|
|
市場調査レポート
商品コード
1100542
マイクロバイオーム治療薬の世界市場 (2022年~2032年):標的治療分野・地域 (10カ国) 別の分析・予測・競合情勢Global Microbiome Therapeutics Market - A Global and Regional Analysis: Focus on Target Therapies, Region (10 Countries), and Competitive Landscape - Analysis and Forecast, 2022-2032 |
||||||
|
● お客様のご希望に応じて、既存データの加工や未掲載情報(例:国別セグメント)の追加などの対応が可能です。 詳細はお問い合わせください。 |
|||||||
| マイクロバイオーム治療薬の世界市場 (2022年~2032年):標的治療分野・地域 (10カ国) 別の分析・予測・競合情勢 |
|
出版日: 2022年07月04日
発行: BIS Research
ページ情報: 英文 196 Pages
納期: 1~5営業日
|
- 全表示
- 概要
- 図表
- 目次
世界のマイクロバイオーム治療薬の市場規模は、2021年の3億630万米ドルから、予測期間中は24.95%のCAGRで推移し、2032年には32億400万米ドルの規模に成長すると予測されています。
マイクロバイオーム治療薬は、さまざまな臨床症状における既存のアンメットニーズに対応できる可能性があり、医学界から大きな注目を集めています。
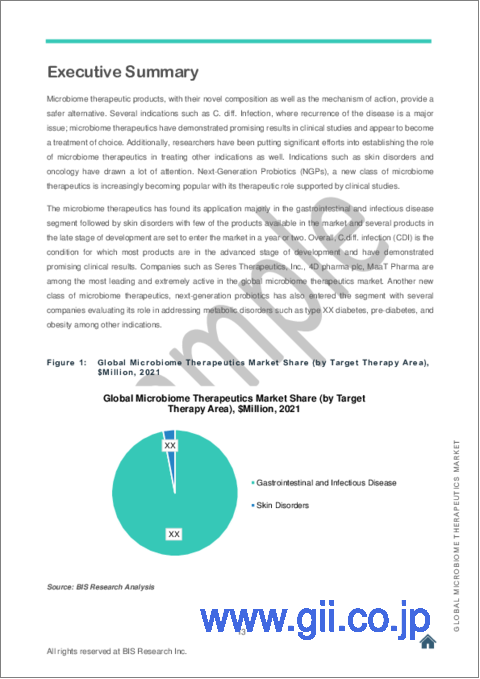
標的治療分野別では、胃腸および皮膚疾患の部門が大きなシェアを示しており、予測期間中も同様の傾向を示すと予測されています。開発が進んでいるマイクロバイオーム治療薬の多くはCDIを適応症としており、最初に市場に参入するものと予想されています。
当レポートでは、世界のマイクロバイオーム治療薬の市場を調査し、市場概要、市場成長への各種影響因子の分析、法規制環境、特許動向、市場規模の推移・予測、各種区分・地域/主要国別の内訳、市場シェア、主要企業のプロファイルなどをまとめています。
第1章 市場の定義
第2章 調査範囲
第3章 調査手法
第4章 市場概要
- 市場規模・将来の成長の可能性
- 歴史的動向
第5章 産業考察
- 規制状況
- 特許情勢
- 提携情勢
- 政府のイニシアチブ
- COVID-19の影響
第6章 市場力学
- 市場促進要因
- 市場抑制要因
- 市場機会
第7章 臨床試験の情勢
- マイクロバイオーム治療パイプラインの情勢
- マイクロバイオーム治療薬市場への参入有望薬・臨床試験設計
- CP-101(Finch Therapeutics Group, Inc.)
- SER-109(Seres Therapeutics, Inc.)
- RBX-2660(Rebiotix Inc.)
- CH-106(Caelus Health)
- LACTIN-V(Osel Inc.)
- MaaT013(MaaT Pharma)
第8章 競合考察
- 主な戦略・展開
- 相乗効果
- 製品の発売・規制当局の承認
- 資金調達・投資
- M&A
- 市場シェア分析
- 成長シェア分析(機会マッピング)
第9章 世界のマイクロバイオーム治療薬市場:標的治療分野別
- 概要
- 胃腸・感染症
- 皮膚疾患
- 癌
- その他
第10章 世界のマイクロバイオーム治療薬市場:地域別
- 概要
- 北米
- 欧州
- アジア太平洋
- その他の地域
第11章 競合ベンチマーキング・企業プロファイル
- 4D pharma plc
- Seres Therapeutics, Inc.
- Microbiotica
- Enterome
- Destiny Pharma plc
- Taisho Pharmaceutical Holdings
- AOBiome Therapeutics, Inc.
- Finch Therapeutics Group, Inc.
- Ferring Pharmaceuticals
- Rebiotix Inc. (A Subsidiary of Ferring Pharmaceuticals)
- MaaT Pharma
- Vedanta Biosciences Inc.
- OxThera AB
- Pendulum Therapeutics
- Caelus Health
- Quorum Innovations
- Sanofi S.A.
- DermBiont, Inc.
- EnteroBiotix Ltd
- YSOPIA Bioscience
- Winclove Probiotics
- TargEDys
- Evelo Biosciences, Inc.
- BiomX
- Biomica Ltd.
- Scioto Biosciences, Inc.
- Lactobio A/S
第12章 付録
List of Figures
- Figure 1: Global Microbiome Therapeutics Market Share (by Target Therapy Area), $Million, 2021
- Figure 2: Global Microbiome Therapeutics Market Share (by Region), $Million, 2021 and 2032
- Figure 3: Growth-Share Analysis (by Region), 2020-2021
- Figure 4: Global Microbiome Therapeutics Market Segmentation
- Figure 5: Global Microbiome Therapeutics Market Methodology
- Figure 6: Primary Research Methodology
- Figure 7: Bottom-Up Approach (Segment Wise Analysis)
- Figure 8: Top-Down Approach (Segment-Wise Analysis)
- Figure 9: Global Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 10: Historical Evolution of Microbiome Therapeutics
- Figure 11: Chemistry, Manufacturing, and Control (CMC) Information of LBPs by FDA
- Figure 12: Patent Landscape of the Microbiome Therapeutics Market (by Region), 2019-2021
- Figure 13: COVID-19 Impact on the Global Microbiome Therapeutics Market
- Figure 14: Number of Strategic Activities in the Global Microbiome Therapeutics Market, 2019-2021
- Figure 15: Number of Agreements for Leading Companies
- Figure 16: Share of Microbiome Therapeutics Products in Global Clinical Trials (by Development Phase)
- Figure 17: Share of Microbiome Therapeutics Pipeline Candidates (by Route of Administration)
- Figure 18: Share of Microbiome Therapeutics Products (by Type of Microbiome Strategy)
- Figure 19: Number of Microbiome Therapeutic Products in Global Clinical Trials (by Indication)
- Figure 20: CP-101: Phase II Clinical Design for Recurrent C. diff. Infection (CDI)
- Figure 21: CP-101 Phase III Clinical Design for Recurrent C. diff. Infection (CDI)
- Figure 22: SER-109: Mechanism of Action
- Figure 23: SER-109: Clinical Trial Details for Recurrent C. diff. Infection (CDI)
- Figure 24: RBX-2660: Key Regulatory Milestones
- Figure 25: RBX-2660: Clinical Progress for Recurrent C. Diff Infection (CDI)
- Figure 26: RBX-2660: Clinical Trial Details for Recurrent C. diff. Infection (CDI)
- Figure 27: CH-106: Clinical Design for Metabolic Syndrome/Prediabetes
- Figure 28: Share of Key Developments and Strategies, January 2019-October 2021
- Figure 29: Share of Synergistic Activities (by Company), January 2019-October 2021
- Figure 30: Share of Product Launch and Regulatory Approvals (by Company), January 2019-October 2021
- Figure 31: Share of Funding and Investment (by Company), January 2019-October 2021
- Figure 32: Share of Mergers and Acquisitions (by Company), January 2019-October 2021
- Figure 33: Market Share Analysis for the Global Microbiome Therapeutics Market, $Million, 2020 and 2021
- Figure 34: Growth-Share Analysis (by Region), 2020-2021
- Figure 35: Growth-Share Analysis (by Company), 2020-2021
- Figure 36: Global Microbiome Therapeutics Market (by Target Therapy Area), $Million, 2021 and 2032
- Figure 37: Number of Pipeline Candidates for Gastrointestinal and Infectious Diseases
- Figure 38: Global Microbiome Therapeutics Market (Gastrointestinal and Infectious Diseases), $Million, 2021-2032
- Figure 39: Number of Pipeline Products for Skin Disorders
- Figure 40: Global Microbiome Therapeutics Market (Skin Disorders), $Million, 2021-2032
- Figure 41: Number of Pipeline Products for Cancer Indications
- Figure 42: Number of Pipeline Products for Other Indications
- Figure 43: Global Microbiome Therapeutics Market (by Region), 2022-2032
- Figure 44: Global Microbiome Therapeutics Market Share (by Region), $Million, 2021-2032
- Figure 45: North America Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 46: North America: Market Dynamics
- Figure 47: North America Microbiome Therapeutics Market Share (by Country), $Million, 2021 and 2032
- Figure 48: U.S. Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 49: Canada Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 50: Europe Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 51: Europe: Market Dynamics
- Figure 52: Europe Microbiome Therapeutics Market Share (by Country), $Million, 2021 and 2032
- Figure 53: France Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 54: Germany Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 55: Sweden Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 56: U.K Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 57: Italy Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 58: Spain Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 59: Rest-of-Europe Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 60: Asia-Pacific Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 61: Asia-Pacific: Market Dynamics
- Figure 62: Asia-Pacific Microbiome Therapeutics Market Share (by Country), $Million, 2021-2032
- Figure 63: Japan Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 64: Australia Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 65: China Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 66: India Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 67: Rest-of-Asia-Pacific Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 68: Rest-of-the-World Microbiome Therapeutics Market, $Million, 2021-2032
- Figure 69: 4D pharma plc: Product Portfolio
- Figure 70: 4D pharma plc: Overall Financials, $Million, 2019-2021
- Figure 71: 4D pharma plc: R&D Expenditure, $Million, 2019-2021
- Figure 72: Seres Therapeutics, Inc.: Product Portfolio
- Figure 73: Seres Therapeutics, Inc.: Overall Financials, $Million, 2019-2021
- Figure 74: Seres Therapeutics, Inc.: R&D Expenditure, $Million, 2019-2021
- Figure 75: Microbiotica: Product Portfolio
- Figure 76: Enterome: Product Portfolio
- Figure 77: Destiny Pharma plc: Product Portfolio
- Figure 78: Destiny Pharma plc: Overall Financials, $Million, 2019-2021
- Figure 79: Destiny Pharma plc: R&D Expenditure, $Million, 2019-2021
- Figure 80: Taisho Pharmaceutical Holdings: Product Portfolio
- Figure 81: Taisho Pharmaceutical Holdings: Overall Financials, $Million, 2019-2021
- Figure 82: Taisho Pharmaceutical Holdings: Net Revenue (by Segment), $Million, 2019-2021
- Figure 83: Taisho Pharmaceutical Holdings: Net Revenue (by Region), $Million, 2019-2021
- Figure 84: Taisho Pharmaceutical Holdings: R&D Expenditure, $Million, 2019-2021
- Figure 85: AOBiome Therapeutics, Inc.: Product Portfolio
- Figure 86: Finch Therapeutics Group, Inc.: Product Portfolio
- Figure 87: Finch Therapeutics Group, Inc.: Overall Financials, $Million, 2019-2021
- Figure 88: Finch Therapeutics Group, Inc.: R&D Expenditure, $Million, 2020-2021
- Figure 89: Rebiotix Inc.: Product Portfolio
- Figure 90: MaaT Pharma: Product Portfolio
- Figure 91: MaaT Pharma: Overall Financials, $Million, 2020-2021
- Figure 92: MaaT Pharma: R&D Expenditure, $Million, 2020-2021
- Figure 93: Vedanta Biosciences Inc.: Product Portfolio
- Figure 94: OxThera AB: Product Portfolio
- Figure 95: Pendulum Therapeutics: Product Portfolio
- Figure 96: Caelus Health: Product Portfolio
- Figure 97: Quorum Innovations: Product Portfolio
- Figure 98: Sanofi S.A.: Product Portfolio
- Figure 99: Sanofi S.A.: Overall Financials, $Million, 2019-2021
- Figure 100: Sanofi S.A.: Net Revenue (by Segment), $Million, 2019-2021
- Figure 101: Sanofi S.A.: Net Revenue by Region), $Million, 2019-2021
- Figure 102: Sanofi S.A.: R&D Expenditure, $Million, 2019-2021
- Figure 103: DermBiont, Inc.: Product Portfolio
- Figure 104: EnteroBiotix Ltd: Product Portfolio
- Figure 105: YSOPIA Bioscience: Product Portfolio
- Figure 106: Winclove Probiotics: Product Portfolio
- Figure 107: TargEDys: Product Portfolio
- Figure 108: Evelo Biosciences, Inc.: Product Portfolio
- Figure 109: Evelo Biosciences, Inc.: Pipeline Products
- Figure 110: Evelo Biosciences, Inc.: Overall Financials, $Million, 2019-2021
- Figure 111: Evelo Biosciences, Inc.: R&D Expenditure, $Million, 2019-2021
- Figure 112: BiomX: Product Portfolio
- Figure 113: BiomX: Pipeline Products
- Figure 114: BiomX: Overall Financials, $Million, 2019-2021
- Figure 115: BiomX: R&D Expenditure, $Million, 2019-2021
- Figure 116: Biomica Ltd.: Pipeline Product
- Figure 117: Scioto Biosciences, Inc.: Product Portfolio
- Figure 118: Scioto Biosciences, Inc.: Pipeline Product
- Figure 119: Lactobio A/S: Product Portfolio
- Figure 120: Lactobio A/S: Pipeline Product
List of Tables
- Table 1: Global Microbiome Therapeutics Market: Key Players Patent Portfolio
- Table 2: Patent Expiration Analysis
- Table 3: Partnership Landscaping
- Table 4: Government Initiatives in Microbiome Therapeutics
- Table 5: Impact of COVID-19 on Microbiome Therapeutics Developing Companies
- Table 6: Key Players/Investors Portfolio
- Table 7: Emerging Microbiome Therapeutics Products Pipeline
- Table 8: Probable Potential First Entrants to Global Microbiome Therapeutics Market
- Table 9: 4D pharma plc: Key Competitors
- Table 10: Seres Therapeutics, Inc.: Key Competitors
- Table 11: Microbiotica: Key Competitors
- Table 12: Enterome: Key Competitors
- Table 13: Destiny Pharma plc: Key Competitors
- Table 14: Taisho Pharmaceutical Holdings: Key Competitors
- Table 15: AOBiome Therapeutics, Inc.: Key Competitors
- Table 16: Finch Therapeutics Group, Inc.: Key Competitors
- Table 17: Rebiotix Inc.: Key Competitors
- Table 18: MaaT Pharma: Key Competitors
- Table 19: Vedanta Biosciences Inc.: Key Competitors
- Table 20: Pendulum Therapeutics: Key Competitors
- Table 21: Caelus Health: Key Competitors
- Table 22: Quorum Innovations: Key Competitors
- Table 23: Sanofi S.A.: Key Competitors
- Table 24: DermBiont Inc.: Key Competitors
- Table 25: EnteroBiotix Ltd: Key Competitors
- Table 26: YSOPIA Bioscience: Key Competitors
- Table 27: Winclove Probiotics: Key Competitors
- Table 28: TargEDys: Key Competitors
- Table 29: Evelo Biosciences, Inc.: Key Competitors
- Table 30: BiomX: Key Competitors
- Table 31: Biomica Ltd.: Key Competitors
- Table 32: Scioto Biosciences, Inc.: Key Competitors
- Table 33: Lactobio A/S: Key Competitors
“Global Microbiome Therapeutics Market to Reach $3,204.0 Million by 2032.”
Market Overview
Microbiome therapeutics have drawn significant attention from the medical community with their potential to address the existing unmet need of various clinical conditions. The global microbiome therapeutics market which accounted for $306.3 million in 2021 is expected to reach $3,204 million by 2032, reporting a CAGR of 24.95% during the forecast period 2022-2032.
The existing microbiome therapeutics market is favored by multiple factors, including the rising geriatric populations coupled with the increasing adoption of inorganic growth strategies by key players in the market.
Impact of COVID-19
There were numerous consequences due to the COVID-19 pandemic, especially because of the reduced access to care for other illnesses. As the number of individuals becoming ill from COVID-19 kept increasing and for the protection of healthy individuals from being affected by the disease, all non-urgent healthcare facilities were suspended as per respective government directives. Even though these measures were necessary, it had mostly negative impact on the Global microbiome therapeutics market. Some the major impacts were; interruption of key clinical trial activities, modification of clinical trial guidelines bringing in additional safety measures, and delay in regulatory agencies' review and approval timeline.
Market Segmentation:
Within the research report, the market is segmented on the basis of:
- target therapy area (gastrointestinal and infectious diseases, skin disorders, cancer indications, and other indications)
- region (North America, Europe, Asia-Pacific, and Rest-of-the-World).
This segmentation highlights value propositions and business models useful for industry leaders and stakeholders. The research also comprises country-level analysis, go-to-market strategies of leading players, and future opportunities, among others, to detail the scope and provide a 360-coverage of the domain.
Segmentation 1: by Target Therapy Area
Disorders related to gastrointestinal (GI) and skin disorders command a major share of the microbiome therapeutics segment, and is expected to continue during the forecast period. Most of the microbiome therapeutics in advanced development stage are indicated to treat C.diff. infection (CDI) and expected to enter the market first. The indications selected were on the basis of research intensity in the specific therapy area.
Segmentation 2: by Region
Asia-Pacific (APAC) is expected to dominate the global microbiome therapeutics market for the forecast period 2022-2032 continuuing with it overall contribution of approximately 40% of the total market size in 2021 followed by Europe with 32.59%. Increasing healthcare expenditure, coupled with government initiatives, are among the leading factors contributing to the growth of the market.
Demand - Drivers and Challenges
Some of the potential drivers identified by BIS Research includes:
- Growing Strategic Activities in Microbiome Therapeutics Segment
- Potential of Microbiome Therapeutics to Address Unmet Needs of Existing Treatment Options
- Microbiome Therapeutic Products as a Safer Alternative to Conventional Drug Treatments
There are some challenges identified for the global microbiome therapeutics market are; Lack of standard regulatory guidelines and safety issues associated with live biotherapeutic products (LBPs).
How Can This Report Add Value to an Organization?
- Product/Innovation Strategy: The report provides an exhaustive list of pipeline products which are currently in the development along with their developmental details in various indications. This can help organizations in understanding the research activities, which is the promising indication to pursue or which organization has the potential product to enter first into the market as well it can help the organization to assess where should they place themselves in order to get maximum benefit.
- Growth/Marketing Strategy: In addition to pursuing research on exploring the benefits of microbiome therapeutics for new indications, companies are evaluating next-generation-probiotics (NGPs) to address clinical conditions. Looking at the storage issues of microbiome therapeutics, companies are exploring extracellular vesicles as an alternative option. Furthermore, companies are working on increasing consumer awareness about Microbiome therapeutics and its potential benefits of being natural product containing live microorganism derived from human body as well as pursuing synergistic activities to bring an effective microbiome therapeutics for the affected population.
- Competitive Strategy: Key players in the global microbiome therapeutics market were analyzed and profiled in the study, who are developing products containing live biotherapeutic products (LBPs), fecal microbiota transplantation (FMT) as well as next-generation-probiotics (NGPs). Moreover, a detailed competitive benchmarking of the players operating in the global microbiome therapeutics market has been done to help the reader understand how players stand against each other, presenting a clear market landscape. Additionally, comprehensive competitive strategies such as partnerships, agreements, and collaborations will aid the reader in understanding the untapped revenue pockets in the market.
Key Market Players and Competition Synopsis
The companies that are profiled have been selected based on inputs gathered from primary experts and analyzing company coverage, product portfolio, and market penetration.
The top segment players leading the market include Sanofi S.A and Taisho Pharmaceutical Holding that capture around 88% of the market with Sanofi S.A holding 64.31% while Taisho Pharmaceutical Holdings 24.49% respectively.
Some of the prominent established names in this market are:
|
|
Table of Contents
1 Market Definition
- 1.1 Inclusion and Exclusion Criteria
2 Research Scope
- 2.1 Target Audience
- 2.2 Key Questions Answered in the Report
3 Research Methodology
- 3.1 Global Microbiome Therapeutics: Research Methodology
- 3.2 Primary Data Sources
- 3.3 Secondary Data Sources
- 3.4 Market Estimation Model
- 3.5 Criteria for Company Profiling
4 Market Overview
- 4.1 Market Size and Future Growth Potential
- 4.2 Historical Trends
5 Industry Insights
- 5.1 Regulatory Landscape
- 5.1.1 Regulatory Requirements for Live Biotherapeutic Products (LBPs)
- 5.1.2 Quality Check of LBPs
- 5.1.3 FDA Approved Fecal Microbiota Transplantation (FMT) Quality Check Procedure
- 5.2 Patent Landscape
- 5.2.1 Patent Filing Analysis
- 5.2.2 Key Players Patent Portfolio
- 5.2.3 Key Players Patent Expiration Analysis
- 5.3 Partnership Landscape
- 5.4 Government Initiatives
- 5.5 Impact of COVID-19 on the Global Microbiome Therapeutics Market
- 5.5.1 Clinical Trial Disruptions and Resumptions
6 Market Dynamics
- 6.1 Market Drivers
- 6.1.1 Growing Strategic Activities in Microbiome Therapeutics Segment
- 6.1.2 Potential of Microbiome Therapeutics to Address Unmet Needs of Existing Treatment Options
- 6.1.3 Microbiome Therapeutic Products as a Safer Alternative to Conventional Drug Treatments
- 6.2 Market Restraints
- 6.2.1 Lack of Uniformed Standardized Regulatory Guidelines
- 6.2.2 Associated Safety Issues with LBPs
- 6.2.3 Storage Issues with Live Micro-Organisms
- 6.2.4 Challenges Associated with Manufacturing of LBPs
- 6.3 Market Opportunities
- 6.3.1 Need for Current Good Manufacturing Practice (cGMP) Certified Contract Manufacturing Facilities
- 6.3.2 Growing Disease Dimension
- 6.3.3 Entry of Major Players
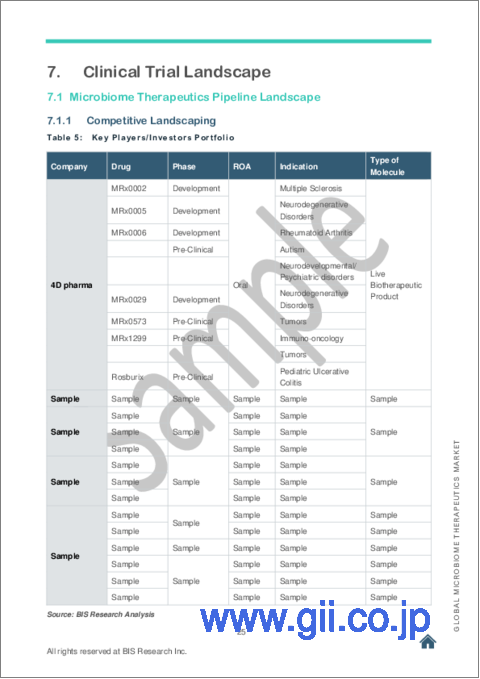
7 Clinical Trial Landscape
- 7.1 Microbiome Therapeutics Pipeline Landscape
- 7.1.1 Competitive Landscaping
- 7.1.2 Pipeline Analysis
- 7.1.2.1 By Development Phase
- 7.1.2.2 By Route of Administration
- 7.1.2.3 By Type of Microbiome Strategy
- 7.1.2.4 By Indication
- 7.1.3 Emerging Microbiome Therapeutics Products
- 7.2 Probable Potential First Entrants to Global Microbiome Therapeutics Market/ Microbiome Therapeutics Clinical Trial Design
- 7.2.1 CP-101 (Finch Therapeutics Group, Inc.)
- 7.2.1.1 CP-101: Product Profile
- 7.2.2 SER-109 (Seres Therapeutics, Inc.)
- 7.2.2.1 SER-109: Product Profile
- 7.2.3 RBX-2660 (Rebiotix Inc.)
- 7.2.3.1 RBX-2660: Product Profile
- 7.2.4 CH-106 (Caelus Health)
- 7.2.4.1 CH-106: Product Profile
- 7.2.5 LACTIN-V (Osel Inc.)
- 7.2.5.1 LACTIN-V: Product Profile
- 7.2.6 MaaT013 (MaaT Pharma)
- 7.2.6.1 MaaT013: Product Profile
- 7.2.1 CP-101 (Finch Therapeutics Group, Inc.)
8 Competitive Insights
- 8.1 Key Strategies and Developments
- 8.2 Synergistic Activities
- 8.3 Product Launch and Regulatory Approvals
- 8.4 Funding and Investment
- 8.5 Mergers and Acquisitions
- 8.6 Market Share Analysis
- 8.7 Growth-Share Analysis (Opportunity Mapping)
- 8.7.1 By Region
- 8.7.2 By Company
9 Global Microbiome Therapeutics Market (by Target Therapy Area)
- 9.1 Overview
- 9.2 Gastrointestinal and Infectious Diseases
- 9.3 Skin Disorders
- 9.4 Cancer Indications
- 9.5 Other Indications
10 Global Microbiome Therapeutics Market (by Region)
- 10.1 Overview
- 10.2 North America
- 10.2.1 Overview
- 10.2.2 U.S.
- 10.2.3 Canada
- 10.3 Europe
- 10.3.1 Overview
- 10.3.2 France
- 10.3.3 Germany
- 10.3.4 Sweden
- 10.3.5 U.K.
- 10.3.6 Italy
- 10.3.7 Spain
- 10.3.8 Rest-of-Europe
- 10.4 Asia-Pacific (APAC)
- 10.4.1 Overview
- 10.4.2 Japan
- 10.4.3 Australia
- 10.4.4 China
- 10.4.5 India
- 10.4.6 Rest-of-Asia-Pacific
- 10.5 Rest-of-the-World
- 10.5.1 Overview
11 Competitive Benchmarking and Company Profiles
- 11.1 4D pharma plc
- 11.1.1 Company Overview
- 11.1.2 Role of 4D pharma plc in the Global Microbiome Therapeutics Market
- 11.1.3 Key Competitors of the Company
- 11.1.4 Financials
- 11.1.5 Recent Developments
- 11.1.6 Analyst's Perspective
- 11.2 Seres Therapeutics, Inc.
- 11.2.1 Company Overview
- 11.2.2 Role of Seres Therapeutics, Inc. in the Global Microbiome Therapeutics Market
- 11.2.3 Key Competitors of the Company
- 11.2.4 Financials
- 11.2.5 Recent Developments
- 11.2.6 Analyst Perspective
- 11.3 Microbiotica
- 11.3.1 Company Overview
- 11.3.2 Role of Microbiotica in the Global Microbiome Therapeutics Market
- 11.3.3 Key Competitors of the Company
- 11.3.4 Recent Developments
- 11.3.5 Analyst's Perspective
- 11.4 Enterome
- 11.4.1 Company Overview
- 11.4.2 Role of Enterome in the Global Microbiome Therapeutics Market
- 11.4.3 Key Competitors of the Company
- 11.4.4 Recent Developments
- 11.4.5 Analyst's Perspective
- 11.5 Destiny Pharma plc
- 11.5.1 Company Overview
- 11.5.2 Role of Destiny Pharma plc in the Global Microbiome Therapeutics Market
- 11.5.3 Key Competitors of the Company
- 11.5.4 Financials
- 11.5.5 Recent Developments
- 11.5.6 Analyst's Perspective
- 11.6 Taisho Pharmaceutical Holdings
- 11.6.1 Company Overview
- 11.6.2 Role of Taisho Pharmaceutical Holdings in the Global Microbiome Therapeutics Market
- 11.6.3 Key Competitors of the Company
- 11.6.4 Financials
- 11.6.5 Recent Developments
- 11.6.6 Analyst's Perspective
- 11.7 AOBiome Therapeutics, Inc.
- 11.7.1 Company Overview
- 11.7.2 Role of AOBiome Therapeutics, Inc. in the Global Microbiome Therapeutics Market
- 11.7.3 Key Competitors of the Company
- 11.7.4 Recent Developments
- 11.7.5 Analyst's Perspective
- 11.8 Finch Therapeutics Group, Inc.
- 11.8.1 Company Overview
- 11.8.2 Role of Finch Therapeutics Group, Inc. in the Global Microbiome Therapeutics Market
- 11.8.3 Key Competitors of the Company
- 11.8.4 Financials
- 11.8.5 Recent Developments
- 11.8.6 Analyst's Perspective
- 11.9 Ferring Pharmaceuticals
- 11.9.1 Company Overview
- 11.1 Rebiotix Inc. (A Subsidiary of Ferring Pharmaceuticals)
- 11.10.1 Company Overview
- 11.10.2 Role of Rebiotix Inc. in the Global Microbiome Therapeutics Market
- 11.10.3 Key Competitors of the Company
- 11.10.4 Recent Developments
- 11.10.5 Analyst's Perspective
- 11.11 MaaT Pharma
- 11.11.1 Company Overview
- 11.11.2 Role of MaaT Pharma in the Global Microbiome Therapeutics Market
- 11.11.3 Key Competitors of the Company
- 11.11.4 Financials
- 11.11.5 Recent Developments
- 11.11.6 Analyst's Perspective
- 11.12 Vedanta Biosciences Inc.
- 11.12.1 Company Overview
- 11.12.2 Role of Vedanta Biosciences Inc. in the Global Microbiome Therapeutics Market
- 11.12.3 Key Competitors of the Company
- 11.12.4 Recent Developments
- 11.12.5 Analyst's Perspective
- 11.13 OxThera AB
- 11.13.1 Company Overview
- 11.13.2 Role of OxThera AB in the Global Microbiome Therapeutics Market
- 11.13.3 Key Competitors of the Company
- 11.13.4 Recent Developments
- 11.13.5 Analyst's Perspective
- 11.14 Pendulum Therapeutics
- 11.14.1 Company Overview
- 11.14.2 Role of Pendulum Therapeutics in the Global Microbiome Therapeutics Market
- 11.14.3 Key Competitors of the Company
- 11.14.4 Recent Developments
- 11.14.5 Analyst's Perspective
- 11.15 Caelus Health
- 11.15.1 Company Overview
- 11.15.2 Role of Caelus Health in the Global Microbiome Therapeutics Market
- 11.15.3 Key Competitors of the Company
- 11.15.4 Recent Developments
- 11.15.5 Analyst's Perspective
- 11.16 Quorum Innovations
- 11.16.1 Company Overview
- 11.16.2 Role of Quorum Innovations in the Global Microbiome Therapeutics Market
- 11.16.3 Key Competitors of the Company
- 11.16.4 Recent Developments
- 11.16.5 Analyst's Perspective
- 11.17 Sanofi S.A.
- 11.17.1 Company Overview
- 11.17.2 Role of Sanofi S.A. in the Global Microbiome Therapeutics Market
- 11.17.3 Key Competitors of the Company
- 11.17.4 Financials
- 11.17.5 Recent Developments
- 11.17.6 Analyst's Perspective
- 11.18 DermBiont, Inc.
- 11.18.1 Company Overview
- 11.18.2 Role of DermBiont, Inc. in the Global Microbiome Therapeutics Market
- 11.18.3 Key Competitors of the Company
- 11.18.4 Recent Developments
- 11.18.5 Analyst's Perspective
- 11.19 EnteroBiotix Ltd
- 11.19.1 Company Overview
- 11.19.2 Role of EnteroBiotix Ltd in the Global Microbiome Therapeutics Market
- 11.19.3 Key Competitors of the Company
- 11.19.4 Recent Developments
- 11.19.5 Analyst's Perspective
- 11.2 YSOPIA Bioscience
- 11.20.1 Company Overview
- 11.20.2 Role of YSOPIA Bioscience in the Global Microbiome Therapeutics Market
- 11.20.3 Key Competitors of the Company
- 11.20.4 Recent Developments
- 11.20.5 Analyst's Perspective
- 11.21 Winclove Probiotics
- 11.21.1 Company Overview
- 11.21.2 Role of Winclove Probiotics in the Global Microbiome Therapeutics Market
- 11.21.3 Key Competitors of the Company
- 11.21.4 Recent Developments
- 11.21.5 Analyst's Perspective
- 11.22 TargEDys
- 11.22.1 Company Overview
- 11.22.2 Role of TargEDys in the Global Microbiome Therapeutics Market
- 11.22.3 Key Competitors of the Company
- 11.22.4 Recent Developments
- 11.22.5 Analyst's Perspective
- 11.23 Evelo Biosciences, Inc.
- 11.23.1 Company Overview
- 11.23.2 Role of Evelo Biosciences, Inc. in the Global Microbiome Therapeutics Market
- 11.23.3 Key Competitors of the Company
- 11.23.4 Financials
- 11.23.5 Recent Developments
- 11.23.6 Analyst's Perspective
- 11.24 BiomX
- 11.24.1 Company Overview
- 11.24.2 Role of BiomX in the Global Microbiome Therapeutics Market
- 11.24.3 Key Competitors of the Company
- 11.24.4 Financials
- 11.24.5 Recent Developments
- 11.24.6 Analyst's Perspective
- 11.25 Biomica Ltd.
- 11.25.1 Company Overview
- 11.25.2 Role of Biomica Ltd. in the Global Microbiome Therapeutics Market
- 11.25.3 Key Competitors of the Company
- 11.25.4 Recent Developments
- 11.25.5 Analyst's Perspective
- 11.26 Scioto Biosciences, Inc.
- 11.26.1 Company Overview
- 11.26.2 Role of Scioto Biosciences, Inc. in the Global Microbiome Therapeutics Market
- 11.26.3 Key Competitors of the Company
- 11.26.4 Recent Developments
- 11.26.5 Analyst's Perspective
- 11.27 Lactobio A/S
- 11.27.1 Company Overview
- 11.27.2 Role of Lactobio A/S in the Global Microbiome Therapeutics Market
- 11.27.3 Key Competitors of the Company
- 11.27.4 Recent Developments
- 11.27.5 Analyst's Perspective