7.2.2 Europe Leadless Pacemakers Market, By End-use
- 7.2.2.1 Europe Hospitals Market, By Country
- 7.2.2.2 Europe Outpatient Facilities Market, By Country
- 7.2.3 Europe Leadless Pacemakers Market, By Country
- 7.2.3.1 Germany Leadless Pacemakers Market
- 7.2.3.1.1 Germany Leadless Pacemakers Market, By Type
- 7.2.3.1.2 Germany Leadless Pacemakers Market, By End-use
- 7.2.3.2 UK Leadless Pacemakers Market
- 7.2.3.2.1 UK Leadless Pacemakers Market, By Type
- 7.2.3.2.2 UK Leadless Pacemakers Market, By End-use
- 7.2.3.3 France Leadless Pacemakers Market
- 7.2.3.3.1 France Leadless Pacemakers Market, By Type
- 7.2.3.3.2 France Leadless Pacemakers Market, By End-use
- 7.2.3.4 Russia Leadless Pacemakers Market
- 7.2.3.4.1 Russia Leadless Pacemakers Market, By Type
- 7.2.3.4.2 Russia Leadless Pacemakers Market, By End-use
- 7.2.3.5 Spain Leadless Pacemakers Market
- 7.2.3.5.1 Spain Leadless Pacemakers Market, By Type
- 7.2.3.5.2 Spain Leadless Pacemakers Market, By End-use
- 7.2.3.6 Italy Leadless Pacemakers Market
- 7.2.3.6.1 Italy Leadless Pacemakers Market, By Type
- 7.2.3.6.2 Italy Leadless Pacemakers Market, By End-use
- 7.2.3.7 Rest of Europe Leadless Pacemakers Market
- 7.2.3.7.1 Rest of Europe Leadless Pacemakers Market, By Type
- 7.2.3.7.2 Rest of Europe Leadless Pacemakers Market, By End-use
- 7.2.3.1 Germany Leadless Pacemakers Market
- 7.2.1 Europe Leadless Pacemakers Market, By Type
- 7.3 Asia Pacific Leadless Pacemakers Market
- 7.3.1 Asia Pacific Leadless Pacemakers Market, By Type
- 7.3.1.1 Asia Pacific Micra Transcatheter Pacing System Market, By Country
- 7.3.1.2 Asia Pacific Others Market, By Country
- 7.3.2 Asia Pacific Leadless Pacemakers Market, By End-use
- 7.3.2.1 Asia Pacific Hospitals Market, By Country
- 7.3.2.2 Asia Pacific Outpatient Facilities Market, By Country
- 7.3.3 Asia Pacific Leadless Pacemakers Market, By Country
- 7.3.3.1 China Leadless Pacemakers Market
- 7.3.3.1.1 China Leadless Pacemakers Market, By Type
- 7.3.3.1.2 China Leadless Pacemakers Market, By End-use
- 7.3.3.2 Japan Leadless Pacemakers Market
- 7.3.3.2.1 Japan Leadless Pacemakers Market, By Type
- 7.3.3.2.2 Japan Leadless Pacemakers Market, By End-use
- 7.3.3.3 India Leadless Pacemakers Market
- 7.3.3.3.1 India Leadless Pacemakers Market, By Type
- 7.3.3.3.2 India Leadless Pacemakers Market, By End-use
- 7.3.3.4 South Korea Leadless Pacemakers Market
- 7.3.3.4.1 South Korea Leadless Pacemakers Market, By Type
- 7.3.3.4.2 South Korea Leadless Pacemakers Market, By End-use
- 7.3.3.5 Singapore Leadless Pacemakers Market
- 7.3.3.5.1 Singapore Leadless Pacemakers Market, By Type
- 7.3.3.5.2 Singapore Leadless Pacemakers Market, By End-use
- 7.3.3.6 Malaysia Leadless Pacemakers Market
- 7.3.3.6.1 Malaysia Leadless Pacemakers Market, By Type
- 7.3.3.6.2 Malaysia Leadless Pacemakers Market, By End-use
- 7.3.3.7 Rest of Asia Pacific Leadless Pacemakers Market
- 7.3.3.7.1 Rest of Asia Pacific Leadless Pacemakers Market, By Type
- 7.3.3.7.2 Rest of Asia Pacific Leadless Pacemakers Market, By End-use
- 7.3.3.1 China Leadless Pacemakers Market
- 7.3.1 Asia Pacific Leadless Pacemakers Market, By Type
- 7.4 LAMEA Leadless Pacemakers Market
- 7.4.1 LAMEA Leadless Pacemakers Market, By Type
- 7.4.1.1 LAMEA Micra Transcatheter Pacing System Market, By Country
- 7.4.1.2 LAMEA Others Market, By Country
- 7.4.2 LAMEA Leadless Pacemakers Market, By End-use
- 7.4.2.1 LAMEA Hospitals Market, By Country
- 7.4.2.2 LAMEA Outpatient Facilities Market, By Country
- 7.4.3 LAMEA Leadless Pacemakers Market, By Country
- 7.4.3.1 Brazil Leadless Pacemakers Market
- 7.4.3.1.1 Brazil Leadless Pacemakers Market, By Type
- 7.4.3.1.2 Brazil Leadless Pacemakers Market, By End-use
- 7.4.3.2 Argentina Leadless Pacemakers Market
- 7.4.3.2.1 Argentina Leadless Pacemakers Market, By Type
- 7.4.3.2.2 Argentina Leadless Pacemakers Market, By End-use
- 7.4.3.3 UAE Leadless Pacemakers Market
- 7.4.3.3.1 UAE Leadless Pacemakers Market, By Type
- 7.4.3.3.2 UAE Leadless Pacemakers Market, By End-use
- 7.4.3.4 Saudi Arabia Leadless Pacemakers Market
- 7.4.3.4.1 Saudi Arabia Leadless Pacemakers Market, By Type
- 7.4.3.4.2 Saudi Arabia Leadless Pacemakers Market, By End-use
- 7.4.3.5 South Africa Leadless Pacemakers Market
- 7.4.3.5.1 South Africa Leadless Pacemakers Market, By Type
- 7.4.3.5.2 South Africa Leadless Pacemakers Market, By End-use
- 7.4.3.6 Nigeria Leadless Pacemakers Market
- 7.4.3.6.1 Nigeria Leadless Pacemakers Market, By Type
- 7.4.3.6.2 Nigeria Leadless Pacemakers Market, By End-use
- 7.4.3.7 Rest of LAMEA Leadless Pacemakers Market
- 7.4.3.7.1 Rest of LAMEA Leadless Pacemakers Market, By Type
- 7.4.3.7.2 Rest of LAMEA Leadless Pacemakers Market, By End-use
- 7.4.3.1 Brazil Leadless Pacemakers Market
- 7.4.1 LAMEA Leadless Pacemakers Market, By Type
Chapter 8. Company Profiles
- 8.1 Medtronic PLC
- 8.1.1 Company overview
- 8.1.2 Financial Analysis
- 8.1.3 Segmental and Regional Analysis
- 8.1.4 Research & Development Expenses
- 8.1.5 Recent strategies and developments:
- 8.1.5.1 Partnerships, Collaborations, and Agreements:
- 8.1.5.2 Product Launches and Product Expansions:
- 8.1.5.3 Acquisition and Mergers:
- 8.1.5.4 Geographical Expansions:
- 8.1.6 SWOT Analysis
- 8.2 Abbott Laboratories
- 8.2.1 Company Overview
- 8.2.2 Financial Analysis
- 8.2.3 Segmental and Regional Analysis
- 8.2.4 Research & Development Expense
- 8.2.5 Recent strategies and developments:
- 8.2.5.1 Product Launches and Product Expansions:
- 8.2.5.2 Acquisition and Mergers:
- 8.2.6 SWOT Analysis
- 8.3 Boston Scientific Corporation
- 8.3.1 Company Overview
- 8.3.2 Financial Analysis
- 8.3.3 Segmental and Regional Analysis
- 8.3.4 Research & Development Expense
- 8.3.5 Recent strategies and developments:
- 8.3.5.1 Partnerships, Collaborations, and Agreements:
- 8.3.5.2 Product Launches and Product Expansions:
- 8.3.5.3 Acquisition and Mergers:
- 8.3.5.4 Trials & Approval:
- 8.3.6 SWOT Analysis
- 8.4 Biotronik SE & Co. KG
- 8.4.1 Company Overview
- 8.4.2 Recent strategies and developments:
- 8.4.2.1 Trials & Approval:
- 8.4.3 SWOT Analysis
- 8.5 Lepu Medical Technology Co., Ltd
- 8.5.1 Company Overview
- 8.5.2 SWOT Analysis
- 8.6 Integer Holdings Corporation
- 8.6.1 Company Overview
- 8.6.2 Financial Analysis
- 8.6.3 Segmental and Regional Analysis
- 8.6.4 Research & Development Expenses
- 8.6.5 SWOT Analysis
- 8.7 MicroPort Scientific Corporation
- 8.7.1 Company Overview
- 8.7.2 Financial Analysis
- 8.7.3 Segmental and Regional Analysis
- 8.7.4 Research & Development Expense
- 8.7.5 SWOT Analysis
- 8.8 OSYPKA AG
- 8.8.1 Company Overview
- 8.8.2 SWOT Analysis
- 8.9 Terumo Corporation
- 8.9.1 Company Overview
- 8.9.2 Financial Analysis
- 8.9.3 Segmental and Regional Analysis
- 8.9.4 Research & Development Expenses
- 8.9.5 Recent strategies and developments:
- 8.9.5.1 Partnerships, Collaborations, and Agreements:
- 8.9.5.2 Acquisition and Mergers:
- 8.9.6 SWOT Analysis
- 8.10. EBR Systems, Inc.
- 8.10.1 Company Overview
- 8.10.2 SWOT Analysis