|
|
市場調査レポート
商品コード
1391875
血液学検査市場:パイプライン(開発段階、セグメント、地域、規制経路、主要企業)Hematology Tests Pipeline by Stages of Development, Segments, Region, Regulatory Path and Key Companies |
||||||
カスタマイズ可能
適宜更新あり
|
|||||||
| 血液学検査市場:パイプライン(開発段階、セグメント、地域、規制経路、主要企業) |
|
出版日: 2023年11月21日
発行: GlobalData
ページ情報: 英文 91 Pages
納期: 即納可能
|
- 全表示
- 概要
- 図表
- 目次
血液学検査には、様々な状態の診断やモニタリングに役立つ様々な血液検査が含まれます。完全血球計算(CBC)検査、ヘモグロビン検査室検査、ヘモグロビンPOC(ポイントオブケア)検査、ヘマトクリットPOC(ポイントオブケア)検査、ヘモグロビン&ヘマトクリットPOC検査、赤血球POC(ポイントオブケア)検査、白血球POC(ポイントオブケア)検査、血小板POC(ポイントオブケア)検査のサブセグメントなどです。
当レポートでは、血液学検査市場について調査し、製品概要と開発中のパイプライン製品動向、様々な開発段階にある製品の比較分析や進行中の臨床試験に関する情報、参入企業の最近の動向などを提供しています。
目次
第1章 目次
第2章 イントロダクション
- 血液学検査の概要
第3章 開発中の製品
- 血液学検査 - 開発段階別パイプライン製品
- 血液学検査 - セグメント別パイプライン製品
- 血液学検査 - 地域別パイプライン製品
- 血液学検査 - 規制経路別パイプライン製品
- 血液学検査 - 推定承認日別パイプライン製品
第4章 血液学検査- 企業が開発中のパイプライン製品
- 血液学検査企業- 開発段階別のパイプライン製品
- 血液学検査- 開発段階別のパイプライン製品
第5章 血液検査企業と製品概要
- Aptitude Medical Systems Inc
- Biochip Labs Inc
- BioMedomics Inc
- Biosurfit, SA
- DxDiscovery Inc
- Dynasil Corporation of America
- Epimune GmbH
- Flobio LLC
- Gendrive PLC
- Group K Diagnostics
- hemCheck Sweden AB
- Hemex Health Inc
- McMaster University
- MiCo BioMed Co Ltd
- Ohio State University
- Optolane Technologies Inc
- PortaScience Inc
- Retham Technologies
- rHealth
- Sanguina LLC
- ShanMukha Innovations Pvt Ltd
- Siemens Healthcare Diagnostics Inc
- Silver Lake Research Corporation
- T2 Biosystems Inc
- University of Florida
- University of Melbourne
- VitaMe Technologies Inc
第6章 血液学検査- 最近の開発
第7章 付録
List of Tables
List of Tables
- Hematology Tests - Pipeline Products by Stage of Development
- Hematology Tests - Pipeline Products by Segment
- Hematology Tests - Pipeline Products by Territory
- Hematology Tests - Pipeline Products by Regulatory Path
- Hematology Tests - Pipeline Products by Estimated Approval Date
- Hematology Tests Companies - Pipeline Products by Stage of Development
- Hematology Tests - Pipeline Products by Stage of Development
- Aptitude Medical Systems Inc Pipeline Products & Ongoing Clinical Trials Overview
- POC Test - Trauma Coagulopathy - Product Status
- POC Test - Trauma Coagulopathy - Product Description
- Biochip Labs Inc Pipeline Products & Ongoing Clinical Trials Overview
- Endothelium-on-a-Chip Assay - Product Status
- Endothelium-on-a-Chip Assay - Product Description
- BioMedomics Inc Pipeline Products & Ongoing Clinical Trials Overview
- Fetomaternal Hemorrhage Test - Product Status
- Fetomaternal Hemorrhage Test - Product Description
- Hemo SCAN - Product Status
- Hemo SCAN - Product Description
- Hemo SCAN S - Product Status
- Hemo SCAN S - Product Description
- Rapid Quantitative Heparin-Induced Thrombocytopenia (H.I.T.) Test - Product Status
- Rapid Quantitative Heparin-Induced Thrombocytopenia (H.I.T.) Test - Product Description
- Biosurfit, SA Pipeline Products & Ongoing Clinical Trials Overview
- Spinit Device - Blood Quantitation Anemia Assay - Product Status
- Spinit Device - Blood Quantitation Anemia Assay - Product Description
- Spinit Device - Thrombocytopenia Assay - Product Status
- Spinit Device - Thrombocytopenia Assay - Product Description
- DxDiscovery Inc Pipeline Products & Ongoing Clinical Trials Overview
- Diagnostic Assay - Sickle Cell Disease - Product Status
- Diagnostic Assay - Sickle Cell Disease - Product Description
- Dynasil Corporation of America Pipeline Products & Ongoing Clinical Trials Overview
- Diagnostic Test - Hemophilia - Product Status
- Diagnostic Test - Hemophilia - Product Description
- Epimune GmbH Pipeline Products & Ongoing Clinical Trials Overview
- i.Mune Tbnk Test - Product Status
- i.Mune Tbnk Test - Product Description
- Flobio LLC Pipeline Products & Ongoing Clinical Trials Overview
- DOAC Diagnostic Assay - Product Status
- DOAC Diagnostic Assay - Product Description
- genedrive plc Pipeline Products & Ongoing Clinical Trials Overview
- Genedrive - Factor V Leiden - Product Status
- Genedrive - Factor V Leiden - Product Description
- Group K Diagnostics Pipeline Products & Ongoing Clinical Trials Overview
- KromaHealth Kit - Complete Blood Count - Product Status
- KromaHealth Kit - Complete Blood Count - Product Description
- hemCheck Sweden AB Pipeline Products & Ongoing Clinical Trials Overview
- v-Test - Serum Tubes - Product Status
- v-Test - Serum Tubes - Product Description
- Hemex Health Inc Pipeline Products & Ongoing Clinical Trials Overview
- Gazelle Hb Variant Test - Product Status
- Gazelle Hb Variant Test - Product Description
- McMaster University Pipeline Products & Ongoing Clinical Trials Overview
- aFXa DOAC Assay - Product Status
- aFXa DOAC Assay - Product Description
- MiCo BioMed Co Ltd Pipeline Products & Ongoing Clinical Trials Overview
- Labchip Based Test - Hemoglobin - Product Status
- Labchip Based Test - Hemoglobin - Product Description
- Ohio State University Pipeline Products & Ongoing Clinical Trials Overview
- Diagnostic Assay - Thrombotic Thrombocytopenic Purpura - Product Status
- Diagnostic Assay - Thrombotic Thrombocytopenic Purpura - Product Description
- Optolane Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview
- Molecular Dx - Thrombosis Panel - Product Status
- Molecular Dx - Thrombosis Panel - Product Description
- PortaScience Inc Pipeline Products & Ongoing Clinical Trials Overview
- PortaWBC - Product Status
- PortaWBC - Product Description
- Retham Technologies LLC Pipeline Products & Ongoing Clinical Trials Overview
- HITDx - Product Status
- HITDx - Product Description
- rHealth Pipeline Products & Ongoing Clinical Trials Overview
- Diagnostic Test - Thrombocytopenia - Product Status
- Diagnostic Test - Thrombocytopenia - Product Description
- Sanguina LLC Pipeline Products & Ongoing Clinical Trials Overview
- AnemoCheck Home - Product Status
- AnemoCheck Home - Product Description
- ShanMukha Innovations Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview
- SickleFind - Product Status
- SickleFind - Product Description
- Siemens Healthcare Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview
- ADVIA 120 Hematology System - IRF Test - Product Status
- ADVIA 120 Hematology System - IRF Test - Product Description
- ADVIA 120 Hematology System - MPC Test - Product Status
- ADVIA 120 Hematology System - MPC Test - Product Description
- ADVIA 120 Hematology System - MPM Test - Product Status
- ADVIA 120 Hematology System - MPM Test - Product Description
- ADVIA 2120 Hematology System - MPC Test - Product Status
- ADVIA 2120 Hematology System - MPC Test - Product Description
- ADVIA 2120 Hematology System - MPM Test - Product Status
- ADVIA 2120 Hematology System - MPM Test - Product Description
- ADVIA 2120i Hematology System - MPC Test - Product Status
- ADVIA 2120i Hematology System - MPC Test - Product Description
- ADVIA 2120i Hematology System - MPM Test - Product Status
- ADVIA 2120i Hematology System - MPM Test - Product Description
- Silver Lake Research Corporation Pipeline Products & Ongoing Clinical Trials Overview
- HemoTypeBT - Product Status
- HemoTypeBT - Product Description
- T2 Biosystems Inc Pipeline Products & Ongoing Clinical Trials Overview
- T2HemoStat - Product Status
- T2HemoStat - Product Description
- T2MR Assay - Platelet Function Testing - Product Status
- T2MR Assay - Platelet Function Testing - Product Description
- University of Florida Pipeline Products & Ongoing Clinical Trials Overview
- Hemoglobin Measurement Device - Product Status
- Hemoglobin Measurement Device - Product Description
- University of Melbourne Pipeline Products & Ongoing Clinical Trials Overview
- Thrombin Generation Assay - Product Status
- Thrombin Generation Assay - Product Description
- VitaMe Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview
- Iron.Scan - Product Status
- Iron.Scan - Product Description
- Glossary
List of Figures
List of Figures
- Hematology Tests - Pipeline Products by Stage of Development
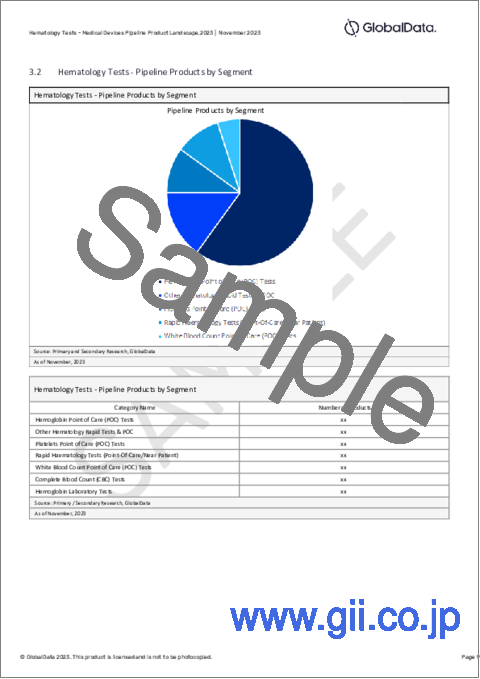
- Hematology Tests - Pipeline Products by Segment
- Hematology Tests - Pipeline Products by Territory
- Hematology Tests - Pipeline Products by Regulatory Path
- Hematology Tests - Pipel
Abstract
Hematology tests include various blood tests that help diagnose and monitor various conditions. It includes the sub-segments Complete Blood Count (CBC) Tests, Hemoglobin Laboratory Tests, Hemoglobin Point of Care (POC) Tests, Hematocrit Point of Care (POC) Tests, Hemoglobin & Hematocrit POC Tests, Erythrocytes Point of Care (POC) Tests, White Blood Count Point of Care (POC) Tests and Platelets Point of Care (POC) Tests. GlobalData's Medical Devices sector report, "Hematology Tests Pipeline Report including Stages of Development, Segments, Region and Countries, Regulatory Path and Key Companies, 2023 Update" provides comprehensive information about the Hematology Tests pipeline products with comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
The Hematology Tests report provides key information and data related to:
Extensive coverage of the Hematology Tests under development
Review details of major pipeline products which include product description, licensing and collaboration details and other developmental activities including pipeline territories, regulatory paths, and estimated approval dates
Reviews of major players involved in the pipeline product development.
Provides key clinical trial data related to ongoing clinical trials such as trial phase, trial status, trial start and end dates, and the number of trials of the major Hematology Tests pipeline products.
Review of Recent Developments in the segment / industry
The Hematology Tests report enables you to:
Access significant competitor information, analysis, and insights to improve your R&D strategies
Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage
Identify and understand important and diverse types of Hematology Tests under development
Formulate market-entry and market expansion strategies
Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
Note: Certain sections in the report may be removed or altered based on the availability and relevance of data in relation to the equipment type.
The GlobalData Differentiation
This report is prepared using data sourced from in-house databases, secondary and primary research by GlobalData's team of industry experts.
The data and analysis within this report are driven by GlobalData Medical Intelligence Center (GDMIC) database. GDMIC gives you the key information required to drive sales, investment and deal-making activity in your business. It includes the following:
- 15,000+ data tables showing market size across more than 780 medical equipment segments and 39 countries, from 2015 and forecast to 2025
- 6,700+ industry-leading analysis reports covering sector reports, medipoint reports, country analysis, expert insights and industry analysis (devices and procedures) reports
- 64,000+ medical equipment company profiles
- 5,600+ company profiles of medical equipment manufacturers in China and India
- 2,200+ company profiles of medical equipment manufacturers in Japan
- 1,200+ companies' revenue splits and market shares
- 1,600+ quarterly and annual medical equipment company financials
- 850+ medical equipment company SWOTs
- 28,000+ pipeline product profiles
- 56,400+ marketed product profiles
- 47,000+ clinical trials
- 41,500+ trial investigators
- 7,000+ reports on companies with products in development
- 44,000+ deals in the medical equipment industry
- 1,100+ surgical and diagnostic procedures by therapy area
- 50+ key healthcare indicators by country
- 431,000+ Themes Content Items
- 600+ Influencers
- 1,900+ Analysts & Researchers
- 0.5m+ Community Members
- 141,000+ Macroeconomic Indicators
- 1,013,000+ City Indicators
For more information or to receive a free demonstration of the service, please visit:
Custom Requirements
Contact us to discuss the areas of your business where you need external input, and we will work with you to identify the strongest way forward to meet your needs.
Scope
- Extensive coverage of the Hematology Tests under development
- The report reviews details of major pipeline products which includes, product description, licensing and collaboration details and other developmental activities
- The report reviews the major players involved in the development of Hematology Tests and list all their pipeline projects
- The coverage of pipeline products based on various stages of development ranging from Early Development to Approved / Issued stage
- The report provides key clinical trial data of ongoing trials specific to pipeline products
- Recent developments in the segment / industry
Reasons to Buy
The report enables you to -
- Formulate significant competitor information, analysis, and insights to improve R&D strategies
- Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage
- Identify and understand important and diverse types of Hematology Tests under development
- Develop market-entry and market expansion strategies
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the product's current stage of development, territory and estimated launch date
Table of Contents
1 Table of Contents
- 1.1 List of Tables
- 1.2 List of Figures
2 Introduction
- 2.1 Hematology Tests Overview
3 Products under Development
- 3.1 Hematology Tests - Pipeline Products by Stage of Development
- 3.2 Hematology Tests - Pipeline Products by Segment
- 3.3 Hematology Tests - Pipeline Products by Territory
- 3.4 Hematology Tests - Pipeline Products by Regulatory Path
- 3.5 Hematology Tests - Pipeline Products by Estimated Approval Date
4 Hematology Tests - Pipeline Products under Development by Companies
- 4.1 Hematology Tests Companies - Pipeline Products by Stage of Development
- 4.2 Hematology Tests - Pipeline Products by Stage of Development
5 Hematology Tests Companies and Product Overview
- 5.1 Aptitude Medical Systems Inc Company Overview
- 5.1.1 Aptitude Medical Systems Inc Pipeline Products & Ongoing Clinical Trials Overview
- 5.2 Biochip Labs Inc Company Overview
- 5.2.1 Biochip Labs Inc Pipeline Products & Ongoing Clinical Trials Overview
- 5.3 BioMedomics Inc Company Overview
- 5.3.1 BioMedomics Inc Pipeline Products & Ongoing Clinical Trials Overview
- 5.4 Biosurfit, SA Company Overview
- 5.4.1 Biosurfit, SA Pipeline Products & Ongoing Clinical Trials Overview
- 5.5 DxDiscovery Inc Company Overview
- 5.5.1 DxDiscovery Inc Pipeline Products & Ongoing Clinical Trials Overview
- 5.6 Dynasil Corporation of America Company Overview
- 5.6.1 Dynasil Corporation of America Pipeline Products & Ongoing Clinical Trials Overview
- 5.7 Epimune GmbH Company Overview
- 5.7.1 Epimune GmbH Pipeline Products & Ongoing Clinical Trials Overview
- 5.8 Flobio LLC Company Overview
- 5.8.1 Flobio LLC Pipeline Products & Ongoing Clinical Trials Overview
- 5.9 genedrive plc Company Overview
- 5.9.1 genedrive plc Pipeline Products & Ongoing Clinical Trials Overview
- 5.10 Group K Diagnostics Company Overview
- 5.10.1 Group K Diagnostics Pipeline Products & Ongoing Clinical Trials Overview
- 5.11 hemCheck Sweden AB Company Overview
- 5.11.1 hemCheck Sweden AB Pipeline Products & Ongoing Clinical Trials Overview
- 5.12 Hemex Health Inc Company Overview
- 5.12.1 Hemex Health Inc Pipeline Products & Ongoing Clinical Trials Overview
- 5.13 McMaster University Company Overview
- 5.13.1 McMaster University Pipeline Products & Ongoing Clinical Trials Overview
- 5.14 MiCo BioMed Co Ltd Company Overview
- 5.14.1 MiCo BioMed Co Ltd Pipeline Products & Ongoing Clinical Trials Overview
- 5.15 Ohio State University Company Overview
- 5.15.1 Ohio State University Pipeline Products & Ongoing Clinical Trials Overview
- 5.16 Optolane Technologies Inc Company Overview
- 5.16.1 Optolane Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview
- 5.17 PortaScience Inc Company Overview
- 5.17.1 PortaScience Inc Pipeline Products & Ongoing Clinical Trials Overview
- 5.18 Retham Technologies LLC Company Overview
- 5.18.1 Retham Technologies LLC Pipeline Products & Ongoing Clinical Trials Overview
- 5.19 rHealth Company Overview
- 5.19.1 rHealth Pipeline Products & Ongoing Clinical Trials Overview
- 5.20 Sanguina LLC Company Overview
- 5.20.1 Sanguina LLC Pipeline Products & Ongoing Clinical Trials Overview
- 5.21 ShanMukha Innovations Pvt Ltd Company Overview
- 5.21.1 ShanMukha Innovations Pvt Ltd Pipeline Products & Ongoing Clinical Trials Overview
- 5.22 Siemens Healthcare Diagnostics Inc Company Overview
- 5.22.1 Siemens Healthcare Diagnostics Inc Pipeline Products & Ongoing Clinical Trials Overview
- 5.23 Silver Lake Research Corporation Company Overview
- 5.23.1 Silver Lake Research Corporation Pipeline Products & Ongoing Clinical Trials Overview
- 5.24 T2 Biosystems Inc Company Overview
- 5.24.1 T2 Biosystems Inc Pipeline Products & Ongoing Clinical Trials Overview
- 5.25 University of Florida Company Overview
- 5.25.1 University of Florida Pipeline Products & Ongoing Clinical Trials Overview
- 5.26 University of Melbourne Company Overview
- 5.26.1 University of Melbourne Pipeline Products & Ongoing Clinical Trials Overview
- 5.27 VitaMe Technologies Inc Company Overview
- 5.27.1 VitaMe Technologies Inc Pipeline Products & Ongoing Clinical Trials Overview
6 Hematology Tests- Recent Developments
- 6.1 Sep 11, 2023: Capitainer-backed initiative in personalized medicine and diagnostics secures a SEK 12 million boost from KK-stiftelsen
- 6.2 Aug 28, 2023: Bio-Rad to Participate in Fireside Chat During Wells Fargo's 2023 Healthcare Conference
- 6.3 Aug 09, 2023: Biohit Group Announces Half Year Financial Report 2023
- 6.4 Aug 09, 2023: Halo Collective Releases Q4 2022, Q1 2023 Financial Results
- 6.5 Jun 14, 2023: Biohit Announces Decisions of the Annual General Meeting
- 6.6 Jun 14, 2023: Biohit Decisions of the Annual General Meeting
- 6.7 May 03, 2023: Halo Collective Announces Change to Board of Directors
- 6.8 Apr 04, 2023: Capitainer Launches Fully Automated Sample Handler
- 6.9 Mar 28, 2023: EKF Diagnostics Holdings Announces its final results for the year ended 31 December 2022
- 6.10 Mar 22, 2023: Biohit Announces Annual Report 2022
- 6.11 Feb 16, 2023: Terumo Blood and Cell Technologies' IMUGARD platelet pooling set cleared by FDA
- 6.12 Feb 15, 2023: Biohit Announces Group Financial Statements Release
- 6.13 Jan 27, 2023: Halo Collective Reports Q4 Revenue Increase For its Budega(TM) Retail Stores in California
- 6.14 Jan 27, 2023: New test could detect Alzheimer's disease 3.5 years before clinical diagnosis
- 6.15 Jan 10, 2023: Molecular You launches a new preventive solution shown to improve health outcomes over 100 days
- 6.16 Jan 06, 2023: Bio-Rad to Report Fourth Quarter and Full Year 2022 Financial Results on Thursday, February 16, 2023
- 6.17 Dec 15, 2022: Biohit Announces Change of R&D Director
- 6.18 Dec 13, 2022: Capitainer launches a new product for a larger blood volume in self-sampling
- 6.19 Nov 30, 2022: Biohit Oyj's Financial Reporting and Annual General Meeting in 2023
7 Appendix
- 7.1 Methodology
- 7.2 About GlobalData
- 7.3 Contact Us
- 7.4 Disclaimer