|
|
市場調査レポート
商品コード
1761730
アルツハイマー病向け疾患修飾薬:世界市場Disease-Modifying Therapies for Alzheimer's Disease: Global Markets |
||||||
|
|||||||
| アルツハイマー病向け疾患修飾薬:世界市場 |
|
出版日: 2025年06月23日
発行: BCC Research
ページ情報: 英文 65 Pages
納期: 即納可能
|
全表示
- 概要
- 図表
- 目次
現在、アルツハイマー病に対する疾患修飾薬の市場は発展初期の段階にあります。
これは、最初のアルツハイマー病に対する疾患修飾薬(レカネマブ)が2023年7月に米国FDAから正式承認を受けたばかりであるためです。現在、複数の疾患修飾が後期段階(第2相以降)の臨床試験に入っており、これらが承認されれば、アルツハイマー病向け疾患修飾薬の市場は大きく拡大する可能性があります。
当レポートでは、世界のアルツハイマー病向け疾患修飾薬の市場を調査し、市場概要、市場影響因子および市場機会の分析、市場規模の推移・予測、各種区分・地域別の詳細分析、主要企業のプロファイルなどをまとめています。
目次
第1章 エグゼクティブサマリー
- 市場見通し
- 調査範囲
- 市場サマリー
第2章 市場概要
- アルツハイマー病の概要
- アルツハイマー病の病態生理
- アルツハイマー病の薬物治療
第3章 市場力学
- 市場力学スナップショット
- 促進要因
- アルツハイマー病治療における高いアンメットニーズ
- バイオマーカーの活用によるアルツハイマー病向け疾患修飾薬の承認の迅速化
- 課題
- アルツハイマー病治療薬の開発における高い失敗率
- 承認された疾患修飾薬の高コストと副作用
- 機会
- アルツハイマー病の前臨床段階における疾患修飾薬
第4章 規制状況
- 米国
- 標準的な医薬品承認プロセス
- 迅速な承認手続き
- 国/地域別の疾患修飾薬承認状況
第5章 新興技術と開発
- 多標的治療フレームワーク
- アルツハイマー病のスクリーニングと診断のための血液検査
- パイプライン分析
- 重要ポイント:
- フェーズ3臨床試験中の小分子疾患修飾薬
- フェーズ3臨床試験中の高分子疾患修飾薬
第6章 市場セグメント分析
- セグメンテーションの内訳
- 市場分析:標的タイプ別
- 抗アミロイド
- 疾患修飾薬の新たなアルツハイマー病病理標的
- 市場分析:医薬品別
- 市場分析:分子タイプ別
- 巨大分子
- 小分子
- 地理的内訳
- 市場分析:地域別
- 北米
- アジア太平洋
- 欧州
- 中東・アフリカ
- 南米
第7章 競合情報
- 概要
- アルツハイマー病向け疾患修飾薬を開発している注目企業
- 最近の動向/戦略分析
第8章 付録
- 調査手法
- 参考文献
- 略語
- 企業プロファイル
- ALZHEON INC.
- ANAVEX LIFE SCIENCES CORP.
- ANNOVIS BIO INC.
- BIOGEN
- BIOVIE INC.
- EISAI CO. LTD.
- JOHNSON & JOHNSON SERVICES INC.
- LILLY
- NOVO NORDISK A/S
- TAURX PHARMACEUTICALS LTD.
List of Tables
- Summary Table : Global Market for DMTs for AD, by Region, Through 2030
- Table 1 : Key Biomarkers Commonly Used to Evaluate and Support the Clinical Significance of AD DMTs
- Table 2 : Recent Late-Stage Therapeutic Candidates Failures in Clinical Trials, 2025
- Table 3 : DMTs Approval Status, by Country or Region, 2025
- Table 4 : Selected Blood Tests in Development for AD Screening/Diagnosis
- Table 5 : Disease-Modifying Small Molecule AD Drug Candidates in Phase 3 Clinical Trials
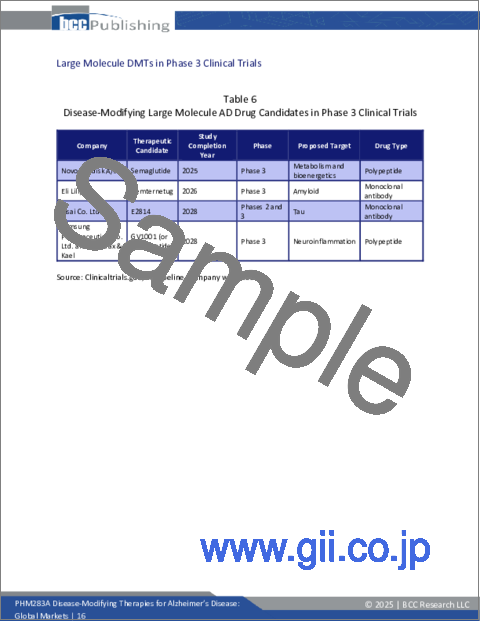
- Table 6 : Disease-Modifying Large Molecule AD Drug Candidates in Phase 3 Clinical Trials
- Table 7 : Global Market for DMTs for AD, by Target Type, Through 2030
- Table 8 : Commercially Available Anti-Amyloid Drugs
- Table 9 : Global Market for DMTs for AD, by Drug, Through 2030
- Table 10 : Small Molecule versus Large Molecule Drugs
- Table 11 : Global Market for DMTs for AD, by Drug Molecule Type, Through 2030
- Table 12 : Global Market for DMTs for AD, by Region, Through 2030
- Table 13 : Notable Companies Developing AD DMTs, 2025
- Table 14 : Abbreviations Used in the Report
- Table 15 : Alzheon Inc.: Company Snapshot
- Table 16 : Alzheon Inc.: Product Portfolio
- Table 17 : Alzheon Inc.: News/Key Developments, 2024
- Table 18 : Anavex Life Sciences Corp.: Company Snapshot
- Table 19 : Anavex Life Sciences Corp.: Financial Performance, FY 2023 and 2024
- Table 20 : Anavex Life Sciences Corp.: Product Portfolio
- Table 21 : Anavex Life Sciences Corp.: News/Key Developments, 2024
- Table 22 : Annovis Bio Inc.: Company Snapshot
- Table 23 : Annovis Bio Inc.: Financial Performance, FY 2023 and 2024
- Table 24 : Annovis Bio Inc.: Product Portfolio
- Table 25 : Biogen: Company Snapshot
- Table 26 : Biogen: Financial Performance, FY 2023 and 2024
- Table 27 : Biogen: Product Portfolio
- Table 28 : Biogen: News/Key Developments, 2024 and 2025
- Table 29 : BioVie Inc.: Company Snapshot
- Table 30 : BioVie Inc.: Financial Performance, FY 2022 and 2023
- Table 31 : BioVie Inc.: Product Portfolio
- Table 32 : Eisai Co. Ltd.: Company Snapshot
- Table 33 : Eisai Co. Ltd.: Financial Performance, FY 2023 and 2024
- Table 34 : Eisai Co. Ltd.: Product Portfolio
- Table 35 : Eisai Co. Ltd.: News/Key Developments, 2023-2025
- Table 36 : Johnson & Johnson Services Inc.: Company Snapshot
- Table 37 : Johnson & Johnson Services Inc.: Financial Performance, FY 2023 and 2024
- Table 38 : Johnson & Johnson Services Inc.: Product Portfolio
- Table 39 : Johnson & Johnson Services Inc.: News/Key Developments, 2024
- Table 40 : Lilly: Company Snapshot
- Table 41 : Lilly: Financial Performance, FY 2023 and 2024
- Table 42 : Lilly: Product Portfolio
- Table 43 : Lilly: News/Key Developments, 2024
- Table 44 : Novo Nordisk A/S: Company Snapshot
- Table 45 : Novo Nordisk A/S: Financial Performance, FY 2023 and 2024
- Table 46 : Novo Nordisk A/S: Product Portfolio
- Table 47 : TauRx Pharmaceuticals Ltd.: Company Snapshot
- Table 48 : TauRx Pharmaceuticals Ltd.: Product Portfolio
- Table 49 : TauRx Pharmaceuticals Ltd.: News/Key Developments, 2024
List of Figures
- Summary Figure : Global Market Shares for DMTs for AD, by Region, 2024
- Figure 1 : Disease-Modifying Therapies (DMTs) Market Snapshot for Alzheimer's Disease
- Figure 2 : People Living with Dementia Around the World, 2020-2050
- Figure 3 : Global Market Shares of DMTs for AD, by Region, 2024
- Figure 4 : Global Market Shares of DMTs for AD, by Leading Companies, 2024
- Figure 5 : Biogen: Revenue Shares, by Business Unit, FY 2024
- Figure 6 : Biogen: Revenue Shares, by Country/Region, FY 2024
- Figure 7 : Eisai Co. Ltd.: Revenue Shares, by Business Unit, FY 2024
- Figure 8 : Eisai Co. Ltd.: Revenue Shares, by Country/Region, FY 2024
- Figure 9 : Johnson & Johnson Services Inc.: Revenue Shares, by Business Unit, FY 2024
- Figure 10 : Johnson & Johnson Services Inc.: Revenue Shares, by Country/Region, FY 2024
- Figure 11 : Lilly: Revenue Shares, by Business Unit, FY 2024
- Figure 12 : Lilly: Revenue Shares, by Country/Region, FY 2024
- Figure 13 : Novo Nordisk A/S: Revenue Shares, by Business Unit, FY 2024
- Figure 14 : Novo Nordisk A/S: Revenue Shares, by Country/Region, FY 2024
This report provides an overview of the global and regional markets for disease-modifying therapies for Alzheimer’s disease. It includes global revenue ($ million) for base year data of 2024, estimates for 2025, and CAGR forecasts through 2030.
Report Scope
Currently, the disease-modifying therapies (DMTs) market for Alzheimer's disease (AD) is in its early stage of development, as the first DMT for AD (lecanemab) was granted full approval by the U.S. FDA in July 2023. Several DMTs are in late-stage (phase 2 and above) clinical trials. If approved, they can expand the AD DMTs market significantly. BCC Research estimates market data for 2024 (the base year) and forecasts values for 2025 through 2030. Other than the approved products, the revenue forecast also considers the potential therapeutic candidates expected to enter during the forecast period and their targeted market potential.
The report analyzes the AD DMTs market by target type (anti-amyloid and other emerging targets), drug (Leqembi, Kisunla, and others), molecule type (large and small), and region (Asia-Pacific, North America, Europe, South America, the Middle East, and Africa).
Report Includes
- Analysis of the current and future global market for disease-modifying therapies for Alzheimer's disease (AD)
- Analyses of the global market trends, with market revenue data (sales figures) from 2023 to 2025, forecasts for 2026, and projected CAGRs through 2030
- Estimates of the market's size and revenue prospects for the global market, accompanied by a market share analysis based on target type, drug type, drug molecule type, and region
- Facts and figures pertaining to market dynamics, technological advancements, regulations, prospects, and the impacts of macroeconomic variables
- Discussion of the underlying opportunities and potential in the disease-modifying therapies market
- Information on products currently available for the diagnosis and treatment of Alzheimer's, as well as the promising new drug candidates and diagnostic imaging agents
- Review of key marketed products, clinical trials, competitive scenario and R&D activities
- An analysis of emerging trends and the industry structure, including companies' market shares and rankings, strategic alliances, M&A activity and a venture funding outlook
- Profiles of the leading companies
Table of Contents
Chapter 1 Executive Summary
- Market Outlook
- Scope of Report
- Market Summary
Chapter 2 Market Overview
- Alzheimer's Disease Overview
- Alzheimer's Disease Pathophysiology
- Pharmaceutical Treatment of AD
Chapter 3 Market Dynamics
- Market Dynamics Snapshot
- Drivers
- High Unmet Need in AD Treatment
- Use of Biomarkers to Accelerate AD DMTs Approvals
- Challenges
- High Failure Rate in AD Drug Development
- High Cost and Side Effects of Approved DMTs
- Opportunity
- DMTs for the Preclinical-Stage of AD
Chapter 4 Regulatory Landscape
- U.S.
- Standard Drug Approval Process
- Expedited Approval Pathways
- DMTs Approval Status by Country/ Region
Chapter 5 Emerging Technologies and Developments
- A Multi-target Therapeutic Framework
- Blood Tests for AD Screening and Diagnosis
- Pipeline Analysis
- Key Takeaways:
- Small Molecule DMTs in Phase 3 Clinical Trials
- Large Molecule DMTs in Phase 3 Clinical Trials
Chapter 6 Market Segment Analysis
- Segmentation Breakdown
- Market Analysis, by Target Type
- Anti-Amyloid
- Other Emerging Alzheimer's Pathology Targets for DMTs
- Market Analysis, by Drug
- Market Analysis, by Drug Molecule Type
- Large Molecule
- Small-Molecule
- Geographic Breakdown
- Market Analysis by Region
- North America
- Asia-Pacific
- Europe
- Middle East and Africa
- South America
Chapter 7 Competitive Intelligence
- Overview
- Notable Companies Developing AD DMTs
- Recent Developments/Strategic Analysis
Chapter 8 Appendix
- Methodology
- References
- Abbreviations
- Company Profiles
- ALZHEON INC.
- ANAVEX LIFE SCIENCES CORP.
- ANNOVIS BIO INC.
- BIOGEN
- BIOVIE INC.
- EISAI CO. LTD.
- JOHNSON & JOHNSON SERVICES INC.
- LILLY
- NOVO NORDISK A/S
- TAURX PHARMACEUTICALS LTD.