|
|
市場調査レポート
商品コード
1605439
筋強直性ジストロフィー - 市場考察、疫学、市場予測(2034年)Myotonic Dystrophy Market Insight, Epidemiology And Market Forecast - 2034 |
||||||
カスタマイズ可能
適宜更新あり
|
|||||||
| 筋強直性ジストロフィー - 市場考察、疫学、市場予測(2034年) |
|
出版日: 2024年10月01日
発行: DelveInsight
ページ情報: 英文 210 Pages
納期: 2~10営業日
|
全表示
- 概要
- 図表
- 目次
主なハイライト
- 筋強直性ジストロフィー市場は、予測期間(2024年~2034年)を通して安定した成長が見込まれます。主要7市場の筋強直性ジストロフィーの市場規模は、診断の向上と新治療法の上市により、拡大が見込まれます。
- 主要7市場の診断された筋強直性ジストロフィーの有病者のうち、約51%が米国の患者です。2023年、米国で5万4,068人の筋強直性ジストロフィー有病者が診断されると予測しています。
- 筋強直性ジストロフィー治療市場の総市場規模は、Tideglusib(AMO-02)という新しく効果的な治療薬の登場により、予測期間に成長が見込まれます。
- 筋強直性ジストロフィーを標的とする治療薬の市場規模は、2020年~2034年にCAGRで18.4%の力強い成長を示すと予測されます。このダイナミックな拡大は、先進の治療オプションに対する需要の増加を反映したものであり、この深刻な疾患への対応に向けた投資が拡大していることを裏付けています。
- 2024年6月、Avidity Therapeuticsは、筋強直性ジストロフィー1型を対象とした世界のフェーズ3 HARBOR試験において、del-desiranの投与を開始しました。この重要な臨床試験は、この遺伝性疾患の患者を対象に、del-desiranの有効性と安全性を評価することを目的としています。HARBOR試験は、筋力低下と筋強直を特徴とする筋強直性ジストロフィー1型の潜在的治療オプションを前進させる重要なステップとなります。世界中の複数の施設で多様な患者を対象とすることで、Avidityは、将来の規制当局による承認への道を開き、この疾患に罹患している人々の生活の質を向上させる可能性のある包括的なデータを収集したいと考えています。
筋強直性ジストロフィーの市場は、遺伝性疾患の有病率の増加や希少疾患に対する意識の高まりなどの複数の重大な要因によって形成されています。ゲノム研究の進行と希少疾患治療への投資の拡大が市場成長の促進要因となっています。とはいえ、研究資金の制限、新治療法の高いコスト、厳しい規制といった課題は依然として大きな障害となっています。さらに、効果的な筋強直性ジストロフィー治療に対する大きなアンメットニーズは、現在の治療情勢における大きなギャップを浮き彫りにしており、企業にとって、多様な患者人口のニーズに対応し、イノベーションを起こす絶好の機会となっています。
筋強直性ジストロフィー市場の見通し
現在のところ、筋強直性ジストロフィーを治癒または進行を遅らせる治療法は承認されていません。しかし、対症療法は可能です。対症療法は苦痛を軽減し、QoLを改善するのに役立ちます。筋強直性ジストロフィーの症状の中で筋強直症はもっとも一般的な症状であり、mexiletine、lamotrigine、carbamazepine、oxcarbazepine、flecainide、propaphenone、phenytoin、ranolazineなどの抗筋強直薬が適応外治療として処方されています。さらに、慢性筋肉痛に対する薬理学的アプローチは、WHOの4段はしごに従っており、第1段階ではNSAIDsを使用し、必要に応じて抗けいれん薬(pregabalin、gabapentin)、抗うつ薬(duloxetine、amitriptyline、nortriptyline)、筋弛緩薬(baclofen、tizanidine)、外用薬(lidocaineまたはcapsaicin patches)などの補助療法を追加します。
日中の過度の眠気は、2型に比べ1型筋強直性ジストロフィーに多く見られますが、methylphenidateやmodafinilで対処できます。これらの薬剤は、筋強直性ジストロフィー患者のこの症状に対する有効性と良好な忍容性を示しており、貴重な治療オプションとなっています。さらに、metforminのような抗糖尿病薬は、血糖値を正常化し、筋強直性ジストロフィーにおける軽度の糖尿病症状に対処するために使用されます。それでもなお、症状や合併症の管理には、その他にも複数の薬物療法、リハビリテーション療法、手術、医療機器が用いられます。
当レポートでは、筋強直性ジストロフィーの主要7市場(米国、ドイツ、スペイン、イタリア、フランス、英国、日本)について調査分析し、各地域の市場規模、現在の治療法、アンメットニーズ、新薬などの情報を提供しています。
目次
第1章 重要考察
第2章 レポートのイントロダクション
第3章 筋強直性ジストロフィー市場の概要
- 筋強直性ジストロフィーの市場シェアの分布(2020年)
- 筋強直性ジストロフィーの市場シェアの分布(2034年)
第4章 筋強直性ジストロフィーのエグゼクティブサマリー
第5章 主な出来事
第6章 疾患の背景と概要
- イントロダクション
- 臨床症状
- 分類
- 病因
- 病態生理学
- 診断
- 治療
第7章 疫学と患者人口
- 主な調査結果
- 主要7市場の筋強直性ジストロフィーと診断された総有病者数
- 前提条件と根拠
- 米国
- 欧州4ヶ国・英国
- 日本
第8章 ペイシェントジャーニー
第9章 新治療法
- 主な競合:新治療法
- AMO-02(tideglusib):AMO Pharma Limited
- Mexiletine:Lupin Ltd.
- Pitolisant:Harmony Biosciences, LLC
- Delpacibart etedesiran(AOC-1001):Avidity Biosciences, Inc.
第10章 筋強直性ジストロフィー:主要7市場の分析
- 主な調査結果
- 主な市場予測の前提条件
- 市場見通し
- コンジョイント分析
- 主要7市場の筋強直性ジストロフィーの総市場規模
- 主要7市場の筋強直性ジストロフィーの市場規模:治療法別
- 米国の市場規模
- 米国の筋強直性ジストロフィーの総市場規模
- 米国の筋強直性ジストロフィーの市場規模:治療法別
- 欧州4ヶ国・英国の市場規模
- ドイツ
- フランス
- イタリア
- スペイン
- 英国
- 日本の市場規模
- 日本の筋強直性ジストロフィーの総市場規模
- 日本の筋強直性ジストロフィーの市場規模
第11章 SWOT分析
第12章 KOLの見解
第13章 アンメットニーズ
第14章 市場参入と償還
第15章 付録
第16章 レポートの調査手法
第17章 DelveInsightのサービス内容
第18章 免責事項
List of Tables
- Table 1: Summary of myotonic dystrophy, Epidemiology, and Key Events (2020-2034)
- Table 2: Summary of Clinical Manifestations of Congenital Myotonic Dystrophy Type 1, Myotonic Dystrophy Type 1, and Myotonic Dystrophy Type 2
- Table 3: Correlation of CTG Repeat Size and Clinical Subtype of Myotonic Dystrophy Type 1
- Table 4: Genetics of Myotonic Dystrophy Type 1 versus Myotonic Dystrophy Type 2
- Table 5: Postulated Pathomechanisms
- Table 6: Reporting Guidelines for Myotonic Dystrophy Type 1
- Table 7: Reporting Guidelines for Myotonic Dystrophy Type 2
- Table 8: Consensus-based Diagnostic Recommendations for Adults with Myotonic Dystrophy Type 1
- Table 9: Consensus-based Care Recommendations for Adults with Myotonic Dystrophy : Respiratory management
- Table 10: Consensus-based Care Recommendations for Adults with myotonic dystrophy: Cardiovascular Management
- Table 11: Consensus-based Care Recommendations for Adults with myotonic dystrophy: Pregnancy and Obstetrics Management
- Table 12: Consensus-based Care Recommendations for Adults with myotonic dystrophy: Skeletal Muscle Weakness and Rehabilitation
- Table 13: Consensus-based Care Recommendations for Adults with myotonic dystrophy: Ocular Management
- Table 14: Consensus-based Care Recommendations for Adults with myotonic dystrophy: Gastrointestinal Management
- Table 15: Consensus-based Care Recommendations for Adults with myotonic dystrophy: Neuropsychiatric management
- Table 16: Consensus-based Care Recommendations for Adults with myotonic dystrophy: Endocrine and Metabolic Management
- Table 17: Consensus-based Care Recommendations for Adults with myotonic dystrophy: Surgery and Anesthesia Management
- Table 18: Consensus-based Care Recommendations for Adults with myotonic dystrophy: Congenital Myotonic Dystrophy 1
- Table 19: Consensus-based Care Recommendations for Adults with myotonic dystrophy: Genetic Counseling
- Table 20: Total Diagnosed Prevalent Cases of Myotonic Dystrophy in the 7MM (2020-2034)
- Table 21: Diagnosed Prevalent Cases of Myotonic Dystrophy in the United States (2020-2034)
- Table 22: Type-Specific Diagnosed Cases of Myotonic Dystrophy in the United States (2020-2034)
- Table 23: Type-Specific Diagnosed Cases of Myotonic Dystrophy Type 1 in the United States (2020-2034)
- Table 24: Age-Specific Diagnosed Cases of Myotonic Dystrophy in the United States (2020-2034)
- Table 25: Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in the United States (2020-2034)
- Table 26: Diagnosed Prevalent Cases of Myotonic Dystrophy in Germany (2020-2034)
- Table 27: Type-specific Diagnosed Cases of Myotonic Dystrophy in Germany (2020-2034)
- Table 28: Type-specific Diagnosed Cases of Myotonic Dystrophy Type 1 in Germany (2020-2034)
- Table 29: Age-specific Diagnosed Cases of Myotonic Dystrophy in Germany (2020-2034)
- Table 30: Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in Germany (2020-2034)
- Table 31: Diagnosed Prevalent Cases of Myotonic Dystrophy in France (2020-2034)
- Table 32: Type-specific Diagnosed Cases of Myotonic Dystrophy in France (2020-2034)
- Table 33: Type-specific Diagnosed Cases of Myotonic Dystrophy Type 1 in France (2020-2034)
- Table 34: Age-specific Diagnosed Cases of Myotonic Dystrophy in France (2020-2034)
- Table 35: Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in France (2020-2034)
- Table 36: Diagnosed Prevalent Cases of Myotonic Dystrophy in Italy (2020-2034)
- Table 37: Type-specific Diagnosed Cases of Myotonic Dystrophy in Italy (2020-2034)
- Table 38: Type-specific Diagnosed Cases of Myotonic Dystrophy Type 1 in Italy (2020-2034)
- Table 39: Age-specific Diagnosed Cases of Myotonic Dystrophy in Italy (2020-2034)
- Table 40: Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in Italy (2020-2034)
- Table 41: Age-specific Diagnosed Cases of Myotonic Dystrophy in Spain (2020-2034)
- Table 42: Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in Spain (2020-2034)
- Table 43: Diagnosed Prevalent Cases of Myotonic Dystrophy in the UK(2020-2034)
- Table 44: Type-specific Diagnosed Cases of Myotonic Dystrophy in the UK(2020-2034)
- Table 48: Diagnosed Prevalence of Myotonic Dystrophy in Japan (2020-2034)
- Table 49: Type-specific Diagnosed Cases of Myotonic Dystrophy in Japan (2020-2034)
- Table 50: Type-specific Diagnosed Cases of Myotonic Dystrophy Type 1 in Japan (2020-2034)
- Table 51: Age-specific Diagnosed Cases of Myotonic Dystrophy in Japan (2020-2034)
- Table 52: Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in Japan (2020-2034)
- Table 53: Key cross of emerging drugs
- Table 54: AMO-02, Clinical Trial Description, 2024
- Table 55: Mexiletine, Clinical Trial Description, 2024
- Table 56: Pitolisant, Clinical Trial Description, 2024
- Table 57: AOC 1001, Clinical Trial Description, 2024
- Table 58: Forecast assumptions for AMO-02
- Table 59: Forecast assumptions for AOC 1001
- Table 60: Total Market Size of Myotonic Dystrophy in the 7MM in USD Million (2020-2034)
- Table 61: Market Size of Myotonic Dystrophy in the United States by Therapies in USD Million (2020-2034)
- Table 62: Total Market Size of Myotonic Dystrophy in the United States in USD Million (2020-2034)
- Table 63: Market Size of Myotonic Dystrophy in the United States by Therapies in USD Million (2020-2034)
- Table 64: Total EU4 and the UK Market Size of Myotonic Dystrophy in USD Million (2020-2034)
- Table 65: Market Size of Myotonic Dystrophy in EU4 and the UK by Therapies in USD Million (2020-2034)
- Table 66: Total Market Size of Myotonic Dystrophy in Japan in USD Million (2020-2034)
- Table 67: Market Size of Myotonic Dystrophy in Japan by Therapies in USD Million (2020-2034)
List of Figures
- Figure 1: Symptoms Associated With myotonic dystrophy
- Figure 2: Classification of myotonic dystrophy
- Figure 3: Postulated Pathomechanisms of myotonic dystrophy
- Figure 4: Molecular Diagnostic Tests in Myotonic Dystrophy Type 1
- Figure 5: Molecular Diagnostic Tests in Myotonic Dystrophy Type 2
- Figure 6: Panel Showing Muscle Histology in Myotonic Dystrophy Type 1 and Myotonic Dystrophy Type 2
- Figure 7: Therapeutic Approaches for myotonic dystrophy
- Figure 8: Respiratory Care Recommendations Flowchart for Myotonic Dystrophy Type 1
- Figure 9: Respiratory Care Recommendations Flowchart for Myotonic Dystrophy Type 2
- Figure 10: Cardiac Care Recommendations Flowchart for Myotonic Dystrophy Type 1
- Figure 11: Cardiac Care Recommendations Flowchart for Myotonic Dystrophy Type 2
- Figure 12: Ocular Care Recommendations Flowchart for Myotonic Dystrophy
- Figure 13: Myotonic Dystrophy Endocrine and Metabolic Recommendations Flowchart
- Figure 14: Total Diagnosed Prevalent Cases of Myotonic Dystrophy in the 7MM (2020-2034)
- Figure 15: Diagnosed Prevalent Cases of Myotonic Dystrophy in the United States (2020-2034)
- Figure 16: Type-Specific Diagnosed Cases of Myotonic Dystrophy in the United States (2020-2034)
- Figure 17: Type-Specific Diagnosed Cases of Myotonic Dystrophy Type 1 in the United States (2020-2034)
- Figure 18: Age-Specific Diagnosed Cases of Myotonic Dystrophy in the United States (2020-2034)
- Figure 19: Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in the United States (2020-2034)
- Figure 20: Diagnosed Prevalent Cases of Myotonic Dystrophy in Germany (2020-2034)
- Figure 21: Type-specific Diagnosed Cases of Myotonic Dystrophy in Germany (2020-2034)
- Figure 22: Type-specific Diagnosed Cases of Myotonic Dystrophy Type 1 in Germany (2020-2034)
- Figure 23: Age-specific Diagnosed Cases of Myotonic Dystrophy in Germany (2020-2034)
- Figure 24: Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in Germany (2020-2034)
- Figure 25: Diagnosed Prevalent Cases of Myotonic Dystrophy in France (2020-2034)
- Figure 26: Type-specific Diagnosed Cases of Myotonic Dystrophy in France (2020-2034)
- Figure 27: Type-specific Diagnosed Cases of Myotonic Dystrophy Type 1 in France (2020-2034)
- Figure 28: Age-specific Diagnosed Cases of Myotonic Dystrophy in France (2020-2034)
- Figure 29: Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in France (2020-2034)
- Figure 30: Diagnosed Prevalent Cases of Myotonic Dystrophy in Italy (2020-2034)
- Figure 31: Type-specific Diagnosed Cases of Myotonic Dystrophy in Italy (2020-2034)
- Figure 32: Type-specific Diagnosed Cases of Myotonic Dystrophy Type 1 in Italy (2020-2034)
- Figure 33: Age-specific Diagnosed Cases of Myotonic Dystrophy in Italy (2020-2034)
- Figure 34: Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in Italy (2020-2034)
- Figure 35: Diagnosed Prevalent Cases of Myotonic Dystrophy in Spain (2020-2034)
- Figure 36: Type-specific Diagnosed Cases of Myotonic Dystrophy in Spain (2020-2034)
- Figure 37: Type-specific Diagnosed Cases of Myotonic Dystrophy Type 1 in Spain (2020-2034)
- Figure 38: Age-specific Diagnosed Cases of Myotonic Dystrophy in Spain (2020-2034)
- Figure 39: Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in Spain (2020-2034)
- Figure 40: Diagnosed Prevalent Cases of Myotonic Dystrophy in the UK(2020-2034)
- Figure 41: Type-specific Diagnosed Cases of Myotonic Dystrophy in the UK(2020-2034)
- Figure 42: Type-specific Diagnosed Cases of Myotonic Dystrophy Type 1 in the UK(2020-2034)
- Figure 43: Age-specific Diagnosed Cases of Myotonic Dystrophy in the UK(2020-2034)
- Figure 44: Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in the UK(2020-2034)
- Figure 45: Diagnosed Prevalence of Myotonic Dystrophy in Japan (2020-2034)
- Figure 46: Type-specific Diagnosed Cases of Myotonic Dystrophy in Japan (2020-2034)
- Figure 47: Type-specific Diagnosed Cases of Myotonic Dystrophy Type 1 in Japan (2020-2034)
- Figure 48: Age-specific Diagnosed Cases of Myotonic Dystrophy in Japan (2020-2034)
- Figure 49: Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in Japan (2020-2034)
- Figure 50: Patient Journey
- Figure 51: Total Market Size of Myotonic Dystrophy in the 7MM (2020-2034)
- Figure 52: Market Size of Myotonic Dystrophy in the 7MM by Therapies (2020-2034)
- Figure 53: Total Market Size of Myotonic Dystrophy in the United States (2020-2034)
- Figure 54: Market Size of Myotonic Dystrophy in the United States by Therapies (2020-2034)
- Figure 55: EU4 and the UK Total Market Size of Myotonic Dystrophy (2020-2034)
- Figure 56: Market Size of Myotonic Dystrophy in EU4 and the UK by Therapies (2020-2034)
- Figure 57: Total Market Size of Myotonic Dystrophy in Japan (2020-2034)
- Figure 58: Market Size of Myotonic Dystrophy in Japan by Therapies (2020-2034)
- Figure 59: SWOT Analysis
- Figure 60: Unmet Needs
- Figure 61: Health Technology Assessment
- Figure 62: Reimbursement Process in Germany
- Figure 63: Reimbursement Process in France
- Figure 64: Reimbursement Process in Italy
- Figure 65: Reimbursement Process in Spain
- Figure 66: Reimbursement Process in the United Kingdom
- Figure 67: Reimbursement Process in Japan
Key Highlights:
- The Myotonic Dystrophy market is projected to witness consistent growth throughout the forecast period (2024-2034). The market size of Myotonic Dystrophy in the 7MM is expected to increase, driven by better diagnosis and the launch of emerging therapies.
- DelveInsight's analysis estimates that among the total diagnosed prevalent cases of Myotonic Dystrophy in 7MM approximately 51% of cases were from the US. As per our estimations, in 2023, the US accounted for nearly 54,068 diagnosed prevalent cases of Myotonic Dystrophy.
- The total market size of the Myotonic Dystrophy treatment market is anticipated to experience growth during the forecast period due to the emergence of new and effective treatments, namely, Tideglusib (AMO-02).
- The market for therapeutics targeting Myotonic Dystrophy is projected to experience robust growth, at a significant compound annual growth rate (CAGR) of 18.4% from 2020 to 2034. This dynamic expansion reflects increasing demand for advanced treatment options and underscores the growing investment in addressing this serious condition.
- In June 2024, Avidity Therapeutics initiated the administration of del-desiran in the global Phase III HARBOR trial for myotonic dystrophy type 1. This significant clinical trial aims to evaluate the efficacy and safety of del-desiran in patients suffering from this genetic disorder. The HARBOR trial represents a critical step in advancing potential treatment options for myotonic dystrophy type 1, a condition characterized by muscle weakness and myotonia. By engaging a diverse patient population across multiple sites worldwide, Avidity hopes to gather comprehensive data that could pave the way for future regulatory approval and improve the quality of life for those affected by this condition.
The market for Myotonic Dystrophy is shaped by several critical factors, including the increasing prevalence of genetic disorders and heightened awareness about rare diseases. Advances in genomic research and growing investments in rare disease therapies are driving market growth. Nonetheless, challenges such as limited funding for research, high costs of novel treatments, and rigorous regulatory hurdles remain significant obstacles. Moreover, the substantial unmet need for effective Myotonic Dystrophy treatments underscores a major gap in the current therapeutic landscape, presenting a prime opportunity for companies to innovate and address the needs of a diverse patient population.
DelveInsight's "Myotonic Dystrophy - Market Insights, Epidemiology, and Market Forecast - 2034" report delivers an in-depth understanding of the myotonic dystrophy, historical and forecasted epidemiology and the Myotonic Dystrophy market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The Myotonic Dystrophy market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Myotonic Dystrophy market size from 2020 to 2034. The report also covers current Myotonic Dystrophy treatment practices/algorithms and unmet medical needs to curate the best of the opportunities and assesses the underlying potential of the market.
Geography Covered:
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Study Period: 2020-2034
Disease Understanding and Treatment Algorithm
Myotonic Dystrophy Overview
Myotonic dystrophy is a form of muscular dystrophy, a group of disorders characterized by the weakness and degeneration of various voluntary muscles in the body. Each type of muscular dystrophy has distinct abnormalities, such as variations in muscle fiber size, muscle fiber necrosis, scar tissue formation, and inflammation, which can be observed in muscle biopsies from affected patients.
There are two primary forms of myotonic dystrophy: type 1 and type 2. Myotonic dystrophy type 1 is commonly referred to as Steinert disease, named after Dr. Steinert, who, along with his colleagues, first documented the classic form of the condition in 1909. Type 2 is known as Ricker syndrome or proximal myotonic dystrophy (PROMM).
Myotonic Dystrophy Diagnosis
Myotonic dystrophy should be suspected in patients with weakness symptoms, a family history of myotonic dystrophy, and characteristic physical exam findings. Genetic testing for CTG repeats has replaced other modalities and become the gold standard test in diagnosing myotonic dystrophy. Other diagnostic testing modalities may often be obtained before genetic testing and involve serum creatinine kinase, hepatobiliary function testing, muscle biopsies, and electrocardiographic findings for cardiomyopathy.
Myotonic Dystrophy Treatment
Myotonic dystrophy types 1 and 2 are among the most common forms of muscular dystrophy that manifest during adulthood. Understanding the clinical differences between these two types is crucial for determining the most appropriate treatment strategies for patients. At present, there are no therapies available that modify the disease itself, but a range of symptomatic treatments can help manage the condition. Encouragingly, next-generation therapies are on the horizon and may soon provide new options for affected individuals. Effectively managing the symptoms of myotonic dystrophy is essential, as it can significantly reduce the suffering experienced by patients and enhance their overall quality of life. Regular monitoring of the disease is also vital, as it can help identify and mitigate potential complications during critical periods.
Myotonic Dystrophy Epidemiology
As the market is derived using the patient-based model, the Myotonic Dystrophy epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Prevalent Cases of Myotonic Dystrophy, Type-specific Cases of Myotonic Dystrophy, Type-specific Cases of Myotonic Dystrophy 1, Age-specific Cases of Myotonic Dystrophy, and Comorbidities associated with Myotonic Dystrophy in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan, from 2020 to 2034. As per DelveInsight's estimations, the total diagnosed prevalent cases of Myotonic Dystrophy in the 7MM was approximately 105,362 cases in 2023 and are projected to decrease during the forecast period.
- Among the 7MM, EU4 and the UK accounted for nearly 353,191 diagnosed prevalent cases of myotonic dystrophy, and these cases are expected to increase during the forecast period (2024-2034).
- Among EU4 and the UK, Germany had the highest diagnosed prevalent population of myotonic dystrophy, with 8,938 cases, followed by the UK and France in 2023. On the other hand, Spain had the lowest diagnosed prevalent population in EU4 and the UK in 2023.
- In Japan, there were around 12,735 diagnosed prevalent cases of Myotonic Dystrophy in 2023. These cases are expected to increase at a significant CAGR.
- In 2023, in Japan, approximately 1,834 cases of individuals with myotonic dystrophy were associated with gastrointestinal symptoms, 1,605 cases with cardiac dysrhythmias, 1,414 cases with sleep disorders, and 3,566 cases with other comorbidities.
- In 2023, the highest proportion of myotonic dystrophy cases in the 7MM was estimated to be among adults, with 96,287 cases, compared to 9,075 cases among children.
- According to the analysis conducted by DelveInsight, among diagnosed cases of myotonic dystrophy, approximately 80% were of myotonic dystrophy type 1 and 20% of myotonic dystrophy type 2 within the US in 2023. This analysis indicates a higher prevalence of myotonic dystrophy type 1.
Myotonic Dystrophy Drug Chapters
The drug chapter segment of the Myotonic Dystrophy report encloses a detailed analysis of Myotonic Dystrophy marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also understands Myotonic Dystrophy clinical trial details, expressive pharmacological action, agreements and collaborations, approval, and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Emerging Drugs
Tideglusib (AMO-02): AMO Pharma
AMO-02 (tideglusib) is in development for the treatment of congenital myotonic dystrophy and has the potential for use in additional CNS, neuromuscular, and other orphan indications. AM0-02 is a clinical-stage investigational medicine for the treatment of the severe form of congenital myotonic dystrophy known as myotonic dystrophy type 1 or Steinert disease. In cellular and animal models of myotonic dystrophy type 1, as well as in muscle biopsies from patients, the activity of glycogen synthase kinase 3 beta (GSK3B) has been shown to increase. AMO-02 is an inhibitor that has been shown to normalize levels of GSK3B in transgenic models and in ex vivo tissue samples in patients with myotonic dystrophy type 1 and to reduce levels of the mRNA that is pathogenic for myotonic dystrophy type 1.
Delpacibart etedesiran (AOC-1001): Avidity Biosciences, Inc.
Delpacibart etedesiran (AOC-1001), also known as del-desiran, is a monoclonal Antibody Oligonucleotide Conjugate (AOC), is designed to address the root cause of myotonic dystrophy type 1 by reducing levels of a disease-related mRNA called DMPK in skeletal, cardiac, and smooth muscle. Del-desiran consists of a proprietary Monoclonal Antibody (mAb) that binds to the Transferrin Receptor 1 (TfR1) conjugated with a small interfering RNA (siRNA) targeting DMPK mRNA. This allows del-desiran to address the underlying cause of disease by reducing DMPK mRNA, releasing MBNL as shown in MARINA participants where del-desiran treatment led to dose-dependent increases in inferred MBNL and, potentially alleviating the spectrum of symptoms that people with myotonic dystrophy type 1 experience.
Del-desiran, Avidity's investigational lead product candidate utilizing its AOC platform, is being studied in the Phase III global HARBOR trial in adults living with myotonic dystrophy type 1. Del-desiran is also being studied in the ongoing MARINA-OLE trial with all of the participants who completed the Phase I/II MARINA trial. Data from the MARINA-OLE trial showed a reversal of disease progression in people living with myotonic dystrophy type 1 across multiple endpoints, including vHOT, muscle strength, and activities of daily living when compared to END-myotonic dystrophy type 1 natural history data.
Myotonic Dystrophy Market Outlook
Myotonic dystrophy is a dominantly inherited type of muscular dystrophy that affects the muscles and other body systems. The disease can lead patients to experience early cataracts, myotonia, muscle weakness/atrophy, fatigue, excessive daytime sleepiness, central/obstructive apnoea, respiratory failure, cardiac arrhythmia, insulin resistance, dysphagia, mood disorders, and others. Myotonic dystrophy is of two types; myotonic dystrophy type 1 is caused by the expansion of a CTG triplet repeat in DMPK, whereas the expansion of a CCTG tetramer causes myotonic dystrophy type 2 repeat in CNBP. However, the symptoms of myotonic dystrophy type 2 are usually milder than those of myotonic dystrophy type 1. Moreover, myotonic dystrophy type 2 lacks a congenital form, and the first clinical manifestations usually begin after the third decade of life with muscular symptoms such as weakness, musculoskeletal pain, stiffness, myotonia, fatigue, and exercise intolerance, which usually represent the reason for the first referral to neurologists. Hence, it is important to adopt the most appropriate interventions to improve the patient's QoL.
Currently, no approved therapy exists that can cure or slow the progression of myotonic dystrophy. However, symptomatic treatments are available. The symptomatic management helps reduce the suffering and improve QoL. Among the myotonic dystrophy symptoms, myotonia is the most common symptom treated with antimyotonic agents like mexiletine, lamotrigine, carbamazepine, oxcarbazepine, flecainide, propaphenone, phenytoin, ranolazine, all prescribed as an off-label treatment. Further, the pharmacological approach for chronic muscle pain follows the WHO four steps ladder, where the first step corresponds to the use of NSAIDs with the addition, if needed, of adjuvant therapy as anticonvulsants (pregabalin, gabapentin), antidepressants (duloxetine, amitriptyline, nortriptyline), muscle relaxants (baclofen, tizanidine), or topical agents (lidocaine or capsaicin patches).
Excessive daytime sleepiness, more prevalent in myotonic dystrophy type 1 compared to type 2, can be managed with methylphenidate and modafinil. These medications have shown efficacy and good tolerability in treating this symptom in myotonic dystrophy patients, providing a valuable therapeutic option. Additionally, anti-diabetic drugs like metformin are used to normalize blood sugar levels and address mild diabetic symptoms in myotonic dystrophy. Nevertheless, several other medications, rehabilitative therapy, surgeries, and medical devices are used in managing symptoms and complications.
Additionally, it is important to note that while mexiletine is currently used off-label for myotonic dystrophy, it is also under development for formal approval and is in the pipeline for myotonic dystrophy. This highlights its potential efficacy and underscores the need for its consideration as an authorized treatment in regions such as the European Union, the US, and Japan.
Myotonic Dystrophy Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to launch in the market during 2020-2034.
Myotonic Dystrophy Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I stage. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Myotonic Dystrophy emerging therapies.
KOL Views
To keep up with current market trends, we take KOLs and SMEs' opinions working in the domain through primary research to fill the data gaps and validate the secondary research. Industry Experts were contacted for insights on Myotonic Dystrophy evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including KOL from Virginia Commonwealth University, University of Rochester Medical Center, Johns Hopkins Hospital, Baltimore, Mount Sinai Hospital, New York, Stanford Health Care, Stanford, Massachusetts General Hospital, Boston, University of California, San Francisco (UCSF) Medical Center, San Francisco, University of Pennsylvania Hospital, Philadelphia, Neurological Institute at the Columbia University Medical Center, New York, Toronto Western Hospital, and others.
Delveinsight's analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapies, treatment patterns, or Myotonic Dystrophy market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst's discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies' safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
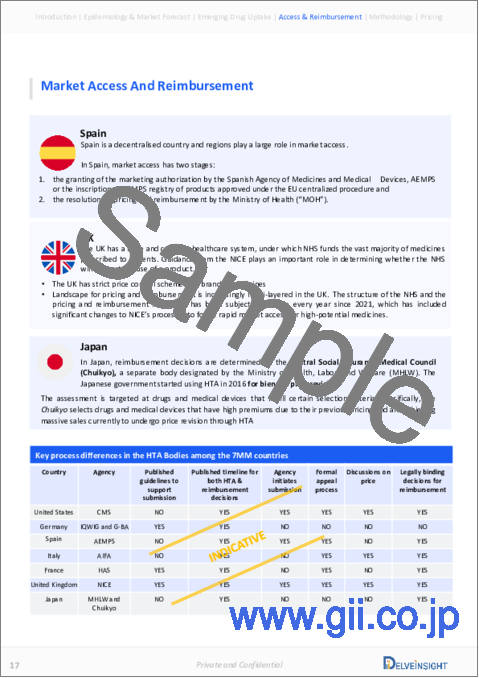
Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the global drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Report:
- The report covers a segment of key events, an executive summary, descriptive overview of myotonic dystrophy, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Myotonic Dystrophy market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind the approach is included in the report covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Myotonic Dystrophy market.
Myotonic Dystrophy Report Insights
- Patient Population
- Therapeutic Approaches
- Myotonic Dystrophy Pipeline Analysis
- Myotonic Dystrophy Market Size and Trends
- Existing and Future Market Opportunity
Myotonic Dystrophy Report Key Strengths
- 11 years Forecast
- The 7MM Coverage
- Myotonic Dystrophy Epidemiology Segmentation
- Key Cross Competition
- Attribute Analysis
- Drugs Uptake and Key Market Forecast Assumptions
Myotonic Dystrophy Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions:
Market Insights
- What was the Myotonic Dystrophy total market size, the market size by therapies, and market share (%) distribution in 2020, and how would it all look in 2034? What are the contributing factors for this growth?
- What unmet needs are associated with the current treatment market of Myotonic Dystrophy?
- What are the patents of emerging therapies for Myotonic Dystrophy?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Epidemiology Insights
- What are the disease risks, burdens, and unmet needs of Myotonic Dystrophy? What will be the growth opportunities across the 7MM concerning the patient population of Myotonic Dystrophy?
- What is the historical and forecasted Myotonic Dystrophy patient pool in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- Why do only limited patients appear for diagnosis?
- Which country is more prevalent for Myotonic Dystrophy and why?
- What factors are affecting the diagnosis of the indication?
Current Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for treating Myotonic Dystrophy? What are the current guidelines for treating Myotonic Dystrophy in the US and Europe?
- How many companies are developing therapies for treating Myotonic Dystrophy?
- How many emerging therapies are in the mid-stage and late stage of development for treating Myotonic Dystrophy?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What are the key designations that have been granted for the emerging therapies for Myotonic Dystrophy?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies? Focus on reimbursement policies.
- What are the 7MM historical and forecasted markets of Myotonic Dystrophy?
Reasons to Buy:
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Myotonic Dystrophy Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies that will help get ahead of competitors.
- Detailed analysis and ranking of potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Frequently Asked Questions:
1. What is the forecast period covered in the report?
The Myotonic Dystrophy Epidemiology and Market Insight report for the 7MM covers the forecast period from 2024 to 2034, providing a projection of market dynamics and trends during this timeframe.
2. Who are the key players in the Myotonic Dystrophy market?
The Myotonic Dystrophy market is sparse. The major players are Lupin Ltd. which are currently developing drugs for the treatment of Myotonic Dystrophy, the rest of the market is now contributed with off-label therapies.
3. How is the market size estimated in the forecast report?
The market size is estimated through data analysis, statistical modeling, and expert opinions. It may consider factors such as incident cases, treatment costs, revenue generated, and market trends.
4. What is the key driver of the Myotonic Dystrophy market?
The increase in prevalent and diagnosed prevalent cases of Myotonic Dystrophy is attributed to the current population size.
5. What is the expected impact of emerging therapies or advancements in Myotonic Dystrophy treatment on the market?
Introducing new therapies, advancements in diagnostic techniques, and innovations in treatment approaches can significantly impact the Myotonic Dystrophy treatment market. Market forecast reports may provide analysis and predictions regarding the potential impact of these developments.
6. Does the report provide insights into the competitive landscape of the market?
The market forecast report may include information on the competitive landscape, profiling key market players, their product offerings, partnerships, and strategies, and helping stakeholders understand the competitive dynamics of the Myotonic Dystrophy market.
Table of Contents
1. Key Insights
2. Report Introduction
3. Myotonic Dystrophy Market Overview at a Glance
- 3.1. Market Share (%) Distribution of Myotonic Dystrophy in 2020
- 3.2. Market Share (%) Distribution of Myotonic Dystrophy in 2034
4. Executive Summary of Myotonic Dystrophy
5. Key events
6. Disease background and overview
- 6.1. Introduction
- 6.2. Clinical Manifestations
- 6.3. Classification
- 6.4. Etiology
- 6.5. Pathophysiology
- 6.6. Diagnosis
- 6.6.1. Differential diagnosis
- 6.7. Treatment
- 6.7.1. Treatment guidelines
- 6.7.1.1. Management of respiratory complications in Myotonic Dystrophy
- 6.7.1.2. Management of cardiovascular complications in Myotonic Dystrophy
- 6.7.1.3. Pregnancy and obstetrics management
- 6.7.1.4. Management of skeletal muscle weakness and rehabilitation
- 6.7.1.5. Management of ocular complications
- 6.7.1.6. Management of gastrointestinal complications
- 6.7.1.7. Management of gastrointestinal complications
- 6.7.1.8. Management of neuropsychiatric complications and excessive daytime sleepiness
- 6.7.1.9. Management of endocrine and metabolic complications
- 6.7.1.10. Recommendations for surgery and anesthesia in Myotonic Dystrophy patients
- 6.7.1.11. Management of Congenital Myotonic Dystrophy type 1
- 6.7.1.12. Genetic counseling
- 6.7.1. Treatment guidelines
7. Epidemiology and Patient Population
- 7.1. Key Findings
- 7.2. Total Diagnosed Prevalent Cases of Myotonic Dystrophy in the 7MM
- 7.3. Assumption and Rationale
- 7.4. The United States
- 7.4.1. Diagnosed Prevalence of Myotonic Dystrophy in the United States
- 7.4.2. Type-Specific Diagnosed Cases of Myotonic Dystrophy in the United States
- 7.4.3. Type-Specific Diagnosed Cases of Myotonic Dystrophy Type 1 in the United States
- 7.4.4. Age-Specific Diagnosed Cases of Myotonic Dystrophy in the United States
- 7.4.5. Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in the United States
- 7.5. The EU4 and the UK
- 7.5.1. Germany
- 7.5.1.1. Diagnosed prevalence of Myotonic Dystrophy in Germany
- 7.5.1.4. Type-specific Diagnosed Cases of Myotonic Dystrophy in Germany
- 7.5.1.5. Type-specific Diagnosed Cases of Myotonic Dystrophy Type 1 in Germany
- 7.5.1.4. Age-specific Diagnosed Cases of Myotonic Dystrophy in Germany
- 7.5.1.5. Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in Germany
- 7.5.2. France
- 7.5.2.1. Diagnosed prevalence of Myotonic Dystrophy in France
- 7.5.2.2. Type-specific Diagnosed Cases of Myotonic Dystrophy in France
- 7.5.2.3. Type-specific Diagnosed Cases of Myotonic Dystrophy Type 1 in France
- 7.5.2.4. Age-specific Diagnosed Cases of Myotonic Dystrophy in France
- 7.5.2.5. Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in France
- 7.5.3. Italy
- 7.5.3.1. Diagnosed prevalence of Myotonic Dystrophy in Italy
- 7.5.3.2. Type-specific Diagnosed Cases of Myotonic Dystrophy in Italy
- 7.5.3.3. Type-specific Diagnosed Cases of Myotonic Dystrophy Type 1 in Italy
- 7.5.3.4. Age-specific Diagnosed Cases of Myotonic Dystrophy in Italy
- 7.5.3.5. Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in Italy
- 7.5.4. Spain
- 7.5.4.1. Diagnosed prevalence of Myotonic Dystrophy in Spain
- 7.5.4.2. Type-specific Diagnosed Cases of Myotonic Dystrophy in Spain
- 7.5.4.3. Type-specific Diagnosed Cases of Myotonic Dystrophy Type 1 in Spain
- 7.5.4.4. Age-specific Diagnosed Cases of Myotonic Dystrophy in Spain
- 7.5.4.5. Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in Spain
- 7.5.5. The UK
- 7.5.5.1. Diagnosed prevalence of Myotonic Dystrophy in the UK
- 7.5.5.2. Type-specific Diagnosed Cases of Myotonic Dystrophy in the UK
- 7.5.5.3. Type-specific Diagnosed Cases of Myotonic Dystrophy Type 1 in the UK
- 7.5.5.4. Age-specific Diagnosed Cases of Myotonic Dystrophy in the UK
- 7.5.5.5. Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in the UK
- 7.5.1. Germany
- 7.6. Japan
- 7.6.1. Diagnosed Prevalence of Myotonic Dystrophy in Japan
- 7.6.2. Type-specific Diagnosed Cases of Myotonic Dystrophy in Japan
- 7.6.3. Type-specific Diagnosed Cases of Myotonic Dystrophy Type 1 in Japan
- 7.6.4. Age-specific Diagnosed Cases of Myotonic Dystrophy in Japan
- 7.6.5. Comorbidity associated Diagnosed Cases with Myotonic Dystrophy in Japan
8. Patient Journey
9. Emerging Therapies
- 9.1. Key cross: Emerging
- 9.2. AMO-02 (tideglusib): AMO Pharma Limited
- 9.2.1. Drug Description
- 9.2.2. Other Development Activities
- 9.2.3. Clinical Trial Information
- 9.2.4. Safety and Efficacy
- 9.2.5. Analyst Views
- 9.3. Mexiletine: Lupin Ltd.
- 9.3.1. Drug Description
- 9.3.2. Other Development Activities
- 9.3.3. Clinical Trial Information
- 9.3.4. Safety and Efficacy
- 9.3.5. Analyst Views
- 9.4. Pitolisant: Harmony Biosciences, LLC
- 9.4.1. Drug Description
- 9.4.2. Other Development Activities
- 9.4.3. Clinical Trial Information
- 9.4.4. Safety and Efficacy
- 9.4.5. Analyst Views
- 9.5. Delpacibart etedesiran (AOC-1001): Avidity Biosciences, Inc.
- 9.5.1. Drug Description
- 9.5.2. Other Development Activities
- 9.5.3. Clinical Trial Information
- 9.5.4. Safety and Efficacy
- 9.5.5. Analyst Views
10. Myotonic Dystrophy: 7 Major Market Analysis
- 10.1. Key Findings
- 10.2. Key Market Forecast Assumptions
- 10.3. Market Outlook
- 10.4. Conjoint Analysis
- 10.5. Total Market Size of Myotonic Dystrophy in the 7MM
- 10.6. Market Size of Myotonic Dystrophy by Therapies in the 7MM
- 10.7. The United States Market Size
- 10.7.1. Total Market Size of Myotonic Dystrophy in the United States
- 10.7.2. Market Size of Myotonic Dystrophy by Therapies in the United States
- 10.8. The EU4 and the UK Market Size
- 10.8.1. Germany
- 10.8.1.1. Total Market Size of Myotonic Dystrophy in Germany
- 10.8.1.2. Market Size of Myotonic Dystrophy in Germany
- 10.8.2. France
- 10.8.2.1. Total Market Size of Myotonic Dystrophy in France
- 10.8.2.2. Market Size of Myotonic Dystrophy in France
- 10.8.3. Italy
- 10.8.3.1. Total Market Size of Myotonic Dystrophy in Italy
- 10.8.3.2. Market Size of Myotonic Dystrophy in Italy
- 10.8.4. Spain
- 10.8.4.1. Total Market Size of Myotonic Dystrophy in Spain
- 10.8.4.2. Market Size of Myotonic Dystrophy in Spain
- 10.8.5. The UK
- 10.8.5.1. Total Market Size of Myotonic Dystrophy in the UK
- 10.8.5.2. Market Size of Myotonic Dystrophy in the UK
- 10.8.1. Germany
- 10.9. Japan Market Size
- 10.9.1. Total Market Size of Myotonic Dystrophy in Japan
- 10.9.2. Market Size of Myotonic Dystrophy in Japan
11. SWOT Analysis
12. KOL Views
13. Unmet Need
14. Market Access and Reimbursement
15. Appendix
- 15.1. Bibliography
- 15.2. Acronyms and Abbreviations