
|
市場調査レポート
商品コード
1762543
特殊CRO市場:業界動向と世界の予測 - 対象治療領域別、サービスタイプ別、主要地域別Specialty CROs Market: Industry Trends and Global Forecasts - Distribution by Target Therapeutic Area, Type of Service and Key Geographical Regions |
||||||
カスタマイズ可能
|
|||||||
| 特殊CRO市場:業界動向と世界の予測 - 対象治療領域別、サービスタイプ別、主要地域別 |
|
出版日: 2025年07月04日
発行: Roots Analysis
ページ情報: 英文 321 Pages
納期: 即日から翌営業日
|
全表示
- 概要
- 図表
- 目次
特殊CRO市場:概要
世界の特殊CROの市場規模は、今年12億米ドルとなりました。同市場は、予測期間中8.6%の有利なCAGRで成長すると予測されています。
市場セグメンテーションと機会分析は、以下のパラメータでセグメント化されています:
対象治療領域
- 腫瘍疾患
- 代謝疾患
- 心血管疾患
- 糖尿病
- その他
サービスタイプ
- 前臨床サービス
- 臨床サービス
主要地域
- 北米
- 欧州
- アジア太平洋
- その他の地域
特殊CRO市場:成長と動向
長年にわたり、バイオ医薬品業界は研究開発アウトソーシングに対するアプローチに大きな変革を遂げてきました。従来のフルサービスCROがしばしば直面する限界に対処するため、特殊開発業務受託機関(CRO)が有力な参入企業となっています。フルサービスプロバイダーは広範な能力を提供する一方で、小規模なバイオテクノロジー企業や新興企業の具体的なニーズに合わせてサービスを調整するのに苦労することが多いです。対照的に、特殊CROはニッチセグメントに焦点を当て、腫瘍、心臓病、代謝性疾患、中枢神経系(CNS)疾患などの特定の疾患領域に集中しながら、標的を絞った臨床または前臨床サービスの専門知識を提供します。これらの組織に一般的に委託されるサービスには、ファーマコビジランス、標的評価、製剤開発、細胞株開発、データ管理、プロジェクト管理、生物統計学などが含まれます。さらに、特殊CROは、世界のバイオ医薬品セクターの進化するニーズによりよく応えることを目指し、サービス提供の幅を広げ、地理的に拡大する戦略的モデルの採用も増えています。
特殊CRO市場:主要インサイト
当レポートでは、世界の特殊CRO市場の現状を調査し、潜在的な成長機会を特定しています。当レポートの主な調査結果は以下の通りです。
- CROが提供する豊富なサービスにより、CROは医薬品開発に不可欠なプラットフォームとなっています。
- 1,000以上のCROを対象とした詳細なデューデリジェンスの結果、特定の能力と提供するサービスの範囲に基づいて200以上の特殊CROが特定されました。
- 前臨床試験に特化したCROは全体の26%であり、これらのCROのサービスポートフォリオは顧客の幅広い要求に応えています。
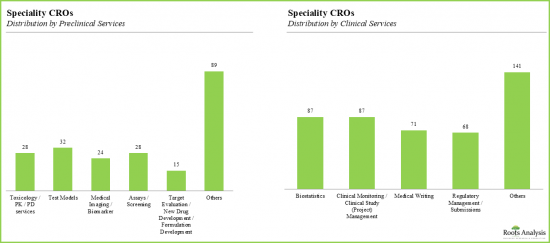
- 毒性学と薬理学は最も人気のある前臨床サービスであり、CROの~14%がそれぞれのポートフォリオで毒性学と薬理学に関連するサービスを提供しています。
- 約32社が試験モデルやメディカルイメージング/バイオマーカーベースの分析に特化しています。
- 特殊CROの40%以上が生物統計関連サービスを提供しており、次いで臨床モニタリングやプロジェクトマネジメントサービスを提供する特殊CROが続きます。
- 特筆すべきは、メディカルライティングと薬事管理・申請業務のアウトソーシングが多いことです。
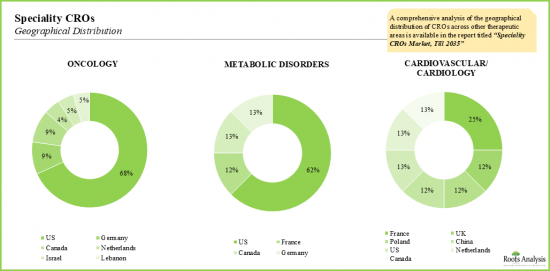
- 市場全体は一握りの大手CROに支配されていますが、特殊CROセグメントの市場は非常に断片化されており、米国が主要なハブであり続けています。
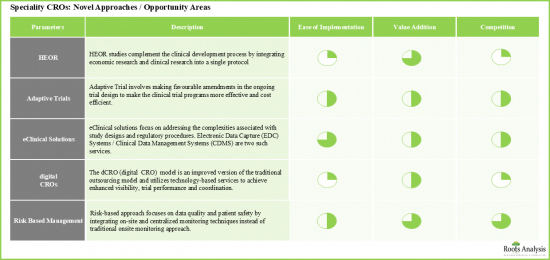
- 新しいアプローチや未開拓の機会領域が、長期的には主要な促進要因として浮上する可能性が高いです。
- 特に、特殊CROがそのサービス・ポートフォリオをさらに確立するにつれて、市場は今後数年間も成長の勢いを維持する可能性が高いと考えられています。
Specialty CROS市場における参入企業例
- Accelovance
- Almedis
- Applied Healthcare Resource Management
- Betagenex
- Biospective
- BRI Biopharmaceutical
- BTS Research
- Cardialysis
- CMX Research
- Crown Bioscience
- Dorizoe Lifesciences
- DSP Clinical Research
- DZS Clinical Services
- EthosExcel(TM)
- Fluofarma
- ICRC-Weyer
- Impact Pharmaceutical Services
- IonsGate Preclinical Services
- KIYATEC
- MedSource
- Novella Clinical
- Physiogenex
- Profil Institute
- Redoxis
- RenaSci
- Research Dynamics Consulting
- RxGen
- SDS Clinical
- Spirovation
- Velesco Pharmaceutical Services
目次
第1章 序文
第2章 エグゼクティブサマリー
第3章 イントロダクション
- 歴史
- 従来のCRO
- 特殊CRO
第4章 特殊CRO:イントロダクション
- 章の概要
- 特殊CROの重要性
第5章 市場概要
- 章の概要
- 調査手法
- 特殊CRO:世界情勢
- 特定のサービス能力に特化した特殊CRO
- 特定の治療領域に特化した特殊CRO
第6章 特殊CRO:サービス重点型
- 章の概要
- 前臨床サービス能力に重点を置くCRO
- BRI Biopharmaceutical Research
- BTS Research
- Dorizoe Lifesciences
- Fluofarma
- KIYATEC
- Redoxis
- Spirovation
- Velesco Pharmaceutical Services
- 臨床サービス能力に重点を置くCRO
- Almedis
- Applied Healthcare Resource Management
- CMX Research
- DSP Clinical Research
- DZS Clinical Services
- EthosExcel(TM)
- ICRC-Weyer
- Impact Pharmaceutical Services
- Research Dynamics Consulting
- SDS Clinical
第7章 特殊CRO:治療領域重点型
- 章の概要
- 腫瘍学に特化した特殊CRO
- Accelovance
- Crown Bioscience
- MedSource
- Novella Clinical
- 循環器系/心臓病に特化した特殊CRO
- Cardialysis
- IonsGate Preclinical Services
- 代謝疾患に特化した特殊CRO
- Betagenex
- Physiogenex
- Profil Institute
- CNSに特化した特殊CRO
- Biospective
- RenaSci
- RxGen
第8章 ケーススタディI:バーチャルCRO
- バーチャルCROのイントロダクション
- Frestedt
- InSymbiosis
- Osiris Pharma
- ProjectPharm
- The Harte Group
- VxP Pharma
第9章 ケーススタディII:フルサービスCRO
- 従来のCROのイントロダクション
- Covance
- Medis Research Group
- Quintiles
- Triclinium Clinical Trial Project Management
第10章 市場予測
- 章の概要
- 予測調査手法
- 世界の特殊CRO市場(2035年まで)
- 世界の特殊CRO市場(2035年まで)、地域別
第11章 将来の機会
第12章 結論
第13章 インタビュー記録
第14章 付録1:表形式データ
第15章 付録2:企業・団体一覧
第16章 付録3:サービスマップ用語集
List of Tables
- Table 5.1 Specialty CROs: Global Landscape
- Table 5.2 List of Specialty CROs and their Service Capabilities
- Table 5.3 Specialty CROs: Classification by Therapeutic Areas
- Table 6.1 BTS Research: Animal Models for Immune and Inflammatory Diseases
- Table 6.2 BTS Research: Animal Models for Oncology
- Table 6.3 BTS Research: Animal Models for CNS
- Table 7.1 Accelovance China: Services
- Table 7.2 MedSource: Research Experience in Oncology by Type of Indication
- Table 7.3 Physiogenex: Animal Models for Diabetes
- Table 7.4 Physiogenex: Technology Platform for Diabetes
- Table 7.5 Physiogenex: Animal Models for Dyslipidemia
- Table 7.6 Physiogenex: Technology Platform for Dyslipidemia
- Table 7.7 Physiogenex: Animal Models for NAFLD/NASH
- Table 7.8 Physiogenex: Technology Platform for NAFLD/NASH
- Table 7.9 Physiogenex: Animal Models for Obesity
- Table 7.10 Physiogenex: Technology Platform for Obesity
- Table 7.11 Physiogenex: Animal Models for Nutraceuticals
- Table 7.12 Physiogenex: Technology Platforms for Nutraceuticals
- Table 8.1 VxP Pharma: Systems Suitable for E/L testing
- Table 14.1 Specialty CROs: Distribution by Year of Foundation
- Table 14.2 Specialty CROs: Distribution by Nature of Specialization
- Table 14.3 Specialty CRO Services: Stage of Development
- Table 14.4 Specialty CROs: Focused Preclinical Services
- Table 14.5 Specialty CROs: Focused Clinical Services
- Table 14.6 Specialty CROs: Distribution by Focused Therapeutic Areas
- Table 14.7 Specialty CROs: Geographical Distribution by Therapeutic Area
- Table 14.8 Novella Clinical: Oncological Trial Experience (%)
- Table 14.9 Quintiles: Service Revenue, Since 2015 (USD Million)
- Table 14.10 Overall Specialty CROs Market, Short-Midterm: Base Scenario (USD Billion)
- Table 14.11 Overall Specialty CROs Market, Long Term Since 2020: Base Scenario (USD Billion)
- Table 14.12 Regional Market Forecast: Base Scenario (USD Billion)
- Table 14.13 Overall Specialty CROs Market, Short-Midterm: Conservative Scenario (USD Billion)
- Table 14.14 Overall Specialty CROs Market, Long Term Since 2020: Conservative Scenario (USD Billion)
- Table 14.15 Regional Market Forecast: Conservative Scenario (USD Billion)
- Table 14.16 Overall Specialty CROs Market, Short-Midterm: Optimistic Scenario (USD Billion)
- Table 14.17 Overall Specialty CROs Market, Long Term Since 2020: Optimistic Scenario (USD Billion)
- Table 14.18 Regional Market Forecast: Optimistic Scenario (USD Billion)
- Table 14.19 Specialty CROs Market: Comparative Market Evolution Scenarios (USD Billion)
List of Figures
- Figure 4.1 Specialty CROs: Benefits
- Figure 4.2 Specialty CROs: Areas of Specialization
- Figure 5.1 Specialty CROs: Distribution on the Basis of Year of Foundation
- Figure 5.2 Specialty CROs: Distribution by Nature of Specialization
- Figure 5.3 Specialty CRO Services: Stage of Development
- Figure 5.4 Specialty CROs: Focused Preclinical Services
- Figure 5.5 Specialty CROs: Focused Clinical Services
- Figure 5.6 Specialty CROs: Distribution by Focused Therapeutic Areas
- Figure 5.7 Specialty CROs: Geographical Distribution by Therapeutic Area
- Figure 6.1 Specialty CROs: Focused Capabilities
- Figure 6.2 BTS Research: Services for Custom Monoclonal and Polyclonal Antibody
- Figure 6.3 Dorizoe Lifesciences: Dosage Forms
- Figure 6.4 Dorizoe Lifesciences: Formulation Development Protocol
- Figure 6.5 Dorizoe Lifesciences: Customized Services
- Figure 6.6 Spirovation: Samples and Biomarkers used for Biomarker Evaluation
- Figure 6.7 Velesco Pharmaceutical Services: Portfolio
- Figure 6.8 ICRC-Weyer: Biostatistical Services
- Figure 6.9 ICRC-Weyer: Safety Writing and Pharmacovigilance
- Figure 6.10 Impact Pharmaceuticals: Medical Writing
- Figure 7.1 Specialty CROs: Focused Therapeutic Area(S)
- Figure 7.2 Novella Clinical: EDC and Supporting Systems
- Figure 7.3 Novella Clinical: Project Management Cycle
- Figure 7.4 Novella Clinical: Oncological Trial Experience (%)
- Figure 7.5 Physiogenex: Preclinical Services for First Level Phenotyping and Screening
- Figure 7.6 Profil Institute: Expertise in Anti-Diabetes Compounds
- Figure 7.7 Profil Institute: Expertise in Anti-Obesity Compounds and Devices
- Figure 7.8 Biospective: Rodent Models
- Figure 7.9 Biospective: Imaging Services For 1.5 and 3T MRI and Brain Pet Studies
- Figure 7.10 Biospective: Therapeutic Areas for Human Imaging
- Figure 7.11 Biospective: Services for Quantitative Image Analysis
- Figure 7.12 Biospective: Stains for Histology and Immunochemistry Studies
- Figure 8.1 Frestedt: Service Portfolio
- Figure 8.2 ProjectPharm: Consulting Services
- Figure 8.3 The Harte Group: Clinical Trial Project Management Services
- Figure 8.4 VxP Pharma: GLP Radiolabelling Studies
- Figure 8.5 VxP Pharma: Mammalian and Viral Cell Banks
- Figure 9.1 Quintiles: Service Revenue (USD Million)
- Figure 10.1 Overall Specialty CROs Market, Short-Midterm: Base Scenario (USD Billion)
- Figure 10.2 Overall Specialty CROs Market, Long Term Since 2020 Base Scenario (USD Billion)
- Figure 10.3 Regional Market Forecast: Base Scenario (USD Billion)
- Figure 11.1 Specialty CROs: Future Opportunities
SPECIALTY CROS MARKET: OVERVIEW
As per Roots Analysis, the global specialty CROs market valued at USD 1.2 billion in the current year is anticipated to grow at a lucrative CAGR of 8.6% during the forecast period.
The market sizing and opportunity analysis has been segmented across the following parameters:
Target Therapeutic Area
- Oncological Disorders
- Metabolic Disorders
- Cardiovascular Disorders
- Diabetes
- Others
Type of Service
- Preclinical Services
- Clinical Services
Key Geographical Regions
- North America
- Europe
- Asia-Pacific
- Rest of the World
SPECIALTY CROS MARKET: GROWTH AND TRENDS
Over the years, the biopharmaceutical industry has undergone a major transformation in its approach towards R&D outsourcing. Specialty contract research organizations (CROs) have become prominent players, addressing limitations often faced with traditional, full-service CROs. While full-service providers offer a broad range of capabilities, they frequently struggle to tailor their services to the specific needs of small-scale biotech companies and start-ups. In contrast, specialty CROs focus on niche segments, delivering expertise in targeted clinical or preclinical services, while concentrating on a particular disease area such as oncology, heart diseases, metabolic disorders, and central nervous system (CNS) conditions. Services commonly outsourced to these organizations include pharmacovigilance, target evaluation, formulation development, cell line development, data management, project management, biostatistics and others. Further, specialty CROs are also increasingly adopting strategic models to broaden their service offerings and expand geographically, aiming to better serve the evolving needs of the global biopharmaceutical sector.

SPECIALTY CROS MARKET: KEY INSIGHTS
The report delves into the current state of the global specialty CROs market and identifies potential growth opportunities within industry. Some key findings from the report include:
- The wealth of services offered by CROs have made them an indispensable platform for drug development.
- Over 200 speciality CROs were identified after detailed due diligence of more than 1,000 CROs based on the specific capabilities and the range of services they provide.
- With 26% of the organizations specializing in preclinical services, the service portfolio of these CROs caters to a wide array of client requirements.

- Toxicology and pharmacology are the most popular preclinical services, with ~14% of the CROs offering the services related to toxicology and pharmacology in their respective portfolios.
- Nearly 32 companies specialize in test models and medical imaging / biomarker-based analysis.
- Over 40% of the speciality CROs offered biostatistics related services, followed by speciality CROs offering clinical monitoring and project management services.
- Notably, medical writing and regulatory management / submission is also frequently outsourced.
- Though the overall market is dominated by a handful of bigger CROs, the market within the speciality CRO segment is highly fragmented; US continues to remain the primary hub.

- Newer approaches / untapped opportunity areas are likely to emerge as key growth drivers in the long-term.
- Notably, as specialty CROs further establish their service portfolios, it is believed that the market is likely to sustain the growth momentum in the coming years.
Example Players in the Specialty CROS Market
- Accelovance
- Almedis
- Applied Healthcare Resource Management
- Betagenex
- Biospective
- BRI Biopharmaceutical
- BTS Research
- Cardialysis
- CMX Research
- Crown Bioscience
- Dorizoe Lifesciences
- DSP Clinical Research
- DZS Clinical Services
- EthosExcel(TM)
- Fluofarma
- ICRC-Weyer
- Impact Pharmaceutical Services
- IonsGate Preclinical Services
- KIYATEC
- MedSource
- Novella Clinical
- Physiogenex
- Profil Institute
- Redoxis
- RenaSci
- Research Dynamics Consulting
- RxGen
- SDS Clinical
- Spirovation
- Velesco Pharmaceutical Services
SPECIALTY CROS MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the specialty CROs market, focusing on key market segments, including [A] target therapeutic area, [B] type of service and [E] key geographical regions.
- Market Landscape: A comprehensive evaluation of specialty CROs, based on several relevant parameters, such as [A] geographical location, [B] year of establishment and [C] R&D capabilities.
- Company Profiles: In-depth profiles of specialty CROs focused on specialized preclinical / clinical service offering and particular therapeutic area, based on [A] overview of the company, [B] service portfolio and [C] recent developments and an informed future outlook.
- Case Study 1: A detailed discussion of the virtually integrated, cross-functional outsourcing approach implemented by virtual CROs.
- Case study 2: A detailed discussion of the one-stop-shop approach followed by traditional CROs.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.2.1. Research Assumptions
- 1.2.2. Project Methodology
- 1.2.3. Forecast Methodology
- 1.2.4. Robust Quality Control
- 1.2.5. Key Considerations
- 1.2.5.1. Demographics
- 1.2.5.2. Economic Factors
- 1.2.5.3. Government Regulations
- 1.2.5.4. Supply Chain
- 1.2.5.5. COVID Impact / Related Factors
- 1.2.5.6. Market Access
- 1.2.5.7. Healthcare Policies
- 1.2.5.8. Industry Consolidation
- 1.3 Key Questions Answered
- 1.4. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. History
- 3.2. Traditional Contract Research Organizations
- 3.2.1. Services
- 3.2.2. Current Industry Environment
- 3.3. Specialty Contract Research Organizations
4. SPECIALTY CROs: AN INTRODUCTION
- 4.1. Chapter Overview
- 4.2. Importance of Specialty CROs
5. MARKET OVERVIEW
- 5.1. Chapter Overview
- 5.2. Methodology
- 5.3. Specialty CROs: Global Landscape
- 5.3.1. Specialty CROs: The Dramatic Rise
- 5.3.2. Specialty CROs: Distribution by Nature of Specialization
- 5.4. Specialty CROs Focused on Specific Service Capability
- 5.4.1. Landscape is Well Distributed across Preclinical and Clinical Services
- 5.4.2. Specialty CROs: Popular Preclinical Services
- 5.4.3. Specialty CROs: Popular Clinical Services
- 5.5. Specialty CROs Focused on Specific Therapeutic Area(s)
- 5.5.1. Oncology is the Most Researched Therapeutic Area
- 5.5.2. CROs Mostly Located in the US; Europe is a Distant Second
6. SPECIALTY CROs: FOCUSED ON SERVICES
- 6.1. Chapter Overview
- 6.2. CROs Focused on Preclinical Service Capabilities
- 6.2.1. BRI Biopharmaceutical Research
- 6.2.1.1. Company Overview
- 6.2.1.2. Focused Clinical Expertise
- 6.2.1.2.1. API / Clinical Product QC, Dosing Solutions & Materials
- 6.2.1.2.2. Bioanalytical Assays for Clinical Trials
- 6.2.1.2.3. Clinical Trial Equipment Rental & PK Sample Collection Kit
- 6.2.1.2.4. Cynomologus & Rhesus Monkey Hepatocytes & Blood Products
- 6.2.1.2.5. Drug Candidate Early In Vitro & In Vivo Screening
- 6.2.1.2.6. In Vitro DMPK & ADME
- 6.2.1.2.7. In Vivo DMPK & ADME
- 6.2.1.2.8. Patient Tumor-Derived Xenograft Models at Oncograph(TM)
- 6.2.1.2.9. Strategic Development of Botanical Drugs
- 6.2.2. BTS Research
- 6.2.2.1. Company Overview
- 6.2.2.2. Focused Clinical Expertise
- 6.2.2.2.1. Custom Services
- 6.2.2.2.2. Disease Models
- 6.2.2.2.3. In Vitro Services
- 6.2.2.2.4. IND Process
- 6.2.2.2.5. Medical Devices
- 6.2.2.2.6. Pharmacology Services
- 6.2.2.2.7. Toxicology Services
- 6.2.2.3. Additional Information
- 6.2.2.3.1. Recent Developments
- 6.2.2.3.2. Acquisitions
- 6.2.3. Dorizoe Lifesciences
- 6.2.3.1. Company Overview
- 6.2.3.2. Focused Clinical Expertise
- 6.2.3.2.1. Services and Capabilities
- 6.2.3.2.2. Customized Services
- 6.2.4. Fluofarma
- 6.2.4.1. Company Overview
- 6.2.4.2. Focused Clinical Expertise
- 6.2.4.2.1. Assay Development Services
- 6.2.4.2.2. Cell-based screening services
- 6.2.4.2.3. In Vitro Drug Profiling Services and Mechanism of Action (MOA) Studies
- 6.2.4.2.4. Predictive Toxicology Services
- 6.2.4.2.5. In Vivo Studies
- 6.2.4.2.6. Biomarker Analysis Services
- 6.2.4.3. Additional Information
- 6.2.4.3.1. Collaborations
- 6.2.4.3.2. Recent Developments
- 6.2.5. KIYATEC
- 6.2.5.1. Company Overview
- 6.2.5.2. Focused Clinical Expertise
- 6.2.5.2.1. Drug Response Profiling (DRP) Services
- 6.2.5.3. Additional Information
- 6.2.5.3.1. Recent Developments
- 6.2.5.3.2. Funding
- 6.2.6. Redoxis
- 6.2.6.1. Company Overview
- 6.2.6.2. Focused Clinical Expertise
- 6.2.6.2.1. In Vivo Models
- 6.2.6.2.2. In Vitro Services
- 6.2.6.3. Additional Information
- 6.2.6.3.1. Collaborations
- 6.2.6.3.2. Recent Developments
- 6.2.6.3.3. Funding
- 6.2.7. Spirovation
- 6.2.7.1. Company Overview
- 6.2.7.2. Focused Clinical Expertise
- 6.2.7.2.1. Clinical Assessment
- 6.2.7.2.2. Functional Screening and Lead Selection
- 6.2.7.2.3. Target ID and Primary Screening
- 6.2.7.3. Additional Information
- 6.2.7.3.1. Facilities
- 6.2.8. Velesco Pharmaceutical Services
- 6.2.8.1. Company Overview
- 6.2.8.2. Focused Clinical Expertise
- 6.2.8.2.1. Analytical Method Development
- 6.2.8.2.2. cGMP Manufacturing
- 6.2.8.2.3. Drug Formulation Development
- 6.2.8.2.4. Instrumentation
- 6.2.8.2.5. Pharmaceutical Consulting
- 6.2.1. BRI Biopharmaceutical Research
- 6.3. CROs Focused on Clinical Service Capabilities
- 6.3.1. Almedis
- 6.3.1.1. Company Overview
- 6.3.1.2. Focused Clinical Expertise
- 6.3.1.2.1. Biostatistics
- 6.3.1.2.2. Clinical Monitoring
- 6.3.1.2.3. International Clinical Trials
- 6.3.1.2.4. Local Studies
- 6.3.1.2.5. Data Management
- 6.3.1.2.6. Medical Writing
- 6.3.1.2.7. Training Programs
- 6.3.2. Applied Healthcare Resource Management
- 6.3.2.1. Company Overview
- 6.3.2.2. Focused Clinical Services
- 6.3.2.3. Additional Information
- 6.3.2.3.1. Collaborations
- 6.3.3. CMX Research
- 6.3.3.1. Company Overview
- 6.3.3.2. Focused Clinical Expertise
- 6.3.3.2.1. CRO Services
- 6.3.3.2.2. Investigator Network
- 6.3.3.2.3. Investigator-Led Trials (ILTs)
- 6.3.3.2.4. MDapps(TM)
- 6.3.4. DSP Clinical Research
- 6.3.4.1. Company Overview
- 6.3.4.2. Focused Clinical Expertise
- 6.3.4.2.1. Data Management
- 6.3.4.2.2. Monitoring
- 6.3.4.2.3. Project Management
- 6.3.4.2.4. Site Management
- 6.3.4.2.5. Statistics and Medical Writing
- 6.3.4.2.6. Others
- 6.3.4.3. Additional Information
- 6.3.4.3.1. Collaboration
- 6.3.4.3.2. Recent Developments
- 6.3.5. DZS Clinical Services
- 6.3.5.1. Company Overview
- 6.3.5.2. Focused Clinical Services
- 6.3.5.2.1. Biostatistics Support and Consulting
- 6.3.5.2.2. Clinical Data Management
- 6.3.5.2.3. Clinical Monitoring
- 6.3.5.2.4. Clinical Project Management
- 6.3.5.2.5. ClinPlus(R) eClinical Platform
- 6.3.5.2.6. Customized FSP Models
- 6.3.5.2.7. DZS Clinical Sourcing and Staffing
- 6.3.5.2.8. Medical Coding
- 6.3.5.2.9. Medical Writing
- 6.3.5.2.10. Statistical Programming and CDISC Implementation
- 6.3.5.2.11. SOP Authoring
- 6.3.6. EthosExcel(TM)
- 6.3.6.1. Company Overview
- 6.3.6.2. Focused Clinical Expertise
- 6.3.6.2.1. Clinical Trial Services
- 6.3.6.2.2. Clinical Trial Resourcing
- 6.3.6.2.3. Diversity Consulting
- 6.3.6.2.4. Investigator and Trial Site Facilitation Services
- 6.3.6.2.5. Site Management
- 6.3.7. ICRC-Weyer
- 6.3.7.1. Company Overview
- 6.3.7.2. Focused Clinical Expertise
- 6.3.7.2.1. Biostatistics
- 6.3.7.2.2. Clinical Data Management
- 6.3.7.2.3. Medical Review
- 6.3.7.2.4. Medical Writing
- 6.3.7.2.5. Safety Writing and Pharmacovigilance
- 6.3.7.2.6. Scientific Consulting
- 6.3.7.3. Additional Information
- 6.3.7.3.1. Expansion of Service Portfolio
- 6.3.8. Impact Pharmaceutical Services
- 6.3.8.1. Company Overview
- 6.3.8.2. Focused Clinical Expertise
- 6.3.8.2.1. Drug Development Consulting
- 6.3.8.2.2. Early Phase Clinical Trial Management
- 6.3.8.2.3. Medical Writing
- 6.3.8.2.4. Project and Program Management
- 6.3.8.2.5. Regulatory Affairs
- 6.3.8.2.6. Regulatory Operations
- 6.3.9. Research Dynamics Consulting
- 6.3.9.1. Company Overview
- 6.3.9.2. Focused Clinical Expertise
- 6.3.9.2.1. Clinical Monitoring
- 6.3.9.2.2. Consulting
- 6.3.9.2.3. GCP Auditing
- 6.3.9.2.4. Investigator Recruitment
- 6.3.9.2.5. Project Management
- 6.3.9.2.6. Site Management
- 6.3.9.3. Additional Information
- 6.3.9.3.1. Collaborations
- 6.3.10. SDS Clinical
- 6.3.10.1. Company Overview
- 6.3.10.2. Focused Clinical Expertise
- 6.3.10.2.1. Clinical Trial Services
- 6.3.10.2.2. Consulting Services
- 6.3.1. Almedis
7. SPECIALTY CROs: FOCUSED ON THERAPEUTIC AREAS
- 7.1. Chapter Overview
- 7.2. Specialty CROs Focused on Oncology
- 7.2.1. Accelovance
- 7.2.1.1. Company Overview
- 7.2.1.2. Focused Clinical Expertise
- 7.2.1.2.1. CRO Services
- 7.2.1.2.2. Patient Recruitment
- 7.2.1.2.3. Clinical Call Center
- 7.2.1.3. Additional Information
- 7.2.1.3.1. Collaborations
- 7.2.1.3.2. Site Expansion
- 7.2.2. Crown Bioscience
- 7.2.2.1. Company Overview
- 7.2.2.2. Focused Clinical Expertise
- 7.2.2.2.1. Biotherapeutics
- 7.2.2.2.2. Drug Discovery
- 7.2.2.2.3. Metabolic Diseases
- 7.2.2.2.4. Oncology
- 7.2.2.3. Additional Information
- 7.2.2.3.1. Collaborations
- 7.2.2.3.2. Service Expansion
- 7.2.2.3.3. Site Expansion
- 7.2.2.3.4. Recent Developments
- 7.2.2.3.5. Funding
- 7.2.3. MedSource
- 7.2.3.1. Company Overview
- 7.2.3.2. Focused Clinical Expertise
- 7.2.3.2.1. Clinical Data Management and Biostatistics
- 7.2.3.2.2. Clinical Support
- 7.2.3.2.3. Clinical Trial Monitoring
- 7.2.3.2.4. Project Management
- 7.2.3.2.5. Regulatory Affairs Management
- 7.2.3.2.6. Study Start-Up
- 7.2.4. Novella Clinical
- 7.2.4.1. Company Overview
- 7.2.4.2. Focused Clinical Expertise
- 7.2.4.2.1. Clinical Monitoring
- 7.2.4.2.2. Data Management
- 7.2.4.2.3. Data Monitoring Committees
- 7.2.4.2.4. Investigator Strategy & Site Coordination (ISSC)
- 7.2.4.2.5. Medical Monitoring
- 7.2.4.2.6. Medical Writing
- 7.2.4.2.7. Project Management
- 7.2.4.2.8. Quality Assurance
- 7.2.4.2.9. Regulatory Affairs
- 7.2.4.2.10. Safety Management
- 7.2.4.2.11. Steering Committees & Clinical Advisory Boards
- 7.2.4.2.12. Training
- 7.2.4.2.13. Clinical Staffing
- 7.2.4.2.14. Expertise in Oncology across Various Phases of Clinical Trials
- 7.2.1. Accelovance
- 7.3. Specialty CROs Focused on Cardiovascular / Cardiology
- 7.3.1. Cardialysis
- 7.3.1.1. Company Overview
- 7.3.1.2. Focused Clinical Expertise
- 7.3.1.2.1. Trial Services
- 7.3.1.2.2. Core Laboratory
- 7.3.1.2.3. Network of Partners
- 7.3.1.3. Additional Information
- 7.3.2. IonsGate Preclinical Services
- 7.3.2.1. Company Overview
- 7.3.2.2. Focused Clinical Expertise
- 7.3.2.2.1. Cell Based Assays
- 7.3.2.2.2. Isolated Tissue Based Assays
- 7.3.2.2.3. In Vivo Models
- 7.3.1. Cardialysis
- 7.4. Specialty CROs Focused on Metabolic Disorders
- 7.4.1. Betagenex
- 7.4.1.1. Company Overview
- 7.4.1.2. Focused Clinical Expertise
- 7.4.1.2.1. Experimental Services
- 7.4.1.2.2. Consulting Services
- 7.4.1.3. Additional Information
- 7.4.2. Physiogenex
- 7.4.2.1. Company Overview
- 7.4.2.2. Focused Clinical Expertise
- 7.4.2.2.1. Research Services
- 7.4.2.2.2. Consultancy Services
- 7.4.2.3. Additional Information
- 7.4.2.3.1. Collaborations
- 7.4.2.3.2. Recent Developments
- 7.4.3. Profil Institute
- 7.4.3.1. Company Overview
- 7.4.3.2. Focused Clinical Expertise
- 7.4.3.2.1. Clinical Development
- 7.4.3.2.2. Clinical Research
- 7.4.3.2.3. Data Management and Statistical Services
- 7.4.3.2.4. Monitoring, Quality & Compliance
- 7.4.3.2.5. Recruitment Services
- 7.4.3.2.6. Regulatory Affairs
- 7.4.3.2.7. Scientific Services
- 7.4.3.2.8. Experience in Metabolic Disorders
- 7.4.3.3. Additional Information
- 7.4.3.3.1. Collaborations
- 7.4.1. Betagenex
- 7.5. Specialty CROs Focused on CNS
- 7.5.1. Biospective
- 7.5.1.1. Company Overview
- 7.5.1.2. Focused Clinical Expertise
- 7.5.1.2.1. Image Processing Technology
- 7.5.1.2.2. Rodent Models
- 7.5.1.2.3. Animal Imaging
- 7.5.1.2.4. Human Imaging and Clinical Trials
- 7.5.1.2.5. Histology and IHC
- 7.5.2. RenaSci
- 7.5.2.1. Company Overview
- 7.5.2.2. Focused Clinical Expertise
- 7.5.2.2.1. Experimental Services
- 7.5.2.2.2. Consultancy Services
- 7.5.2.3. Additional Information
- 7.5.2.3.1. Recent Developments
- 7.5.3. RxGen
- 7.5.3.1. Company Overview
- 7.5.3.2. Focused Clinical Expertise
- 7.5.3.2.1. Efficacy Models
- 7.5.3.2.2. Custom Model Development
- 7.5.3.2.3. Pharmacokinetics, Pharmacodynamics and Delivery Optimization Services
- 7.5.3.2.4. Toxicology and Safety Pharmacology
- 7.5.3.2.5. Supporting Technologies & Capabilities
- 7.5.3.3. Additional Information
- 7.5.3.3.1. Collaboration
- 7.5.3.3.2. Funding
- 7.5.3.3.3. Recent Developments
- 7.5.1. Biospective
8. CASE STUDY I: VIRTUAL CROs
- 8.1. Introduction to Virtual CROs
- 8.2. Frestedt
- 8.2.1. Company Overview
- 8.2.2. Service Portfolio
- 8.2.2.1. Clinical Research
- 8.2.2.2. Regulatory Affairs
- 8.2.2.3. Quality Systems
- 8.3. InSymbiosis
- 8.3.1. Company Overview
- 8.3.2. Service Portfolio
- 8.3.2.1. Drug Discovery
- 8.3.2.2. Lead Optimization and Efficacy Model
- 8.3.2.3. Non-Clinical Safety Studies and Bioanalysis
- 8.3.2.4. Regulatory IND / IMPD filings
- 8.3.2.5. Document Management
- 8.3.2.6. Phase I / II/ III Clinical Studies
- 8.3.2.7. Collaborations
- 8.3.3. Additional Information
- 8.3.3.1. Recent Developments
- 8.4. Osiris Pharma
- 8.4.1. Company Overview
- 8.4.2. Service Portfolio
- 8.4.2.1. Program Management
- 8.4.2.2. Consultancy
- 8.4.2.3. Non-clinical Assessment and Management
- 8.4.2.4. Preclinical Toxicology and Safety Studies
- 8.4.2.5. Communication and Monitoring
- 8.4.2.6. Writing and Reviewing of Reports
- 8.4.2.7. Other Services
- 8.5. ProjectPharm
- 8.5.1. Company Overview
- 8.5.2. Service Portfolio
- 8.5.2.1. Virtual CRO
- 8.5.2.2. Project Management Consulting / Organizational Project Management
- 8.5.2.3. Vendor Selection
- 8.5.2.4. Financial Audits
- 8.5.2.5. Training
- 8.5.2.6. Rescue Studies
- 8.5.2.7. Study Start-Up
- 8.6. The Harte Group
- 8.6.1. Company Overview
- 8.6.2. Service Portfolio
- 8.6.2.1. Virtual CRO
- 8.6.2.2. Consultation
- 8.6.2.3. Clinical Trial Project Management
- 8.6.2.4. Integrated Project Delivery and Accountability
- 8.7. VxP Pharma
- 8.7.1. Company Overview
- 8.7.2. Service Portfolio
- 8.7.2.1. Chemical Development
- 8.7.2.2. Preclinical
- 8.7.2.3. Preformulation and Solid State Chemistry
- 8.7.2.4. Analytical and Bioanalytical
- 8.7.2.4.1. Analytical Method Development and Validation
- 8.7.2.4.2. Extractables and Leachables Studies
- 8.7.2.4.3. Particle Size Determination
- 8.7.2.4.4. Container-API Compatibility
- 8.7.2.4.5. Material Characterization
- 8.7.2.4.6. Forced Degradation and Stability Studies
- 8.7.2.4.7. Drug Device Compatibility Studies
- 8.7.2.4.8. Microbial Testing
- 8.7.2.4.9. Bioanalytical Testing
- 8.7.2.5. Formulation Development and Clinical Trial materials
- 8.7.2.6. Parenteral and Lyophilized Clinical Trial Materials
- 8.7.2.7. Clinical Packaging and Distribution
- 8.7.2.8. Commercial Services
9. CASE STUDY II: FULL SERVICE CROs
- 9.1. Introduction to Traditional CROs
- 9.2. Covance
- 9.2.1. Company Overview
- 9.2.2. Service Portfolio
- 9.2.2.1. Analytical Services
- 9.2.2.2. Clinical Development
- 9.2.2.3. Clinical Testing
- 9.2.2.4. Consulting
- 9.2.2.5. Health Economics & Market Access
- 9.2.2.6. Lead Optimization
- 9.2.2.7. Manufacturing Services
- 9.2.2.8. Research
- 9.2.2.9. Safety Assessment
- 9.2.3. Collaborations
- 9.3. Medis Research Group
- 9.3.1. Company Overview
- 9.3.2. Focused Clinical Expertise
- 9.3.2.1. Biostatistics
- 9.3.2.2. Data Management
- 9.3.2.3. Medical Writing
- 9.3.2.4. Oncology Expertise
- 9.3.2.5. Pharmacovigilance
- 9.3.2.6. Proofreading and Translation
- 9.3.2.7. RDC System ODM QuaSi(R)
- 9.3.2.8. Regulatory Services
- 9.3.2.9. Scientific Consulting
- 9.3.2.10. Study Documentation
- 9.3.2.11. Study Management
- 9.3.2.12. Study Monitoring
- 9.3.2.13. Clinical Trial Phase Expertise
- 9.3.2.14. Additional Information
- 9.4. Quintiles
- 9.4.1. Company Overview
- 9.4.2. Service Portfolio
- 9.4.2.1. Clinical Trial Execution
- 9.4.2.2. Consulting
- 9.4.2.3. Laboratories
- 9.4.2.4. Real-World and Late Phase
- 9.4.2.5. Patient and Provider Engagement
- 9.4.2.6. Product Marketing and Sales
- 9.4.2.7. Technology Solutions
- 9.4.2.8. Portfolio and Strategic Planning
- 9.4.3. Financial Performance
- 9.4.4. Collaborations
- 9.4.5. Recent Developments
- 9.5. Triclinium Clinical Trial Project Management
- 9.5.1. Company Overview
- 9.5.1.1. Focused Clinical Expertise
- 9.5.1.2. Additional Information
- 9.5.1.2.1. Collaborations
- 9.5.1. Company Overview
10. MARKET FORECAST
- 10.1. Chapter Overview
- 10.2. Forecast Methodology
- 10.3. Global Specialty CROs Market, Till 2035
- 10.4. Regional Specialty CROs Market, Till 2035
11. FUTURE OPPORTUNITIES
- 11.1. Chapter Overview
- 11.2. The Changing Scenario of Outsourcing
- 11.3. Health Economics and Outcomes Research (HEOR) Studies
- 11.4. Adaptive Trial Design
- 11.5. eClinical Solutions
- 11.6. Risk Based Monitoring (RBM)
- 11.7. Digital CRO (dCRO)
12. CONCLUSION
- 12.1. A Widening Portfolio of Services Governed by Industry Constraints
- 12.2. Closer Working Collaboration is the Key to Success
- 12.3. Within Therapeutic Areas, Oncology is the Flagbearer
- 12.4. Due to Several Niche Offerings, Specialty CRO Market Remains Fragmented
- 12.5. The Market of Specialty CROs is Likely to Sustain the Growth Momentum
- 12.6. Untapped Opportunity Areas will Emerge as Key Growth Drivers in the Long-Term
- 12.7. Concluding Remarks
13. INTERVIEW TRANSCRIPTS
- 13.1. Chapter Overview
- 13.2. President, CRO and Outcomes Research, Company A
- 13.3. President, Company B


