
|
年間契約型情報サービス
商品コード
1349829
HER2低発現転移性乳がん市場:Tumour DeckHER2-Low Metastatic Breast Cancer - Tumour Deck |
||||||
|
|||||||
| HER2低発現転移性乳がん市場:Tumour Deck |
|
出版日: 年間契約型情報サービス
発行: Mellalta Meets LLP
ページ情報: 英文 300 Pages
|
全表示
- 概要
- 目次
HER2発現レベルが低く(HER2 IHCスコア1+または2+と定義される)、ERBB2増幅が検出されない腫瘍は、このHER2低発現転移性乳がんカテゴリーに分類されます。これは、HER2免疫組織化学的(IHC)スコア1+またはスコア2+/in situ ハイブリタイゼーション(ISH)陰性の表現型を有するHER2陰性BCの新たに定義されたサブセットです。IHC/ISHは、HER2発現を定義するために現在適用されている唯一の標準的技術です。
HER2低発現転移性乳がんに対する治療法は急速に進化しています。最近の臨床試験では、CDK4/6阻害剤と内分泌療法との併用が、標準的な一次治療として有効であることが証明されました。さらに、PI3K阻害剤やAKT阻害剤の使用も臨床試験で検討されており、近い将来、さらなる治療選択肢を提供する可能性があります。
HER2低発現転移性乳がんは、乳がんの新しいサブタイプであり、新たに診断された乳がん症例の約50%~60%を占めています。このことは、HER2低発現転移性乳がんが比較的一般的なサブタイプであることを示しています。HER2低発現乳がんはHER2の発現があっても、一般的にはHER2陰性とみなされ、治療されています。HER2低発現はHR+乳がんでより一般的ですが、HR陰性乳がんでも認められることが研究で示されています。
現在、HER2低発現転移性乳がんの治療の主流は、化学療法、内分泌療法、標的療法などの異なる治療アプローチを組み合わせることです。
近年、標的療法が臨床試験で有望視され、代替治療法として検討されています。HER2低値転移性乳がんに対する現在の標準治療は、最近の標的療法の進歩により急速に進化しています。最近の臨床試験では、新規のHER2指向性抗体薬物複合体(ADC)がHER2低値腫瘍の治療に大きな臨床的利益をもたらすことが実証されています。このようなADCとして承認されているものにトラスツズマブ・デルクステカン(T-Dxd)があり、HER2低発現乳がんにおいて有望な結果を示しています。
標的療法に加えて、内分泌療法もHER2低発現乳がん、特にホルモン受容体陽性患者に対する重要な治療選択肢です。CDK4/6阻害剤と内分泌療法の併用などの併用療法も、HER2低発現乳がん患者の予後改善に有望です。
当レポートでは、世界のHER2低発現転移性乳がん市場について調査し、市場の現状とともに、症例数の動向、患者動向、競合製品の市場における位置づけ、市場の機会などを提供しています。
目次
第1章 エグゼクティブサマリー
第2章 HER2低発現転移性乳がんの概要
- HER2低発現転移性乳がんの定義、症状、病因、病因
- HER2-Lowステータスの臨床的意義
第3章 HER2低発現転移性乳がんの定義と診断
第4章 HER2低発現転移性乳がんの疫学
- 国別の罹患率
第5章 HER2低発現転移性乳がんの治療実践
- 現在の治療法
- 治療アルゴリズム
- 迅速な承認に許容されるエンドポイント
第6章 HER2低発現転移性乳がんの承認された標的治療
- 承認された治療法の概要
第7章 パイプライン臨床試験
- HER2低発現転移性乳がんパイプ情勢の状況の概要と分析
- HER2低発現転移性乳がんの競合情勢
- 臨床試験における主要な分子と結果
- 主要な医薬品の承認と発売のタイムライン
8章 第III相の資産
- 臨床試験と結果
第9章 第II相の資産
- 臨床試験と結果
第10章 第I相の資産
- 臨床試験と結果
第11章 HER2低発現転移性乳がんパイプラインの非臨床分子
第12章 医師/KOLの見解
- 米国、EU、日本の4人のKOLからの洞察
第13章 HER2低発現転移性乳がんにおける主要イベント
- 承認された標的療法の拡大
- 新たな実行可能な目標の作成
第14章 HER2低発現転移性乳がん市場予測-2033年
- 主要薬剤別の市場予測と患者シェア
第15章 付録
The current clinical definition of HER2-low Breast Cancer (HER2-Low BC) used in clinical practice and ongoing clinical trials relies on the standard IHC and ISH approach; thus, tumors with low level of HER2 expression (defined as a HER2 IHC score of 1+ or 2+) and no detectable ERBB2 amplification fall into this category. It is a newly defined subset of HER2-negative BC that has HER2 immunohistochemical (IHC) score of 1+ or score of 2+/in situ hybridization (ISH) negative phenotype. IHC/ISH is the only standard technique currently applied to define HER2 expression.
"The treatment armamentarium for HER2-low metastatic breast cancer is rapidly evolving. Recent clinical trials have demonstrated the efficacy of CDK4/6 inhibitors in combination with endocrine therapy as a standard first-line treatment option. Additionally, the use of PI3K inhibitors and AKT inhibitors is being explored in clinical trials and may provide further treatment options in the near future."
HER2 low metastatic breast cancer is a new subtype of breast cancer which accounts for approximately 50%-60% of newly diagnosed breast cancer cases. This indicates that HER2 low breast cancer is a relatively common subtype of the disease. Even though HER2-low breast cancer has some HER2 expression, it is generally considered and treated as HER2 negative. Studies have shown that HER2-low expression is more common in HR+ breast cancer, but it can also be found in HR negative breast cancer (Won et al., 2021; Tan et al., 2021).
Mellalta Meets HER2-Low Expression in Breast Cancer: Evaluating the Evidence, Challenges, and Opportunities for Expanding Treatment Benefit to More Patients
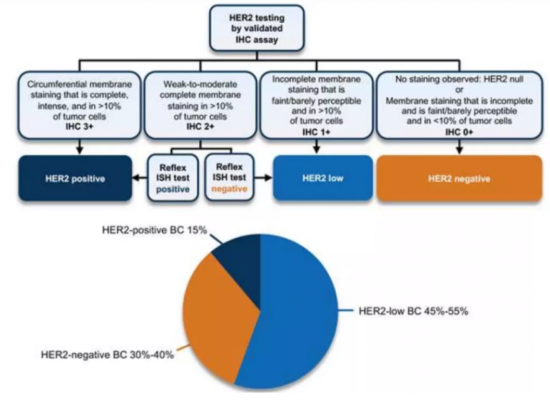
Mellalta's HER2-Low Metastatic Breast Cancer Deck: Current Treatment Landscape
Currently the mainstay of treatment for HER2-Low Metastatic Breast Cancer consist of combination of different therapeutic approaches like chemotherapy, endocrine therapies, targeted therapies.
In recent years, targeted therapies have shown promise in clinical trials and are being explored as alternative treatment options. The current standard of care for HER2-low metastatic breast cancer is rapidly evolving due to recent advancements in targeted therapies. Recent clinical trials have demonstrated significant clinical benefits of novel HER2-directed antibody-drug conjugates (ADCs) in treating HER2-low tumors. One such approved ADC is trastuzumab deruxtecan (T-Dxd), which has shown promising results in HER2-low breast cancer.
In addition to targeted therapies, endocrine therapy is also an important treatment option for HER2-low breast cancer, particularly in patients with hormone receptor-positive disease. Combination therapies, such as CDK4/6 inhibitors in combination with endocrine therapy, have also shown promise in improving outcomes for patients with HER2-low breast cancer.
"It is exciting that we have been able to now translate HER2-targeted therapy to a broader group of patients with HER2-low-expressing breast cancer. Overall, promising responses to T-DXd offer newfound treatment possibilities for a substantial number of patients, many of whom were previously considered to have limited therapeutic options. The recognition of HER2-low status also signals an opportunity to develop more precise, individualized therapeutic approaches through future research."
Mellalta's HER2-Low Metastatic Breast Cancer Deck: Current Unmet Needs
- Need for a clear and universally accepted definition of HER2-low, as the current classification is still evolving and there is ongoing research to determine the minimum threshold of HER2 expression required for treatment efficacy.
- HER2-low breast cancer (BC) has a poor prognosis, making the development of more suitable treatment an unmet clinical need.
- Need for standardized diagnostic criteria and guidelines for HER2-low tumors.
- Limited options for combination therapies that can enhance the efficacy of HER2-targeted treatments in HER2-low tumours.
"We are facing real challenges in terms of [HER2] identification in the clinic, and I would contend that we are in a state of flux in terms of the identification."
Mellalta's HER2-Low Metastatic Breast Cancer Deck: Key Takeaways
- Human epidermal growth factor receptor 2 (HER2) breast cancer, especially in the unresected, metastatic setting, is no longer considered solely binary as positive or negative.
- There is a new generation of approved antibody drug conjugate (ADC) like Trastuzumab deruxtecan (T-DXd) that have a higher drug-to-antibody ratio (DAR) and can deliver the toxin in a more effective manner for advanced unresectable, metastatic breast cancer.
- The 2023 National Comprehensive Cancer Network (NCCN) guidelines for the use of trastuzumab deruxtecan (T-DXd) reflect the clinical trial eligibility for DESTINY-Breast04. This allows health care professionals to use other ADCs approved for hormone receptor (HR)-positive and triple negative disease, irrespective of HER2 status.
- A traditional immunohistochemical (IHC) assay and scoring system have been used in testing to identify HER2-low tumors and the tumors with an IHC score of more than 0, less than 1+. New technologies may help to better identify patients with this subtype of tumor.
- The recently presented data of the DAISY trial suggested meaningful activity of T-DXd even in patients with HER2-0 metastatic breast cancer
Mellalta's HER2-Low Metastatic Breast Cancer Deck: Questions Answered:
- Potential challenges and opportunities in implementing targeted therapies for HER2-low breast cancer
- What is the size of clinically and commercially relevant drug-treatable HER2 low BC populations, and how will drug-treatment rates change over time?
- What is the expected market impact of recent drug approval such as Enhertu in treatment landscape of HER2-low metastatic BC?
- What are the most promising agents in the pipeline, and how will they shape the future of this therapy market?
- What key drivers and constraints will affect the HER2 low metastatic breast cancer therapy market over the forecast period?
Table of Content
1. Executive Summary
- 1.1. Summary of future trends
- 1.2. Potential opportunities to explore.
- 1.3. Drivers/barriers for entry
- 1.4. Unmet needs
- 1.5. What's new in HER2-Low Metastatic Breast Cancer?
2. HER2-Low Metastatic Breast Cancer Overview
- 2.1. HER2-Low Metastatic Breast Cancer definition, symptoms, etiology, Pathogenesis
- 2.2. Clinical Significance of HER2-Low Status
3. HER2-Low Metastatic Breast Cancer Definition & Diagnosis
- 3.1. Diagnostic Algorithm
- 3.2. HER2 Assessment with Immunohistochemistry (IHC) and In Situ Hybridization (ISH) (ASCO/CAP Guidelines)
- 3.3. AI-assisted interpretation of HER2 Status in HER2-Low Metastatic Breast Cancer
4. HER2-Low Metastatic Breast Cancer Epidemiology
- 4.1. Incidence rates by countries
5. HER2-Low Metastatic Breast Cancer Treatment Practices
- 5.1. Current treatment practices
- 5.2. Treatment algorithms
- 5.3. Acceptable endpoints for accelerated approval?
6. HER2-Low Metastatic Breast Cancer Approved Targeted Treatments
- 6.1. Quick overview of approved therapy
7. Pipeline clinical trials
- 7.1. HER2-Low Metastatic Breast Cancer pipeline landscape overview and analysis
- 7.2. Competitive Landscape of HER2-Low Metastatic Breast Cancer
- 7.3. Key molecules in clinical trials and results
- 7.4. Timeline of key drug approvals and launches.
8. Phase III Assets
- 8.1. Clinical trials and results
9. Phase II Assets
- 9.1. Clinical trials and results
10. Phase I Assets
- 10.1. Clinical trials and results
11. HER2-Low Metastatic Breast Cancer Pipeline Non-Clinical Molecules
- 11.1. Pre-clinical molecules
- 11.2. Mechanism of action, catalyst dates, and events
12. Physicians/KOLs Input
- 12.1. Insights from 4 KOLs in the US, EU, and Japan
13. Key Catalyst Events in HER2-Low Metastatic Breast Cancer
- 13.1. Expansion of approved targeted therapies
- 13.2. Creation of new actionable targets
14. HER2-Low Metastatic Breast Cancer Market Forecast -2033
- 14.1. Market Forecast and patient share by key drugs

