|
|
市場調査レポート
商品コード
1632489
アジア太平洋の細胞および遺伝子治療製造QC市場: 分析と予測(2024年~2033年)Asia-Pacific Cell and Gene Therapy Manufacturing QC Market: Analysis and Forecast, 2024-2033 |
||||||
カスタマイズ可能
|
|||||||
| アジア太平洋の細胞および遺伝子治療製造QC市場: 分析と予測(2024年~2033年) |
|
出版日: 2025年01月15日
発行: BIS Research
ページ情報: 英文 142 Pages
納期: 1~5営業日
|
全表示
- 概要
- 図表
- 目次
アジア太平洋の細胞および遺伝子治療製造QCの市場規模は、2023年に2億4,640万米ドルとなりました。
同市場は、2024年~2033年の予測期間中に18.36%のCAGRで拡大し、2033年までに13億1,730万米ドルに達すると予測されています。同市場は、CAR T細胞などの革新的な治療法の採用拡大が主な要因となって、目覚ましい2桁成長を遂げています。細胞や遺伝子を用いた治療の安全性、有効性、一貫性を確保するためには、強固な品質管理プロセスが不可欠です。同市場は、製造過程における厳格な品質管理措置の必要性、製造技術の進歩、規制枠組みの拡大によって牽引されています。市場機会を活用するため、業界各社は革新的なQC技術の開発、堅牢な分析法の確立、規制機関や学術機関との連携に注力しています。規制要件を満たし、このダイナミックで変革の激しい市場で成功するためには、メーカーが包括的なQC戦略を実施することが極めて重要です。
| 主要市場統計 | |
|---|---|
| 予測期間 | 2024年~2033年 |
| 2024年の評価 | 2億8,890万米ドル |
| 2033年の予測 | 13億1,730万米ドル |
| CAGR | 18.36% |
アジア太平洋の細胞および遺伝子治療製造QC市場は、がん、代謝性疾患、自己免疫疾患などの対象疾患の有病率の上昇に牽引され、顕著な成長を遂げています。さらに、細胞・遺伝子治療の承認が増加していることも、市場拡大に寄与しています。さらに、細胞・遺伝子治療分野への新規参入や投資が一貫して増加しており、これがアジア太平洋の細胞および遺伝子治療製造QC市場を強化しているため、これらの治療法の製造に必要な製品やサービス・ソリューションに対する需要が急増しています。
Danaher Corporation(Cytiva)、F. Hoffmann-La Roche Ltd、THERMO FISHER SCIENTIFIC INC.などの主要企業が牽引するアジア太平洋の細胞および遺伝子治療製造QC市場は、細胞・遺伝子治療製造の展望を一変させました。これらの業界リーダーは、細胞・遺伝子治療製造における安全性、有効性、コンプライアンスの確保という複雑な課題に対処するため、革新的なQCソリューションを導入しています。
研究開発投資と臨床試験の増加に支えられたバイオ医薬品イノベーションの急成長により、この地域では高度なQCメカニズムに対する需要が高まっています。自動化、リアルタイムモニタリング、統合分析により、ワークフローが合理化され、コストが削減され、これらの企業の技術が効率性の新たなベンチマークとなっています。
当レポートでは、アジア太平洋の細胞および遺伝子治療製造QC市場について調査し、市場の概要とともに、オファリング別、治療タイプ別、プロセス別、技術別、用途別、地域別の動向、および市場に参入する企業のプロファイルなどを提供しています。
目次
第1章 市場
第2章 細胞および遺伝子治療製造QC市場:規制枠組み
- 食品医薬品局(FDA)による化学、製造、管理(CMC)要件
- 欧州医薬品庁(EMA)による細胞および遺伝子治療製品の品質側面
- 現行適正製造規範(CGMP)規制
- 世界規制枠組み:細胞および遺伝子治療製造QC市場
第3章 アジア太平洋の細胞および遺伝子治療製造QC市場:市場概要
- 市場概要
- 市場の足跡と成長の可能性
- 将来の可能性
- COVID-19による市場への影響
- 市場力学
- 市場規模と予測
- アジア太平洋の細胞および遺伝子治療製造QC市場(オファリング別)
- アジア太平洋の細胞および遺伝子治療製造QC市場(治療タイプ別)
- アジア太平洋の細胞および遺伝子治療製造QC市場(プロセス別)
- アジア太平洋の細胞および遺伝子治療製造QC市場(技術別)
- アジア太平洋の細胞および遺伝子治療製造QC市場(用途別)
第4章 アジア太平洋の細胞および遺伝子治療製造QC市場:国別
- シンガポール
- 日本
- 韓国
- オーストラリア
- インド
- タイ
- マレーシア
- インドネシア
- その他
第5章 企業プロファイル
- Bio-Techne Corporation
- Danaher Corporation(Cytiva)
- F. Hoffmann-La Roche Ltd
- Lonza
- Miltenyi Biotec B.V. & Co. KG
- Sartorius AG
- Thermo Fisher Scientific Inc.
- WuXi AppTec
- Fujifilm Holdings Corporation
- Merck KGaA
List of Figures
- Figure 1: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market, $Million, 2023-2033
- Figure 2: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market, Impact Analysis
- Figure 3: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), % Share, 2023 and 2033
- Figure 4: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Offering), % Share, 2023 and 2033
- Figure 5: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Process), % Share, 2023 and 2033
- Figure 6: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Technology), % Share, 2023 and 2033
- Figure 7: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Application), % Share, 2023 and 2033
- Figure 8: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market Segmentation
- Figure 9: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market: Research Methodology
- Figure 10: Primary Research Methodology
- Figure 11: Bottom-Up Approach (Segment-Wise Analysis)
- Figure 12: Top-Down Approach (Segment-Wise Analysis)
- Figure 13: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market Size and Growth Potential (Realistic Scenario), $Million, 2023-2033
- Figure 14: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market Size and Growth Potential (Optimistic Scenario), $Million, 2023-2033
- Figure 15: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market Size and Growth Potential (Pessimistic Scenario), $Million, 2023-2033
- Figure 16: Impact of COVID-19 on CGT Developmental Activities
- Figure 17: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market, $Million, 2023-2033
- Figure 18: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2023-2033
- Figure 19: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2023-2033
- Figure 20: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2023-2033
- Figure 21: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2023-2033
- Figure 22: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2023-2033
- Figure 23: Singapore Cell and Gene Therapy Manufacturing QC Market, $Million, 2023-2033
- Figure 24: Singapore Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2023-2033
- Figure 25: Singapore Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2023-2033
- Figure 26: Singapore Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2023-2033
- Figure 27: Singapore Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2023-2033
- Figure 28: Singapore Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2023-2033
- Figure 29: Japan Cell and Gene Therapy Manufacturing QC Market, $Million, 2023-2033
- Figure 30: Japan Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2023-2033
- Figure 31: Japan Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2023-2033
- Figure 32: Japan Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2023-2033
- Figure 33: Japan Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2023-2033
- Figure 34: Japan Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2023-2033
- Figure 35: South Korea Cell and Gene Therapy Manufacturing QC Market, $Million, 2023-2033
- Figure 36: South Korea Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2023-2033
- Figure 37: South Korea Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2023-2033
- Figure 38: South Korea Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2023-2033
- Figure 39: South Korea Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2023-2033
- Figure 40: South Korea Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2023-2033
- Figure 41: Australia Cell and Gene Therapy Manufacturing QC Market, $Million, 2023-2033
- Figure 42: Australia Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2023-2033
- Figure 43: Australia Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2023-2033
- Figure 44: Australia Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2023-2033
- Figure 45: Australia Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2023-2033
- Figure 46: Australia Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2023-2033
- Figure 47: India Cell and Gene Therapy Manufacturing QC Market, $Million, 2023-2033
- Figure 48: India Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2023-2033
- Figure 49: India Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2023-2033
- Figure 50: India Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2023-2033
- Figure 51: India Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2023-2033
- Figure 52: India Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2023-2033
- Figure 53: Thailand Cell and Gene Therapy Manufacturing QC Market, $Million, 2023-2033
- Figure 54: Thailand Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2023-2033
- Figure 55: Thailand Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2023-2033
- Figure 56: Thailand Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2023-2033
- Figure 57: Thailand Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2023-2033
- Figure 58: Thailand Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2023-2033
- Figure 59: Malaysia Cell and Gene Therapy Manufacturing QC Market, $Million, 2023-2033
- Figure 60: Malaysia Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2023-2033
- Figure 61: Malaysia Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2023-2033
- Figure 62: Malaysia Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2023-2033
- Figure 63: Malaysia Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2023-2033
- Figure 64: Malaysia Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2023-2033
- Figure 65: Indonesia Cell and Gene Therapy Manufacturing QC Market, $Million, 2023-2033
- Figure 66: Indonesia Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2023-2033
- Figure 67: Indonesia Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2023-2033
- Figure 68: Indonesia Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2023-2033u
- Figure 69: Indonesia Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2023-2033
- Figure 70: Indonesia Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2023-2033
- Figure 71: Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market, $Million, 2023-2033
- Figure 72: Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Offering), $Million, 2023-2033
- Figure 73: Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Therapy Type), $Million, 2023-2033
- Figure 74: Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Process), $Million, 2023-2033
- Figure 75: Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Technology), $Million, 2023-2033
- Figure 76: Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Application), $Million, 2023-2033
- Figure 77: Bio-Techne Corporation: Overall Financials, $Million, 2021-2023
- Figure 78: Danaher Corporation (Cytiva): Overall Financials, $Million, 2021-2023
- Figure 79: F. Hoffmann-La Roche Ltd: Overall Financials, $Million, 2021-2023
- Figure 80: Lonza: Overall Financials, $Million, 2021-2023
- Figure 81: Sartorius AG: Overall Financials, $Million, 2021-2023
- Figure 82: Thermo Fisher Scientific Inc.: Overall Financials, $Million, 2021-2023
- Figure 83: WuXi AppTec: Overall Financials, $Million, 2021-2023
- Figure 84: Fujifilm Holdings Corporation: Overall Financials, $Million, 2021-2023
- Figure 85: Merck KGaA: Overall Financials, $Million, 2021-2023
An Introduction to Asia-Pacific Cell and Gene Therapy Manufacturing QC
The Asia-Pacific cell and gene therapy manufacturing QC market was valued at $246.4 million in 2023 and is anticipated to reach $1,317.3 million by 2033, witnessing a CAGR of 18.36% during the forecast period 2024-2033. The given figure illustrates the market revenue of the Asia-Pacific cell and gene therapy manufacturing QC market from 2023-2033. The market has been witnessing impressive double-digit growth, largely driven by the increasing adoption of innovative therapies such as CAR T-cells and others. Robust quality control processes are essential to ensure cell and gene-based treatments' safety, efficacy, and consistency. The market is driven by the need for stringent QC measures throughout the manufacturing journey, advancements in manufacturing technologies, and expanding regulatory frameworks. To capitalize on market opportunities, industry players are focusing on developing innovative QC technologies, establishing robust analytical methods, and collaborating with regulatory bodies and academic institutions. It is crucial for manufacturers to implement comprehensive QC strategies to meet regulatory requirements and position themselves for success in this dynamic and transformative market.
Market Introduction
| KEY MARKET STATISTICS | |
|---|---|
| Forecast Period | 2024 - 2033 |
| 2024 Evaluation | $288.9 Million |
| 2033 Forecast | $1,317.3 Million |
| CAGR | 18.36% |
The Asia-Pacific cell and gene therapy manufacturing QC market has been experiencing notable growth, driven by the escalating prevalence of target diseases such as cancers, metabolic disorders, autoimmune disorders, and others. Additionally, the increasing approval of cell and gene therapies has further contributed to this market expansion. Furthermore, there has been a consistent rise in the number of new entrants and investments in the field of cell and gene therapy, which has strengthened the Asia-Pacific cell and gene therapy manufacturing QC market, thereby providing a surge in demand for the products and services solutions required in the manufacturing of these therapies. For instance, as per an article titled 'Cell & Gene Therapy in the Asia Pacific: Revolutionizing Disease Treatment,' published in 2023, South Korea has established a $1.3 billion fund to support the development of cell and gene therapies. Further, Singapore has invested in several initiatives to support the development of cell and gene therapies, including the establishment of the Singapore Consortium for Synthetic Biology and the Bioprocessing Technology Institute. Therefore, the impact of the aforementioned factors is expected to drive the Asia-Pacific cell and gene therapy manufacturing QC market in the forecast period 2024-2033.
Industrial Impact
The Asia-Pacific cell and gene therapy manufacturing QC market, driven by leading companies such as Danaher Corporation (Cytiva), F. Hoffmann-La Roche Ltd, and THERMO FISHER SCIENTIFIC INC., has transformed the landscape of cell and gene therapy manufacturing. These industry leaders have introduced innovative QC solutions to address the complexities of ensuring safety, efficacy, and compliance in cell and gene therapy production.
The region's rapid growth in biopharmaceutical innovation, supported by increased R&D investments and clinical trials, has created a robust demand for advanced QC mechanisms. Automation, real-time monitoring, and integrated analytics have streamlined workflows and reduced costs, with technologies from these companies setting new benchmarks for efficiency.
The region's stringent regulatory environment and expanding manufacturing capacities further drive the adoption of scalable and precise QC technologies. With a focus on tailored solutions for diverse regional needs, these advancements are accelerating time-to-market for therapies, enhancing product integrity, and reinforcing regulatory compliance. As the market evolves, trends such as AI-driven predictive analytics and real-time release testing will continue to shape its trajectory in cell and gene therapy manufacturing.
Market Segmentation:
Segmentation 1: by Offering
- Products
- Services
Services Segment to Dominate the Asia-Pacific Cell and Gene Therapy Manufacturing QC market (by Offering)
Based on offering, the Asia-Pacific cell and gene therapy manufacturing QC market was led by the services segment, which held a 70.28% share in 2023. The high growth of the services segment can be attributed to the increase in outsourcing manufacturing and QC operations to CDMOs and CROs.
Segmentation 2: by Therapy Type
- Cell Therapy
- Gene Therapy
Cell Therapy Segment to Dominate the Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
Based on therapy type, the cell therapy segment accounted for the largest share of 58.42% in the Asia-Pacific cell and gene therapy manufacturing QC market in 2023. In addition, cell therapy is expected to witness the highest growth rate of 19.47% in the forecast period 2024-2033. The growth of the cell therapy segment can be attributed to the ongoing advancements and increasing regulatory approvals for CAR-T cell therapies.
Segmentation 3: by Process
- Raw Materials Preparation
- Upstream Processing
- Downstream Processing
- Packaging
Upstream Processing Segment to Dominate the Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Process)
Based on process, the upstream processing segment accounted for the largest share of 47.10% in the Asia-Pacific cell and gene therapy manufacturing QC market in 2023. Moreover, upstream processing is expected to hold the highest growth rate of 19.45% in the forecast period 2024-2033. This can be attributed to the shift toward the usage of single-use bioreactors for cell and gene therapy manufacturing, as these systems reduce contamination risks and allow for streamlined QC protocols.
Segmentation 4: by Technology
- Polymerase Chain Reaction
- Flow Cytometry
- Limulus Amebocyte Lysate (LAL)
- Enzyme-Linked Immunosorbent Assay (ELISA)
- Chromatography
- Mass Spectrometry
- Western Blotting
- Next-Generation Sequencing
- Electrophoresis
- Other Technologies
PCR Segment to Dominate the Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Technology)
Based on technology, the PCR segment accounted for the largest share of 23.98% in the Asia-Pacific cell and gene therapy manufacturing QC market in 2023. However, the next-generation sequencing segment is expected to hold the highest growth rate of 22.68% in the forecast period 2024-2033. The high growth of the next-generation sequencing segment can be attributed to the ability of NGS to provide detailed genetic profiles with high sensitivity, allowing for a thorough assessment of cell lines, vector purity, off-target effects in gene editing, and genetic modifications.
Segmentation 5: by Application
- Safety Testing
- Potency Testing
- Identity Testing
- Stability and Genetic Fidelity Testing
- Others
Safety Testing Segment to Dominate the Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Application)
Based on application, the safety testing segment accounted for the largest share of 46.15% in the Asia-Pacific cell and gene therapy manufacturing QC market in 2023. However, the potency testing segment is expected to hold the highest growth rate of 20.28% in the forecast period 2024-2033. The high growth rate of the potency testing segment can be attributed to the continuous developments in potency assays and their essential role in efficacy validation.
Recent Developments in the Asia-Pacific Cell and Gene Therapy Manufacturing QC Market
- In January 2023, Bio-Techne Corporation announced the expansion of its Advanced Cell Diagnostics (ACD)-branded RNAscope in situ hybridization (ISH) portfolio. It launched an RNAscope Plus smRNA-RNA detection assay that enables simultaneous fluorescence detection of small regulatory RNA together with three target RNAs or RNA biomarkers in the same tissue section at single-cell and subcellular resolution.
- In August 2023, Cytiva collaborated with Genepeutic Bio to establish Thailand's first GMP-certified cell therapy manufacturing facility. The collaboration is expected to boost the capabilities of cancer treatment delivery within Thailand and Southeast Asia.
- In April 2023, Cytiva launched X-platform bioreactors, which aim to streamline single-use upstream bioprocessing operations. These versatile bioreactors can be used for producing monoclonal antibodies, protein-based drugs, cell and gene therapies, and viral vectors. They offer flexibility and efficiency in bioprocessing, facilitating the development and manufacturing of various therapeutic products.
- In February 2023, AcuraBio, a contract development and manufacturing organization (CDMO), is expanding its current good manufacturing practice (cGMP) plasmid DNA services using Cytiva's single-use purification technology. This expansion is aimed at addressing supply constraints for mRNA and cell and gene therapies.
- In July 2022, Forecyte Bio collaborated with Cytiva to expedite the development and manufacturing of cell and gene therapies. This collaboration aimed to standardize processes within the CGT CDMO sector and drive industry growth.
Demand - Drivers, Challenges, and Opportunities
Market Drivers:
Increasing Number of Approvals Leading to an Upsurge in Demand for Cell and Gene Therapies QC Testing: The increasing number of regulatory approvals for cell and gene therapies is driving a significant upsurge in demand for quality control (QC) testing in the Asia-Pacific region. As innovative therapies move from clinical trials to commercial availability, ensuring their safety, efficacy, and consistency becomes paramount. Regulatory bodies across major markets such as Japan, Australia, and South Korea have established stringent approval processes, emphasizing robust QC measures. This has necessitated the adoption of advanced testing technologies capable of addressing the complexities of cell and gene therapies, including sterility, potency, identity, and safety evaluations. Key industry players have been responding to this demand by developing automated and scalable QC solutions, enabling faster and more reliable testing workflows. With the rapid expansion of manufacturing capacities and growing patient access to these therapies, the role of QC testing in maintaining compliance and ensuring product quality continues to grow, marking it as a critical factor in the industry's ongoing evolution.
Market Challenges:
Limited Adoption of Cell and Gene Therapy Due to High Manufacturing and QC Costs: The adoption of cell and gene therapies in the Asia-Pacific region is currently limited by the high costs associated with manufacturing and quality control (QC). These therapies involve complex processes, including cell isolation, genetic modification, and precision delivery, which demand highly specialized equipment, skilled personnel, and stringent QC measures. QC testing, in particular, requires advanced technologies to ensure these therapies' safety, efficacy, and consistency, driving up operational costs. This financial burden is further compounded by the lack of standardized manufacturing protocols and economies of scale, as cell and gene therapies are often produced in small batches tailored to individual patients or specific conditions.
Small and medium-sized biopharmaceutical companies face significant challenges in overcoming these cost barriers, which can delay the scaling of innovative treatments. Additionally, the need for comprehensive compliance with stringent regulatory requirements adds to the financial and operational strain. To address these challenges, industry players and governments are increasingly investing in automation, process optimization, and scalable QC solutions to reduce costs. Partnerships and collaborations among stakeholders also aim to share resources and expertise, paving the way for broader adoption and access to these life-saving therapies. However, overcoming these cost-related barriers remains a critical hurdle for the region's widespread implementation of cell and gene therapies.
How can this report add value to an organization?
Product/Innovation Strategy: The Asia-Pacific cell and gene therapy manufacturing QC market has been extensively segmented based on offering, therapy type, process, technology, and application. This can help readers understand which segments account for the largest share and which are well-positioned to grow in the coming years.
Competitive Strategy: The Asia-Pacific cell and gene therapy manufacturing QC market has numerous established players with significant product portfolios. Key players in the Asia-Pacific cell and gene therapy manufacturing QC market analyzed and profiled in the study involve established players offering products and services for cell and gene therapy.
Methodology
Key Considerations and Assumptions in Market Engineering and Validation
- The base year considered for the calculation of the market size is 2023. A historical year analysis has been done for the period FY2019-FY2022. The market size has been estimated for FY2023 and projected for the period FY2024-FY2033.
- The scope of this report has been carefully derived based on interactions with experts in different companies across the world. This report provides a market study of products and services of the Asia-Pacific cell and gene therapy manufacturing QC market.
- The market contribution of Asia-Pacific cell and gene therapy manufacturing QC is anticipated to be launched and calculated based on the historical analysis of the solutions.
- The company's revenue has been referenced from their annual reports for FY2022 and FY2023. For private companies, revenues have been estimated based on factors such as inputs obtained from primary research, funding history, market collaborations, and operational history.
- The market has been mapped based on the available cell and gene therapy manufacturing QC in the Asia-Pacific. This report has considered and profiled all the key companies with significant offerings in this field.
Primary Research:
The primary sources involve industry experts in cell and gene therapy manufacturing QC, including the market players offering products and services. Resources such as CEOs, vice presidents, marketing directors, and technology and innovation directors have been interviewed to obtain and verify both qualitative and quantitative aspects of this research study.
The key data points taken from the primary sources include:
- Validation and triangulation of all the numbers and graphs
- Validation of the report's segmentation and key qualitative findings
- Understanding the competitive landscape and business model
- Current and proposed production values of a product by market players
- Validation of the numbers of the different segments of the market in focus
- Percentage split of individual markets for regional analysis
Secondary Research
Open Sources
- Certified publications, articles from recognized authors, white papers, directories, and major databases, among others
- Annual reports, SEC filings, and investor presentations of the leading market players
- Company websites and detailed study of their product portfolio
- Gold standard magazines, journals, white papers, press releases, and news articles
- Paid databases
The key data points taken from the secondary sources include:
- Segmentations and percentage shares
- Data for market value
- Key industry trends of the top players of the market
- Qualitative insights into various aspects of the market, key trends, and emerging areas of innovation
- Quantitative data for mathematical and statistical calculations
Key Market Players and Competition Synopsis
The companies profiled have been selected based on inputs gathered from primary experts, who have analyzed company coverage, product portfolio, and market penetration.
Some prominent names established in this market are:
- Bio-Techne Corporation
- Danaher Corporation (Cytiva)
- F. Hoffmann-La Roche Ltd
- Lonza
- Miltenyi Biotec B.V. & Co. KG
- Sartorius AG
- Thermo Fisher Scientific Inc.
- WuXi AppTec
- Fujifilm Holdings Corporation
- Merck KGaA
Table of Contents
1 Markets
- 1.1 Product Definition
- 1.1.1 Product Definition
- 1.1.2 Inclusion and Exclusion Criteria
- 1.2 Market Scope
- 1.2.1 Scope of the Work
- 1.2.2 Key Questions Answered in the Report
- 1.3 Research Methodology
- 1.3.1 Asia-Pacific Cell and Gene Therapy Manufacturing QC Market
- 1.3.2 Data Sources
- 1.3.2.1 Primary Data Sources
- 1.3.2.2 Secondary Data Sources
- 1.3.3 Market Estimation Model
2 Cell and Gene Therapy Manufacturing QC Market: Regulatory Framework
- 2.1 Chemistry, Manufacturing, and Control (CMC) Requirements by the Food and Drug Administration (FDA)
- 2.1.1 Product Testing
- 2.1.2 Microbial Testing
- 2.1.3 Identity
- 2.1.4 Purity
- 2.1.5 Potency
- 2.1.6 Viability
- 2.1.7 Cell Number or Dose
- 2.2 Quality Aspects of Cell and Gene Therapy Products by the European Medicines Agency (EMA)
- 2.2.1 Characterization
- 2.2.2 Identity Testing
- 2.2.3 Purity Testing
- 2.3 Current Good Manufacturing Practice (CGMP) Regulations
- 2.3.1 U.S.
- 2.3.2 Europe
- 2.3.3 Asia-Pacific
- 2.4 Global Regulatory Framework: Cell and Gene Therapy Manufacturing QC Market
3 Asia-Pacific Cell and Gene Therapy Manufacturing QC Market: Market Overview
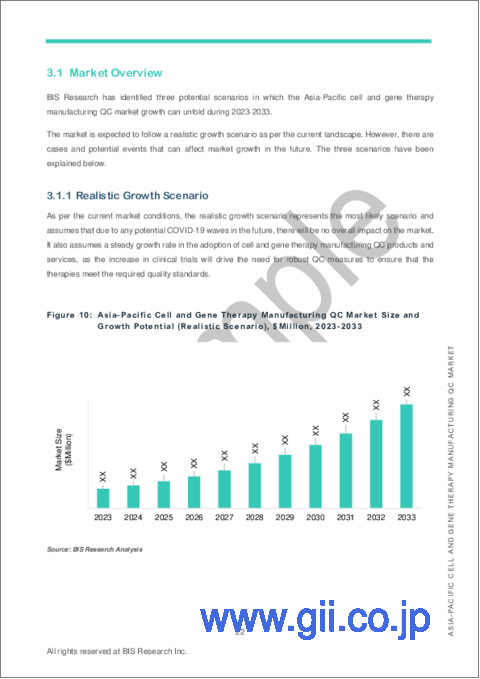
- 3.1 Market Overview
- 3.1.1 Realistic Growth Scenario
- 3.1.2 Optimistic Scenario
- 3.1.3 Pessimistic Scenario
- 3.2 Market Footprint and Growth Potential
- 3.3 Future Potential
- 3.4 COVID-19 Impact on Market
- 3.4.1 Impact on Research and Clinical Operations
- 3.4.2 COVID-19 Impact: Current Scenario of the Market
- 3.4.3 Pre- and Post-COVID-19 Impact Assessment
- 3.4.3.1 Pre-COVID-19 Phase
- 3.4.3.2 Post-COVID-19 Phase
- 3.5 Market Dynamics
- 3.6 Market Sizing and Forecast
- 3.6.1 Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 3.6.2 Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 3.6.3 Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Process)
- 3.6.4 Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 3.6.5 Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Application)
4 Asia-Pacific Cell and Gene Therapy Manufacturing QC Market: by Country
- 4.1 Singapore
- 4.1.1 Singapore Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 4.1.2 Singapore Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 4.1.3 Singapore Cell and Gene Therapy Manufacturing QC Market (by Process)
- 4.1.4 Singapore Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 4.1.5 Singapore Cell and Gene Therapy Manufacturing QC Market (by Application)
- 4.2 Japan
- 4.2.1 Japan Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 4.2.2 Japan Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 4.2.3 Japan Cell and Gene Therapy Manufacturing QC Market (by Process)
- 4.2.4 Japan Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 4.2.5 Japan Cell and Gene Therapy Manufacturing QC Market (by Application)
- 4.3 South Korea
- 4.3.1 South Korea Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 4.3.2 South Korea Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 4.3.3 South Korea Cell and Gene Therapy Manufacturing QC Market (by Process)
- 4.3.4 South Korea Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 4.3.5 South Korea Cell and Gene Therapy Manufacturing QC Market (by Application)
- 4.4 Australia
- 4.4.1 Australia Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 4.4.2 Australia Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 4.4.3 Australia Cell and Gene Therapy Manufacturing QC Market (by Process)
- 4.4.4 Australia Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 4.4.5 Australia Cell and Gene Therapy Manufacturing QC Market (by Application)
- 4.5 India
- 4.5.1 India Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 4.5.2 India Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 4.5.3 India Cell and Gene Therapy Manufacturing QC Market (by Process)
- 4.5.4 India Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 4.5.5 India Cell and Gene Therapy Manufacturing QC Market (by Application)
- 4.6 Thailand
- 4.6.1 Thailand Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 4.6.2 Thailand Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 4.6.3 Thailand Cell and Gene Therapy Manufacturing QC Market (by Process)
- 4.6.4 Thailand Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 4.6.5 Thailand Cell and Gene Therapy Manufacturing QC Market (by Application)
- 4.7 Malaysia
- 4.7.1 Malaysia Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 4.7.2 Malaysia Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 4.7.3 Malaysia Cell and Gene Therapy Manufacturing QC Market (by Process)
- 4.7.4 Malaysia Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 4.7.5 Malaysia Cell and Gene Therapy Manufacturing QC Market (by Application)
- 4.8 Indonesia
- 4.8.1 Indonesia Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 4.8.2 Indonesia Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 4.8.3 Indonesia Cell and Gene Therapy Manufacturing QC Market (by Process)
- 4.8.4 Indonesia Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 4.8.5 Indonesia Cell and Gene Therapy Manufacturing QC Market (by Application)
- 4.9 Rest-of-Asia-Pacific
- 4.9.1 Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Offering)
- 4.9.2 Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Therapy Type)
- 4.9.3 Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Process)
- 4.9.4 Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Technology)
- 4.9.5 Rest-of-Asia-Pacific Cell and Gene Therapy Manufacturing QC Market (by Application)
5 Company Profiles
- 5.1 Bio-Techne Corporation
- 5.1.1 Company Overview
- 5.1.2 Role of Bio-Techne Corporation in the Cell and Gene Therapy Manufacturing QC Market
- 5.1.3 Product Portfolio
- 5.1.4 Key Competitors
- 5.1.5 Financials
- 5.1.6 Analyst Perspective
- 5.2 Danaher Corporation (Cytiva)
- 5.2.1 Company Overview
- 5.2.2 Role of Danaher Corporation (Cytiva) in the Asia-Pacific Cell and Gene Therapy Manufacturing QC Market
- 5.2.3 Product Portfolio
- 5.2.4 Key Competitors
- 5.2.5 Financials
- 5.2.6 Analyst Perspective
- 5.3 F. Hoffmann-La Roche Ltd
- 5.3.1 Company Overview
- 5.3.2 Role of F. Hoffmann-La Roche Ltd in the Asia-Pacific Cell and Gene Therapy Manufacturing QC Market
- 5.3.3 Product Portfolio
- 5.3.4 Key Competitors
- 5.3.5 Financials
- 5.3.6 Analyst Perspective
- 5.4 Lonza
- 5.4.1 Company Overview
- 5.4.2 Role of Lonza in the Asia-Pacific Cell and Gene Therapy Manufacturing QC Market
- 5.4.3 Product Portfolio
- 5.4.4 Key Competitors
- 5.4.5 Financials
- 5.4.6 Analyst Perspective
- 5.5 Miltenyi Biotec B.V. & Co. KG
- 5.5.1 Company Overview
- 5.5.2 Role of Miltenyi Biotec B.V. & Co. KG in the Asia-Pacific Cell and Gene Therapy Manufacturing QC Market
- 5.5.3 Product Portfolio
- 5.5.4 Key Competitors
- 5.5.5 Analyst Perspective
- 5.6 Sartorius AG
- 5.6.1 Company Overview
- 5.6.2 Role of Sartorius AG in the Asia-Pacific Cell and Gene Therapy Manufacturing QC Market
- 5.6.3 Product Portfolio
- 5.6.4 Key Competitors
- 5.6.5 Financials
- 5.6.6 Analyst Perspective
- 5.7 Thermo Fisher Scientific Inc.
- 5.7.1 Company Overview
- 5.7.2 Role of Thermo Fisher Scientific Inc. in the Asia-Pacific Cell and Gene Therapy Manufacturing QC Market
- 5.7.3 Product Portfolio
- 5.7.4 Key Competitors
- 5.7.5 Financials
- 5.7.6 Analyst Perspective
- 5.8 WuXi AppTec
- 5.8.1 Company Overview
- 5.8.2 Role of WuXi AppTec in the Asia-Pacific Cell and Gene Therapy Manufacturing QC Market
- 5.8.3 Product Portfolio
- 5.8.4 Key Competitors
- 5.8.5 Financials
- 5.8.6 Analyst Perspective
- 5.9 Fujifilm Holdings Corporation
- 5.9.1 Company Overview
- 5.9.2 Role of Fujifilm Holdings Corporation in the Asia-Pacific Cell and Gene Therapy Manufacturing QC Market
- 5.9.3 Product Portfolio
- 5.9.4 Key Competitors
- 5.9.5 Financials
- 5.9.6 Analyst Perspective
- 5.10 Merck KGaA
- 5.10.1 Company Overview
- 5.10.2 Role of Merck KGaA in the Asia-Pacific Cell and Gene Therapy Manufacturing QC Market
- 5.10.3 Product Portfolio
- 5.10.4 Key Competitors
- 5.10.5 Financials
- 5.10.6 Analyst Perspective
List of Tables
- Table 1: Summary of Regulatory Requirements in Asia-Pacific
- Table 2: Key Questions Answered in the Report
- Table 3: Global Regulatory Scenario: Cell and Gene Therapy Manufacturing QC Market
- Table 4: List of Approved CGT Drugs in Asian Countries
- Table 5: Asia-Pacific Cell and Gene Therapy Manufacturing QC Market Dynamics, Impact Analysis
- Table 6: Some of the Approved Cell and Gene Therapies in Japan