
|
市場調査レポート
商品コード
1762523
分子診断市場:業界動向と世界の予測-検査タイプ別、オファリングタイプ別、サンプルタイプ別、技術タイプ別、治療領域別、エンドユーザー別、地域別Molecular Diagnostics Market: Industry Trends and Global Forecasts - Distribution by Test Type, Type of Offering, Type of Sample, Type of Technology, Therapeutic Area, End Users, and Geographical Regions |
||||||
カスタマイズ可能
|
|||||||
| 分子診断市場:業界動向と世界の予測-検査タイプ別、オファリングタイプ別、サンプルタイプ別、技術タイプ別、治療領域別、エンドユーザー別、地域別 |
|
出版日: 2025年07月04日
発行: Roots Analysis
ページ情報: 英文 224 Pages
納期: 即日から翌営業日
|
全表示
- 概要
- 図表
- 目次
分子診断市場:概要
世界の分子診断の市場規模は、2035年までの予測期間中に6.2%のCAGRで拡大し、現在の159億米ドルから2035年までに309億米ドルに成長すると予測されています。
市場セグメンテーションでは、市場規模と機会分析を以下のパラメータで区分しています:
検査タイプ
- ラボ検査
- ポイントオブケア検査
オファリングタイプ
- 試薬
- 検査機器
- サービス
サンプルタイプ
- 血液、血清、血漿
- 尿
- その他
技術タイプ
- ポリメラーゼ連鎖反応(PCR)
- in situハイブリダイゼーション
- 等温核酸増幅技術
- 次世代シーケンス
- マイクロアレイ
- 質量分析
- その他
治療領域
- 心血管疾患
- 遺伝子疾患
- 感染症
- 神経疾患
- 腫瘍疾患
- その他
エンドユーザー
- 病院
- 研究所
- その他
主な地域
- 北米(米国、カナダ)
- 欧州(オーストリア、ベルギー、フランス、ドイツ、イタリア、オランダ、ポーランド、スペイン、スイス、英国、その他)
- アジア(中国、インド、インドネシア、日本、シンガポール、韓国、タイ、その他)
- ラテンアメリカ(アルゼンチン、ブラジル、メキシコ、その他)
- 中東・北アフリカ(エジプト、イスラエル、サウジアラビア、その他)
- その他の地域(オーストラリア、ニュージーランド)
分子診断薬市場:成長と動向
分子診断検査は、ゲノムやプロテオーム中の生物学的マーカーを分析するために使用される高度な技術やツールです。これらの診断ソリューションは、疾患の検出とモニタリング、遺伝子異常の特定、個別化治療計画の指導に不可欠です。分子診断領域で使用される主な技術には、ポリメラーゼ連鎖反応、次世代シーケンシング、マイクロアレイなどがあります。PCRは微量のDNAやRNAの増幅と検出を可能にする特異性の高い技術であるのに対し、NGSはゲノム全体のハイスループットなシーケンシングを可能にします。このように、分子診断ソリューションは、がん疾患、感染症、遺伝子検査、個別化医療など、さまざまな医療分野で極めて重要な役割を担っています。これらのソリューションは診断の精度を高め、オーダーメイドの治療戦略を可能にし、サポートすることで、最終的には診断結果の改善と公衆衛生の向上を目指しています。さらに、ヘルスケアの意思決定の70%以上が臨床検査結果に基づいて行われていることは、患者ケアにおけるこうした診断ツールの重要性を反映しています。
さらに、迅速な検査、所要時間の短縮、迅速な意思決定など、分子診断ソリューションが提供するいくつかの利点により、市場は予測期間中、健全なCAGRで成長すると予想されます。
分子診断市場:主要インサイト
当レポートでは、分子診断市場の現状を調査し、潜在的な成長機会を特定しています。当レポートの主な調査結果は以下の通りです。
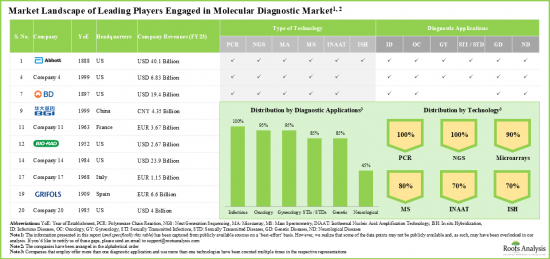
- 分子診断領域は、様々な診断アプリケーションを提供するために様々な種類の先端技術を利用する参入企業によるダイナミックな市場情勢を特徴とします。
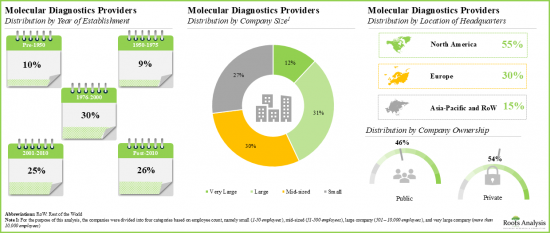
- 本分析で対象とした多くの大手企業は1951年から2000年に設立され、その60%は北米を拠点としています。
- 分子診断ソリューションの多様なポートフォリオと近年の好調な業績により、ロシュはこの分野の主要企業の中で最も実力のある企業として浮上しました。
- 分子診断市場における様々な動向の影響を調査するため、弊社は独自の調査手法を開発し、ポーターのファイブフォースの枠組みの下で様々なパラメータを分析しました。
- 分子診断薬市場は予防ヘルスケアに対する意識の高まりに後押しされているが、規制の複雑さへの対応など、業界参入企業にとっては依然として大きなハードルとなっています。
- 世界中で慢性疾患の有病率が高まっていることに後押しされ、分子診断薬市場は予測期間中6.2%の健全な成長率で成長すると予想されます。
- 予測される将来機会は、検査タイプ、サンプルタイプ、治療領域、エンドユーザーなどの複数のセグメントにうまく分散されると予想されます。
分子診断市場:主要セグメント
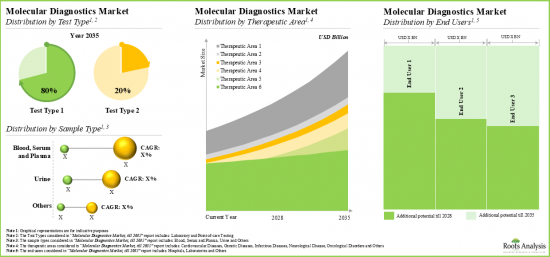
検査タイプにより、市場はラボ検査とポイントオブケア検査に区分されます。現在、分子診断薬市場ではラボ検査セグメントが最大シェアを占めています。この動向は今後も変わらないと思われます。さらに、ポイントオブケア検査分野の分子診断市場は、予測期間中に最も高い市場成長ポテンシャルを示すと予想されます。
オファリングタイプ別では、試薬が世界の分子診断薬市場で最も急成長しています。
試薬、機器、サービスに区分されます。現在のところ、試薬セグメントが世界の分子診断薬市場で最大のシェアを占めています。さらに、試薬は頻繁に補充する必要があり、これが経常収益に貢献することから、試薬セグメントは予測期間中により高いCAGRで成長すると予想されます。
サンプルタイプ別では、市場は血液、血清・血漿、尿、その他の検体に区分されます。現在、血液、血清、血漿セグメントが世界の分子診断薬市場で最も高い割合を占めています。しかし、尿セグメントは予測期間中により高いCAGRで成長すると予想されています。
技術タイプ別では、市場はポリメラーゼ連鎖反応(PCR)、in situハイブリダイゼーション、等温核酸増幅技術、次世代シーケンシング、マイクロアレイ、質量分析、その他に区分されます。ポリメラーゼ連鎖反応(PCR)分野が市場全体の主な促進要因になると予想されるが、次世代シーケンシング分野の世界分子診断市場が比較的高いCAGRで成長する可能性が高いことは注目に値します。これは、ハイスループット、精度の向上、複数の遺伝子の同時配列決定機能など、次世代シーケンサーが提供するいくつかの利点に起因すると考えられます。
市場は治療領域別に、心血管疾患、遺伝性疾患、感染症、神経疾患、腫瘍性疾患、その他に区分されます。現在のところ、感染症分野が分子診断薬市場の最大シェアを占めています。さらに、神経疾患セグメントは予測期間中に最も高い成長ポテンシャルを示し、他のセグメントと比較して高いCAGRで成長すると予想されています。
エンドユーザー別では、世界市場は病院、研究所、その他に区分されます。現在、病院セグメントが最大の市場シェアを占めています。しかし、検査室向け分子診断市場は今後数年で大幅な成長が見込まれます。
主要地域別に見ると、市場は北米、欧州、アジア、ラテンアメリカ、中東・アフリカ、その他ラテンアメリカに区分されます。現在、北米が世界の分子診断薬市場を独占しており、最大の収益シェアを占めています。さらに、アジア太平洋地域の市場は、今後より高いCAGRで成長すると思われます。
分子診断市場における参入企業例
- Abbott Laboratories
- Agilent Technologies
- Becton Dickinson
- BGI Genomics
- bioMerieux
- Bio-Rad
- Danaher
- DiaSorin
- Grifols
- Hologic
- Illumina
- Qiagen
- QuidelOrtho
- Revvity
- Roche
- Sansure
- Seegene
- Siemens Healthineers
- Sysmex
- Thermo Fisher Scientific
目次
第1章 背景
第2章 調査手法
第3章 経済的およびその他のプロジェクト特有の考慮事項
第4章 エグゼクティブサマリー
第5章 イントロダクション
- 分子診断の概要
- 分子診断ソリューションに採用されている主要技術
- 分子診断分野における課題
- 分子診断分野における最近の動向
- 分子診断分野の将来展望
第6章 市場影響分析:促進要因、抑制要因、機会、課題
第7章 世界の分子診断市場
- 主要な前提と調査手法
- 世界の分子診断市場(2035年まで)
第8章 分子診断市場(検査タイプ別)
- 市場変動分析
- 分子診断市場:検査タイプ別
- 2035年までの臨床検査向け分子診断市場
- 2035年までのPOC検査向け分子診断市場
第9章 分子診断市場(オファリングタイプ別)
- 市場変動分析
- 分子診断市場:オファリングタイプ別
第10章 分子診断市場(サンプルタイプ別)
- 市場変動分析
- 分子診断市場:サンプルタイプ別
第11章 分子診断市場(技術別)
- 市場変動分析
- 分子診断市場:技術タイプ別
第12章 分子診断市場(治療領域別)
- 市場変動分析
- 分子診断市場:治療領域別
第13章 分子診断市場(エンドユーザー別)
- 市場変動分析
- 分子診断市場:エンドユーザー別
第14章 分子診断市場(地域別)
- 市場変動分析
- 分子診断市場:地域別
第15章 分子診断市場(主要企業別)
- 分子診断市場:年間売上高別主要企業
第16章 市場概要:主要分子診断ソリューションプロバイダー
- 分子診断ソリューション:市場情勢
- 分子診断:ソリューションプロバイダーの情勢
第17章 企業競争力分析:分子診断ソリューションプロバイダー
- 評価調査手法と主要パラメータ
- 分子診断ソリューションプロバイダー:大手企業の競争力分析
- 分子診断ソリューションプロバイダー:大手企業の競争力分析
- ベンチマーク分析:主要な分子診断ソリューションプロバイダー
第18章 企業プロファイル:北米を拠点とする分子診断ソリューションプロバイダー
- 詳細な企業プロファイル
- Abbott
- Agilent Technologies
- BD
- Danaher
- Thermo Fisher Scientific
- その他の企業プロファイル
- Bio-Rad
- Illumina
- Hologic
- PerkinElmer
- QuidelOrtho
第19章 企業プロファイル:欧州を拠点とする分子診断ソリューションプロバイダー
- 詳細な企業プロファイル
- bioMerieux
- Grifols
- Roche
- Siemens Healthineers
- その他の企業プロファイル
- DiaSorin
- Qiagen
第20章 企業プロファイル:アジアを拠点とする分子診断ソリューションプロバイダー
- 詳細な企業プロファイル
- Sysmex
- その他の企業プロファイル
- BGI Genomics
- Sansure
- Seegene
第21章 ポーターのファイブフォース分析
第22章 付録I:表形式データ
第23章 付録II:企業および組織の一覧
List of Tables
- Table 16.1 Leading Molecular Diagnostics Solution Providers: Information on Year of Establishment, Headquarters, Company Ownership, Type of Technology and Diagnostic Applications
- Table 16.2 Other Leading Molecular Diagnostics Solution Providers: Information on Year of Establishment, Headquarters and Company Ownership
- Table 18.1 Abbott: Company Overview
- Table 18.2 Abbott: Product Portfolio
- Table 18.3 Abbott: Recent Developments and Future Outlook
- Table 18.4 Agilent Technologies: Company Overview
- Table 18.5 Agilent Technologies: Product Portfolio
- Table 18.6 Agilent Technologies: Recent Developments and Future Outlook
- Table 18.7 BD: Company Overview
- Table 18.8 BD: Product Portfolio
- Table 18.9 BD: Recent Developments and Future Outlook
- Table 18.10 Danaher: Company Overview
- Table 18.11 Danaher: Product Portfolio
- Table 18.12 Danaher: Recent Developments and Future Outlook
- Table 18.13 Roche: Company Overview
- Table 18.14 Roche: Product Portfolio
- Table 18.15 Roche: Recent Developments and Future Outlook
- Table 18.16 Thermo Fisher Scientific: Company Overview
- Table 18.17 Thermo Fisher Scientific: Product Portfolio
- Table 18.18 Thermo Fisher Scientific: Recent Developments and Future Outlook
- Table 18.19 Bio-Rad: Company Overview
- Table 18.20 Bio-Rad: Product Portfolio
- Table 18.21 Illumina: Company Overview
- Table 18.22 Illumina: Product Portfolio
- Table 18.23 Hologic: Company Overview
- Table 18.24 Hologic: Product Portfolio
- Table 18.25 PerkinElmer: Company Overview
- Table 18.26 PerkinElmer: Product Portfolio
- Table 18.27 QuidelOrtho: Company Overview
- Table 18.28 QuidelOrtho: Product Portfolio
- Table 19.1 bioMerieux: Company Overview
- Table 19.2 bioMerieux: Product Portfolio
- Table 19.3 bioMerieux: Recent Developments and Future Outlook
- Table 19.4 Grifols: Company Overview
- Table 19.5 Grifols: Product Portfolio
- Table 19.6 Grifols: Recent Developments and Future Outlook
- Table 19.7 Siemens Healthineers: Company Overview
- Table 19.8 Siemens Healthineers: Product Portfolio
- Table 19.9 Siemens Healthineers: Recent Developments and Future Outlook
- Table 19.10 DiaSorin: Company Overview
- Table 19.11 DiaSorin: Product Portfolio
- Table 19.12 Qiagen: Company Overview
- Table 19.13 Qiagen: Product Portfolio
- Table 20.1 Sysmex: Company Overview
- Table 20.2 Sysmex: Product Portfolio
- Table 20.3 Sysmex: Recent Developments and Future Outlook
- Table 20.4 BGI Genomics: Company Overview
- Table 20.5 BGI Genomics: Product Portfolio
- Table 20.6 Sansure: Company Overview
- Table 20.7 Sansure: Product Portfolio
- Table 20.8 Seegene: Company Overview
- Table 20.9 Seegene: Product Portfolio
- Table 22.1 Global Molecular Diagnostics Market, Till 2035 (USD Billion)
- Table 22.2 Global Molecular Diagnostics Market, Conservative Scenario, till 2035 (USD Billion)
- Table 22.3 Global Molecular Diagnostics Market, Optimistic Scenario, till 2035 (USD Billion)
- Table 22.4 Molecular Diagnostics Market: Distribution by Test Type
- Table 22.5 Molecular Diagnostics Market for Laboratory Testing, Till 2035 (USD Billion)
- Table 22.6 Molecular Diagnostics Market for Point-of-Care Testing, Till 2035 (USD Billion)
- Table 22.7 Molecular Diagnostics Market: Distribution by Type of Offering
- Table 22.8 Molecular Diagnostics Market for Instruments, Till 2035 (USD Billion)
- Table 22.9 Molecular Diagnostics Market for In-house Instruments, Till 2035 (USD Billion)
- Table 22.10 Molecular Diagnostics Market for Outsourced Instruments, Till 2035 (USD Billion)
- Table 22.11 Molecular Diagnostics Market for Reagents, Till 2035 (USD Billion)
- Table 22.12 Molecular Diagnostics Market for Services, Till 2035 (USD Billion)
- Table 22.13 Molecular Diagnostics Market: Distribution by Sample Type
- Table 22.14 Molecular Diagnostics Market for Blood, Serum and Plasma, Till 2035 (USD Billion)
- Table 22.15 Molecular Diagnostics Market for Urine, Till 2035 (USD Billion)
- Table 22.16 Molecular Diagnostics Market for Others, Till 2035 (USD Billion)
- Table 22.17 Molecular Diagnostics Market: Distribution by Type of Technology
- Table 22.18 Molecular Diagnostics Market for PCR, Till 2035 (USD Billion)
- Table 22.19 Molecular Diagnostics Market for In situ Hybridization, Till 2035 (USD Billion)
- Table 22.20 Molecular Diagnostics Market for Isothermal Nucleic Acid Amplification Technology, Till 2035 (USD Billion)
- Table 22.21 Molecular Diagnostics Market for Next Generation Sequencing, Till 2035 (USD Billion)
- Table 22.22 Molecular Diagnostics Market for Microarrays, Till 2035 (USD Billion)
- Table 22.23 Molecular Diagnostics Market for Mass Spectrometry, Till 2035 (USD Billion)
- Table 22.24 Molecular Diagnostics Market for Other Technologies, Till 2035 (USD Billion)
- Table 22.25 Molecular Diagnostics Market: Distribution by Therapeutic Area
- Table 22.26 Molecular Diagnostics Market for Infectious Diseases, Till 2035 (USD Billion)
- Table 22.27 Molecular Diagnostics Market for COVID-19, Till 2035 (USD Billion)
- Table 22.28 Molecular Diagnostics Market for Respiratory Infections (Excluding COVID-19), Till 2035 (USD Billion)
- Table 22.29 Molecular Diagnostics Market for Healthcare-associated Infections, Till 2035 (USD Billion)
- Table 22.30 Molecular Diagnostics Market for Hepatitis, Till 2035 (USD Billion)
- Table 22.31 Molecular Diagnostics Market for HIV, Till 2035 (USD Billion)
- Table 22.32 Molecular Diagnostics Market for Sexually Transmitted Diseases, Till 2035 (USD Billion)
- Table 22.33 Molecular Diagnostics Market for Other Infectious Diseases, Till 2035 (USD Billion)
- Table 22.34 Molecular Diagnostics Market for Oncological Disorders, Till 2035 (USD Billion)
- Table 22.35 Molecular Diagnostics Market for Lung Cancer, Till 2035 (USD Billion)
- Table 22.36 Molecular Diagnostics Market for Breast Cancer, Till 2035 (USD Billion)
- Table 22.37 Molecular Diagnostics Market for Colorectal Cancer, Till 2035 (USD Billion)
- Table 22.38 Molecular Diagnostics Market for Prostate Cancer, Till 2035 (USD Billion)
- Table 22.39 Molecular Diagnostics Market for Gastric Cancer, Till 2035 (USD Billion)
- Table 22.40 Molecular Diagnostics Market for Other Oncological Disorders, Till 2035 (USD Billion)
- Table 22.41 Molecular Diagnostics Market for Cardiovascular Diseases, Till 2035 (USD Billion)
- Table 22.42 Molecular Diagnostics Market for Neurological Diseases, Till 2035 (USD Billion)
- Table 22.43 Molecular Diagnostics Market for Genetic Diseases, Till 2035 (USD Billion)
- Table 22.44 Molecular Diagnostics Market for Other Therapeutic Areas, Till 2035 (USD Billion)
- Table 22.45 Molecular Diagnostics Market: Distribution by End Users
- Table 22.46 Molecular Diagnostics Market for Laboratories, Till 2035 (USD Billion)
- Table 22.47 Molecular Diagnostics Market for Large Laboratories, Till 2035 (USD Billion)
- Table 22.48 Molecular Diagnostics Market for Small and Medium-sized Laboratories, Till 2035 (USD Billion)
- Table 22.49 Molecular Diagnostics Market for Hospitals, Till 2035 (USD Billion)
- Table 22.50 Molecular Diagnostics Market for Other End Users, Till 2035 (USD Billion)
- Table 22.51 Molecular Diagnostics Market: Distribution by Geographical Regions
- Table 22.52 Molecular Diagnostics Market in North America, Till 2035 (USD Billion)
- Table 22.53 Molecular Diagnostics Market in the US, Till 2035 (USD Billion)
- Table 22.54 Molecular Diagnostics Market in Canada, Till 2035 (USD Billion)
- Table 22.55 Molecular Diagnostics Market in Europe, Till 2035 (USD Billion)
- Table 22.56 Molecular Diagnostics Market in Austria, Till 2035 (USD Billion)
- Table 22.57 Molecular Diagnostics Market in Belgium, Till 2035 (USD Billion)
- Table 22.58 Molecular Diagnostics Market in France, Till 2035 (USD Billion)
- Table 22.59 Molecular Diagnostics Market in Germany, Till 2035 (USD Billion)
- Table 22.60 Molecular Diagnostics Market in Italy, Till 2035 (USD Billion)
- Table 22.61 Molecular Diagnostics Market in the Netherlands, Till 2035 (USD Billion)
- Table 22.62 Molecular Diagnostics Market in Poland, Till 2035 (USD Billion)
- Table 22.63 Molecular Diagnostics Market in Spain, Till 2035 (USD Billion)
- Table 22.64 Molecular Diagnostics Market in Switzerland, Till 2035 (USD Billion)
- Table 22.65 Molecular Diagnostics Market in the UK, Till 2035 (USD Billion)
- Table 22.66 Molecular Diagnostics Market in Rest of the Europe, Till 2035 (USD Billion)
- Table 22.67 Molecular Diagnostics Market in Asia, Till 2035 (USD Billion)
- Table 22.68 Molecular Diagnostics Market in China, Till 2035 (USD Billion)
- Table 22.69 Molecular Diagnostics Market in India, Till 2035 (USD Billion)
- Table 22.70 Molecular Diagnostics Market in Indonesia, Till 2035 (USD Billion)
- Table 22.71 Molecular Diagnostics Market in Japan, Till 2035 (USD Billion)
- Table 22.72 Molecular Diagnostics Market in Singapore, Till 2035 (USD Billion)
- Table 22.73 Molecular Diagnostics Market in South Korea, Till 2035 (USD Billion)
- Table 22.74 Molecular Diagnostics Market in Thailand, Till 2035 (USD Billion)
- Table 22.75 Molecular Diagnostics Market in Rest of Asia, Till 2035 (USD Billion)
- Table 22.76 Molecular Diagnostics Market in Latin America, Till 2035 (USD Billion)
- Table 22.77 Molecular Diagnostics Market in Brazil, Till 2035 (USD Billion)
- Table 22.78 Molecular Diagnostics Market in Argentina, Till 2035 (USD Billion)
- Table 22.79 Molecular Diagnostics Market in Mexico, Till 2035 (USD Billion)
- Table 22.80 Molecular Diagnostics Market in Rest of Latin America, Till 2035 (USD Billion)
- Table 22.81 Molecular Diagnostics Market in Middle East and North Africa, Till 2035 (USD Billion)
- Table 22.82 Molecular Diagnostics Market in Egypt, Till 2035 (USD Billion)
- Table 22.83 Molecular Diagnostics Market in Israel, Till 2035 (USD Billion)
- Table 22.84 Molecular Diagnostics Market in Saudi Arabia, Till 2035 (USD Billion)
- Table 22.85 Molecular Diagnostics Market in Rest of Middle East and North Africa, Till 2035 (USD Billion)
- Table 22.86 Molecular Diagnostics Market in Rest of the World, Till 2035 (USD Billion)
- Table 22.87 Molecular Diagnostics Market in Australia, Till 2035 (USD Billion)
- Table 22.88 Molecular Diagnostics Market in New Zealand, Till 2035 (USD Billion)
- Table 22.89 Molecular Diagnostics Market: Distribution of Leading Players by Annual Revenues (FY23, USD Billion
- Table 22.90 Molecular Diagnostic Solution: Distribution by Type of Technology
- Table 22.91 Molecular Diagnostic Solution: Distribution by Diagnostic Applications
- Table 22.92 Molecular Diagnostic Solution: Distribution by Type of Technology and Diagnostic Applications
- Table 22.93 Molecular Diagnostics Solution Providers: Distribution by Year of Establishment
- Table 22.94 Molecular Diagnostics Solution Providers: Distribution by Company Size
- Table 22.95 Molecular Diagnostics Solution Providers: Distribution by Location of Headquarters
- Table 22.96 Molecular Diagnostics Solution Providers: Distribution by Company Ownership
- Table 22.97 Abbott: Financial Information (USD Billion)
- Table 22.98 Agilent Technologies: Financial Information (USD Billion)
- Table 22.99 BD: Financial Information (USD Billion)
- Table 22.100 Danaher: Financial Information (USD Billion)
- Table 22.101 Thermo Fisher Scientific: Financial Information (CHF Billion)
- Table 22.102 bioMerieux: Financial Information (EUR Billion)
- Table 22.103 Grifols: Financial Information (EUR Billion)
- Table 22.104 Roche: Financial Information (CHF Billion)
- Table 22.105 Siemens Healthineers: Financial Information (EUR Billion)
- Table 22.106 Sysmex: Financial Information (JPY Billion)
List of Figures
- Figure 2.1 Research Methodology: Research Assumptions
- Figure 2.2 Research Methodology: Project Methodology
- Figure 2.3 Research Methodology: Forecast Methodology
- Figure 2.4 Research Methodology: Robust Quality Control
- Figure 2.5 Research Methodology: Key Market Segmentations
- Figure 5.1 Key Technologies Employed in Molecular Diagnostic Solution
- Figure 5.2 Challenges in the Molecular Diagnostics Domain
- Figure 6.1 Market Drivers
- Figure 6.2 Market Restraints
- Figure 6.3 Market Opportunities
- Figure 6.4 Market Challenges
- Figure 7.1 Global Molecular Diagnostics Market, Till 2035 (USD Billion)
- Figure 7.2 Global Molecular Diagnostics Market, Conservative Scenario, Till 2035 (USD Billion)
- Figure 7.3 Global Molecular Diagnostics Market, Optimistic Scenario, Till 2035 (USD Billion)
- Figure 8.1 Molecular Diagnostics Market: Distribution by Test Type
- Figure 8.2 Molecular Diagnostics Market for Laboratory Testing, Till 2035 (USD Billion)
- Figure 8.3 Molecular Diagnostics Market for Point-of-Care Testing, Till 2035 (USD Billion)
- Figure 9.1 Molecular Diagnostics Market: Distribution by Type of Offering
- Figure 9.2 Molecular Diagnostics Market for Instruments, Till 2035 (USD Billion)
- Figure 9.3 Molecular Diagnostics Market for In-house Instruments, Till 2035 (USD Billion)
- Figure 9.4 Molecular Diagnostics Market for Outsourced Instruments, Till 2035 (USD Billion)
- Figure 9.5 Molecular Diagnostics Market for Reagents, Till 2035 (USD Billion)
- Figure 9.6 Molecular Diagnostics Market for Services, Till 2035 (USD Billion)
- Figure 10.1 Molecular Diagnostics Market: Distribution by Sample Type
- Figure 10.2 Molecular Diagnostics Market for Blood, Serum and Plasma, Till 2035 (USD Billion)
- Figure 10.3 Molecular Diagnostics Market for Urine, Till 2035 (USD Billion)
- Figure 10.4 Molecular Diagnostics Market for Others, Till 2035 (USD Billion)
- Figure 11.1 Molecular Diagnostics Market: Distribution by Type of Technology
- Figure 11.2 Molecular Diagnostics Market for PCR, Till 2035 (USD Billion)
- Figure 11.3 Molecular Diagnostics Market for In Situ Hybridization, Till 2035 (USD Billion)
- Figure 11.4 Molecular Diagnostics Market for Isothermal Nucleic Acid Amplification Technology, Till 2035 (USD Billion)
- Figure 11.5 Molecular Diagnostics Market for Next Generation Sequencing, Till 2035 (USD Billion)
- Figure 11.6 Molecular Diagnostics Market for Microarrays, Till 2035 (USD Billion)
- Figure 11.7 Molecular Diagnostics Market for Mass Spectrometry, Till 2035 (USD Billion)
- Figure 11.8 Molecular Diagnostics Market for Other Technologies, Till 2035 (USD Billion)
- Figure 12.1 Molecular Diagnostics Market: Distribution by Therapeutic Area
- Figure 12.2 Molecular Diagnostics Market for Infectious Diseases, Till 2035 (USD Billion)
- Figure 12.3 Molecular Diagnostics Market for COVID-19, Till 2035 (USD Billion)
- Figure 12.4 Molecular Diagnostics Market for Respiratory Infections (Excluding COVID-19), Till 2035 (USD Billion)
- Figure 12.5 Molecular Diagnostics Market for Healthcare-associated Infections, Till 2035 (USD Billion)
- Figure 12.6 Molecular Diagnostics Market for Hepatitis, Till 2035 (USD Billion)
- Figure 12.7 Molecular Diagnostics Market for HIV, Till 2035 (USD Billion)
- Figure 12.8 Molecular Diagnostics Market for Sexually Transmitted Diseases, Till 2035 (USD Billion)
- Figure 12.9 Molecular Diagnostics Market for Other Infectious Diseases, Till 2035 (USD Billion)
- Figure 12.10 Molecular Diagnostics Market for Oncological Disorders, Till 2035 (USD Billion)
- Figure 12.11 Molecular Diagnostics Market for Lung Cancer, Till 2035 (USD Billion)
- Figure 12.12 Molecular Diagnostics Market for Breast Cancer, Till 2035 (USD Billion)
- Figure 12.13 Molecular Diagnostics Market for Colorectal Cancer, Till 2035 (USD Billion)
- Figure 12.14 Molecular Diagnostics Market for Prostate Cancer, Till 2035 (USD Billion)
- Figure 12.15 Molecular Diagnostics Market for Gastric Cancer, Till 2035 (USD Billion)
- Figure 12.16 Molecular Diagnostics Market for Other Oncological Disorders, Till 2035 (USD Billion)
- Figure 12.17 Molecular Diagnostics Market for Cardiovascular Diseases, Till 2035 (USD Billion)
- Figure 12.18 Molecular Diagnostics Market for Neurological Diseases, Till 2035 (USD Billion)
- Figure 12.19 Molecular Diagnostics Market for Genetic Diseases, Till 2035 (USD Billion)
- Figure 12.20 Molecular Diagnostics Market for Other Therapeutic Areas, Till 2035 (USD Billion)
- Figure 13.1 Molecular Diagnostics Market: Distribution by End Users
- Figure 13.2 Molecular Diagnostics Market for Laboratories, Till 2035 (USD Billion)
- Figure 13.3 Molecular Diagnostics Market for Large Laboratories, Till 2035 (USD Billion)
- Figure 13.4 Molecular Diagnostics Market for Small and Medium-sized Laboratories, Till 2035 (USD Billion)
- Figure 13.5 Molecular Diagnostics Market for Hospitals, Till 2035 (USD Billion)
- Figure 13.6 Molecular Diagnostics Market for Other End Users, Till 2035 (USD Billion)
- Figure 14.1 Molecular Diagnostics Market: Distribution by Geographical Regions
- Figure 14.2 Molecular Diagnostics Market in North America, Till 2035 (USD Billion)
- Figure 14.3 Molecular Diagnostics Market in the US, Till 2035 (USD Billion)
- Figure 14.4 Molecular Diagnostics Market in Canada, Till 2035 (USD Billion)
- Figure 14.5 Molecular Diagnostics Market in Europe, Till 2035 (USD Billion)
- Figure 14.6 Molecular Diagnostics Market in Austria, Till 2035 (USD Billion)
- Figure 14.7 Molecular Diagnostics Market in Belgium, Till 2035 (USD Billion)
- Figure 14.8 Molecular Diagnostics Market in France, Till 2035 (USD Billion)
- Figure 14.9 Molecular Diagnostics Market in Germany, Till 2035 (USD Billion)
- Figure 14.10 Molecular Diagnostics Market in Italy, Till 2035 (USD Billion)
- Figure 14.11 Molecular Diagnostics Market in the Netherlands, Till 2035 (USD Billion)
- Figure 14.12 Molecular Diagnostics Market in Poland, Till 2035 (USD Billion)
- Figure 14.13 Molecular Diagnostics Market in Spain, Till 2035 (USD Billion)
- Figure 14.14 Molecular Diagnostics Market in Switzerland, Till 2035 (USD Billion)
- Figure 14.15 Molecular Diagnostics Market in the UK, Till 2035 (USD Billion)
- Figure 14.16 Molecular Diagnostics Market in Rest of Europe, Till 2035 (USD Billion)
- Figure 14.17 Molecular Diagnostics Market in Asia, Till 2035 (USD Billion)
- Figure 14.18 Molecular Diagnostics Market in China, Till 2035 (USD Billion)
- Figure 14.19 Molecular Diagnostics Market in India, Till 2035 (USD Billion)
- Figure 14.20 Molecular Diagnostics Market in Indonesia, Till 2035 (USD Billion)
- Figure 14.21 Molecular Diagnostics Market in Japan, Till 2035 (USD Billion)
- Figure 14.22 Molecular Diagnostics Market in Singapore, Till 2035 (USD Billion)
- Figure 14.23 Molecular Diagnostics Market in South Korea, Till 2035 (USD Billion)
- Figure 14.24 Molecular Diagnostics Market in Thailand, Till 2035 (USD Billion)
- Figure 14.25 Molecular Diagnostics Market in Rest of the Asia, Till 2035 (USD Billion)
- Figure 14.26 Molecular Diagnostics Market in Latin America, Till 2035 (USD Billion)
- Figure 14.27 Molecular Diagnostics Market in Brazil, Till 2035 (USD Billion)
- Figure 14.28 Molecular Diagnostics Market in Argentina, Till 2035 (USD Billion)
- Figure 14.29 Molecular Diagnostics Market in Mexico, Till 2035 (USD Billion)
- Figure 14.30 Molecular Diagnostics Market in Rest of Latin America, Till 2035 (USD Billion)
- Figure 14.31 Molecular Diagnostics Market in Middle East and North Africa, Till 2035 (USD Billion)
- Figure 14.32 Molecular Diagnostics Market in Egypt, Till 2035 (USD Billion)
- Figure 14.33 Molecular Diagnostics Market in Israel, Till 2035 (USD Billion)
- Figure 14.34 Molecular Diagnostics Market in Saudi Arabia, Till 2035 (USD Billion)
- Figure 14.35 Molecular Diagnostics Market in Rest of Middle East and North Africa, Till 2035 (USD Billion)
- Figure 14.36 Molecular Diagnostics Market in Rest of the World, Till 2035 (USD Billion)
- Figure 14.37 Molecular Diagnostics Market in Australia, Till 2035 (USD Billion)
- Figure 14.38 Molecular Diagnostics Market in New Zealand, Till 2035 (USD Billion)
- Figure 15.1 Molecular Diagnostics Market: Distribution of Leading Players by Annual Revenue (FY23, USD Billion)
- Figure 16.1 Molecular Diagnostic Solution: Distribution by Type of Technology
- Figure 16.2 Molecular Diagnostic Solution: Distribution by Diagnostic Applications
- Figure 16.3 Molecular Diagnostic Solution: Distribution by Type of Technology and Diagnostic Applications
- Figure 16.4 Molecular Diagnostics Solution Providers: Distribution by Year of Establishment
- Figure 16.5 Molecular Diagnostics Solution Providers: Distribution by Company Size
- Figure 16.6 Molecular Diagnostics Solution Providers: Distribution by Location of Headquarters
- Figure 16.7 Molecular Diagnostics Solution Providers: Distribution by Company Ownership
- Figure 17.1 Molecular Diagnostic Solution Providers: Competitiveness Analysis of Very Large Players
- Figure 17.2 Molecular Diagnostic Solution Providers: Competitiveness Analysis of Large Players
- Figure 17.3 Roche: Benchmarking Analysis
- Figure 17.4 Abbott: Benchmarking Analysis
- Figure 17.5 Thermo Fisher Scientific: Benchmarking Analysis
- Figure 17.6 Qiagen: Benchmarking Analysis
- Figure 17.7 bioMerieux: Benchmarking Analysis
- Figure 17.8 DiaSorin: Benchmarking Analysis
- Figure 17.9 Illumina: Benchmarking Analysis
- Figure 17.10 Sysmex: Benchmarking Analysis
- Figure 17.11 Perkin Elmer: Benchmarking Analysis
- Figure 17.12 Bio-Rad: Benchmarking Analysis
- Figure 17.13 Leading Molecular Diagnostic Solution Providers: Benchmarking by Competitiveness
- Figure 17.14 Leading Molecular Diagnostic Solution Providers: Benchmarking by Type of Technology Score
- Figure 17.15 Leading Molecular Diagnostic Solution Providers: Benchmarking by Diagnostic Applications Score
- Figure 18.1 Abbott: Financial Information (USD Billion)
- Figure 18.2 Agilent Technologies: Financial Information (USD Billion)
- Figure 18.3 BD: Financial Information (USD Billion)
- Figure 18.4 Danaher: Financial Information (USD Billion)
- Figure 18.5 Thermo Fisher Scientific: Financial Information (CHF Billion)
- Figure 19.1 bioMerieux: Financial Information (EUR Billion)
- Figure 19.2 Grifols: Financial Information (EUR Billion)
- Figure 19.3 Roche: Financial Information (CHF Billion)
- Figure 19.4 Siemens Healthineers: Financial Information (EUR Billion)
- Figure 20.1 Sysmex: Financial Information (JPY Billion)
- Figure 21.1 Threats of New Entrants
- Figure 21.2 Bargaining Power of Buyers
- Figure 21.3 Bargaining Power of Suppliers
- Figure 21.4 Threats of Substitute Products
- Figure 21.5 Rivalry among Existing Competitors
- Figure 21.6 Porter's Five Force Analysis: Harvey Ball Analysis
MOLECULAR DIAGNOSTICS MARKET: OVERVIEW
As per Roots Analysis, the global molecular diagnostics market is estimated to grow from USD 15.9 billion in the current year to USD 30.9 billion by 2035, at a CAGR of 6.2% during the forecast period, till 2035.
The market sizing and opportunity analysis has been segmented across the following parameters:
Test Type
- Laboratory Testing
- Point-of-Care Testing
Type of Offering
- Reagents
- Instruments
- Services
Type of Sample
- Blood, Serum and Plasma
- Urine
- Others
Type of Technology
- Polymerase Chain Reaction (PCR)
- In situ Hybridization
- Isothermal Nucleic Acid Amplification Technology
- Next Generation Sequencing
- Microarrays
- Mass Spectrometry
- Others
Therapeutic Area
- Cardiovascular Diseases
- Genetic Diseases
- Infectious Diseases
- Neurological Diseases
- Oncological Diseases
- Others
End Users
- Hospitals
- Laboratories
- Others
Key Geographical Regions
- North America (US, Canada)
- Europe (Austria, Belgium, France, Germany, Italy, Netherlands, Poland, Spain, Switzerland, UK, Rest of the Europe)
- Asia (China, India, Indonesia, Japan, Singapore, South Korea, Thailand, Rest of Asia)
- Latin America (Argentina, Brazil, Mexico, Rest of Latin America)
- Middle East and North Africa (Egypt, Israel, Saudi Arabia, Rest of Middle East and North Africa)
- Rest of the World (Australia and New Zealand)
MOLECULAR DIAGNOSTICS MARKET: GROWTH AND TRENDS
Molecular diagnostic tests are advanced techniques and tools used to analyze biological markers in the genome and proteome. These diagnostic solutions are essential for detecting and monitoring diseases, identifying genetic abnormalities, and guiding personalized treatment plans. The primary technologies used in the molecular diagnostics domain include polymerase chain reaction, next-generation sequencing and microarrays. While PCR is a highly specific technique that enables the amplification and detection of trace amounts of DNA or RNA, NGS allows for high-throughput sequencing of entire genomes. Thus, molecular diagnostic solutions are pivotal across various medical fields, including oncological disorders, infectious diseases, genetic testing, and personalized medicine. These solutions enhance the accuracy of diagnosis, enable and support tailored treatment strategies aiming to ultimately improve diagnostic outcomes and advancing public health. In addition, it is worth mentioning that more than 70% of the healthcare decisions are made based on laboratory test results, which reflects the importance of such diagnostic tools in patient care.

Further, owing to the several benefits offered by these molecular diagnostic solutions, such as providing rapid testing, reducing turnaround times and enabling quicker decision-making, the market is expected to grow at a healthy compounded annual growth rate (CAGR) during the forecast period.
MOLECULAR DIAGNOSTICS MARKET: KEY INSIGHTS
The report delves into the current state of the molecular diagnostics market and identifies potential growth opportunities within industry. Some key findings from the report include:
- The molecular diagnostic domain features a dynamic market landscape of players that utilize various types of advanced technologies in order to offer a variety of diagnostic applications.
- A number of leading players considered in this analysis were established during 1951 to 2000; 60% of such players are based in North America.

- Owing to its diverse portfolio of molecular diagnostics solutions and strong financial performance in recent fiscal year, Roche emerged as the most competent company among the leading players in this domain.
- In order to study the impact of various trends in the molecular diagnostics market, we developed our proprietary research methodology to analyze different parameters under Porter's Five Forces framework.
- The molecular diagnostics market is fueled by growing awareness towards preventive healthcare; however, factors, such as navigating through regulatory complexities remain significant hurdles for industry players.
- Driven by the increasing prevalence of chronic disorders across the globe, the global molecular diagnostics market is expected to grow at a healthy growth rate of 6.2% during the forecast period.

- The anticipated future opportunity is expected to be well distributed across multiple segments, such as test type, sample type, therapeutic area, and end users.
MOLECULAR DIAGNOSTICS MARKET: KEY SEGMENTS
Laboratory Testing Segment holds the Largest Share of the Molecular Diagnostics Market
Based on the test type, the market is segmented into laboratory testing and point-of-care testing. At present, the laboratory testing segment holds the maximum share of the molecular diagnostics market. This trend is likely to remain the same in the coming future. Further, the molecular diagnostics market for point-of-care testing segment is expected to show the highest market growth potential during the forecast period.
By Type of Offering, Reagents is the Fastest Growing Segment of the Global Molecular Diagnostics Market
Based on the type of offering, the market is segmented into reagents, instruments and services. At present, the reagents segment holds the maximum share of the global molecular diagnostics market. Further, owing to the fact that reagents are required to be replenished frequently, which contributes to the recurrent revenues, the market for reagents segment is expected to grow at a higher CAGR during the forecast period.
By Type of Sample, Blood, Serum and Plasma Segment Accounts for the Largest Share of the Global Molecular Diagnostics Market
Based on the type of sample, the market is segmented into blood, serum and plasma, urine, and other samples. Currently, the blood, serum and plasma segment capture the highest proportion of the global molecular diagnostics market. However, the urine segment is expected to grow at a higher CAGR during the forecast period.
The Polymerase Chain Reaction (PCR) Segment by Type of Technology Occupy the Largest Share of the Molecular Diagnostics Market
Based on the type of technology, the market is segmented into Polymerase Chain Reaction (PCR), in situ hybridization, isothermal nucleic acid amplification technology, next generation sequencing, microarrays, mass spectrometry and others. While the polymerase chain reaction (PCR) segment is expected to be the primary driver of the overall market, it is worth highlighting that the global molecular diagnostics market for next generation sequencing segment is likely to grow at a relatively higher CAGR. This can be attributed to the several benefits offered by next generation sequencing, such as high-throughput, improved accuracy, and the capability to simultaneously sequence multiple genes.
By Therapeutic Area, Infectious Disease Segment is Likely to Dominate the Molecular Diagnostics Market
Based on the therapeutic area, the market is segmented into cardiovascular diseases, genetic diseases, infectious diseases, neurological diseases, oncological diseases and others. At present the infectious diseases segment holds the maximum share of the molecular diagnostics market. Additionally, the neurological diseases segment is expected to show the highest growth potential during the forecast period, growing at a higher CAGR, compared to the other segments.
Currently, Hospitals Segment Holds the Largest Share of the Molecular Diagnostics Market
Based on end users, the global market is segmented into hospitals, laboratories, and others. Currently, the hospitals segment holds the largest market share. However, the molecular diagnostics market for laboratories segment is expected to witness substantial growth in the coming years.
North America Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia, Latin America, Middle East and North Africa, and Rest of the World. Currently, North America dominates the global molecular diagnostics market and accounts for the largest revenue share. Further, the market in Asia-Pacific is likely to grow at a higher CAGR in the coming future.
Example Players in the Molecular Diagnostics Market
- Abbott Laboratories
- Agilent Technologies
- Becton Dickinson
- BGI Genomics
- bioMerieux
- Bio-Rad
- Danaher
- DiaSorin
- Grifols
- Hologic
- Illumina
- Qiagen
- QuidelOrtho
- Revvity
- Roche
- Sansure
- Seegene
- Siemens Healthineers
- Sysmex
- Thermo Fisher Scientific
MOLECULAR DIAGNOSTICS MARKET: RESEARCH COVERAGE
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the global molecular diagnostics market, focusing on key market segments, including [A] test type, [B] type of offering, [C] type of sample, [D] type of technology, [E] therapeutic area, [F] end users and [D] key geographical regions.
- Market Impact Analysis: A thorough analysis of various factors, such as drivers, restraints, opportunities, and existing challenges that are likely to impact market growth.
- Market Landscape: A comprehensive evaluation of the leading molecular diagnostics companies, based on several relevant parameters, such as [A] year of establishment, [B] company size, [C] company ownership and [D] location of the headquarters. Further, the section includes a comprehensive evaluation of molecular diagnostic solutions, focusing on the parameters, such as [A] type of technology used and [B] diagnostic applications.
- Company Competitiveness Analysis: A comprehensive competitive analysis of molecular diagnostic companies, examining factors, such as [A] years of experience and [B] company competitiveness.
- Regulatory Landscape for Medical Devices: A comprehensive discussion of the various guidelines established by major regulatory bodies for medical device approval across different countries. Additionally, a multi-dimensional bubble analysis was done, focusing on the comparison of contemporary regulatory scenario in key geographies across the globe.
- Company Profiles: In-depth profiles of key players that specialize in molecular diagnostic solutions, focusing on [A] overview of the company, [B] financial information, [C] molecular diagnostic offerings and [D] recent developments and an informed future outlook.
- Porter's Five Forces Analysis: A qualitative assessment of Porter's Five Forces framework based on the five competitive forces, including [A] threats to new entrants, [B] bargaining power of product providers, [C] bargaining power of buyers, [D] threat of substitute products and [E] rivalry among existing competitors.
KEY QUESTIONS ANSWERED IN THIS REPORT
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. BACKGROUND
- 1.1. Context
- 1.2. Project Objectives
2. RESEARCH METHODOLOGY
- 2.1. Chapter Overview
- 2.2. Research Assumptions
- 2.3. Project Methodology
- 2.4. Forecast Methodology
- 2.5. Robust Quality Control
- 2.6. Key Market Segmentations
- 2.7. Key Factors
- 2.7.1. Demographics
- 2.7.2. Economic Factors
- 2.7.3. Government Regulations
- 2.7.4. Supply Chain
- 2.7.5. COVID Impact
- 2.7.6. Market Access
- 2.7.7. Healthcare Policies
- 2.7.8. Industry Consolidation
3. ECONOMIC AND OTHER PROJECT SPECIFIC CONSIDERATIONS
- 3.1. Chapter Overview
- 3.2. Market Dynamics
- 3.2.1. Time Period
- 3.2.1.1. Historical Trends
- 3.2.1.2. Current and Future
- 3.2.2. Currency Coverage and Foreign Exchange Rate
- 3.2.2.1. Major Currencies Affecting the Market
- 3.2.2.2. Factors Affecting Currency Fluctuations and Foreign Exchange Rates
- 3.2.2.3. Impact of Foreign Exchange Rate Volatility on the Market
- 3.2.2.4. Strategies for Mitigating Foreign Exchange Risk
- 3.2.3. Trade Policies
- 3.2.3.1. Impact of Trade Barriers on the Market
- 3.2.3.2. Strategies for Mitigating the Risks Associated with Trade Barriers
- 3.2.4. Recession
- 3.2.4.1. Historical Analysis of Past Recessions and Lessons Learnt
- 3.2.4.2. Assessment of Current Economic Conditions and Potential Impact on the Market
- 3.2.5. Inflation
- 3.2.5.1. Measurement and Analysis of Inflationary Pressures in the Economy
- 3.2.5.2. Potential Impact of Inflation on the Market Evolution
- 3.2.1. Time Period
4. EXECUTIVE SUMMARY
5. INTRODUCTION
- 5.1. Overview of Molecular Diagnostics
- 5.2. Key Technologies Employed in Molecular Diagnostic Solution
- 5.3. Challenges in the Molecular Diagnostics Domain
- 5.4. Recent Developments in the Molecular Diagnostics Domain
- 5.5. Future Perspective in the Molecular Diagnostics Domain
6. MARKET IMPACT ANALYSIS: DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES
- 6.1. Market Drivers
- 6.2. Market Restraints
- 6.3. Market Opportunities
- 6.4. Market Challenges
7. GLOBAL MOLECULAR DIAGNOSTICS MARKET
- 7.1. Key Assumptions and Methodology
- 7.2. Global Molecular Diagnostics Market, Till 2035
- 7.2.1. Scenario Analysis
- 7.2.1.1. Conservative Scenario
- 7.2.1.2. Optimistic Scenario
- 7.2.1. Scenario Analysis
8. MOLECULAR DIAGNOSTICS MARKET, BY TEST TYPE
- 8.1. Market Movement Analysis
- 8.2. Molecular Diagnostics Market: Distribution by Test Type
- 8.2.1. Molecular Diagnostics Market for Laboratory Testing, Till 2035
- 8.2.2. Molecular Diagnostics Market for Point-of-Care Testing, Till 2035
9. MOLECULAR DIAGNOSTICS MARKET, BY TYPE OF OFFERING
- 9.1. Market Movement Analysis
- 9.2. Molecular Diagnostics Market: Distribution by Type of Offering
- 9.2.1. Molecular Diagnostics Market for Instruments, Till 2035
- 9.2.1.1. Molecular Diagnostics Market for In-house Instruments, Till 2035
- 9.2.1.2. Molecular Diagnostics Market for Outsourced Instruments, Till 2035
- 9.2.2. Molecular Diagnostics Marlet for Reagents, till 2035
- 9.2.3. Molecular Diagnostics Market for Services, till 2035
- 9.2.1. Molecular Diagnostics Market for Instruments, Till 2035
10. MOLECULAR DIAGNOSTICS MARKET, BY SAMPLE TYPE
- 10.1. Market Movement Analysis
- 10.2. Molecular Diagnostics Market: Distribution by Sample Type
- 10.2.1. Molecular Diagnostics Market for Blood, Serum and Plasma, till 2035
- 10.2.2. Molecular Diagnostics Market for Urine, till 2035
- 10.2.3. Molecular Diagnostics Market for Other Samples, till 2035
11. MOLECULAR DIAGNOSTICS MARKET, BY TYPE OF TECHNOLOGY
- 11.1. Market Movement Analysis
- 11.2. Molecular Diagnostics Market: Distribution by Type of Technology
- 11.2.1. Molecular Diagnostics Market for PCR, till 2035
- 11.2.2. Molecular Diagnostics Market for In Situ Hybridization, till 2035
- 11.2.3. Molecular Diagnostics Market for Isothermal Nucleic Acid Amplification Technology, till 2035
- 11.2.4. Molecular Diagnostics Market for Next Generation Sequencing, till 2035
- 11.2.5. Molecular Diagnostics Market for Microarrays, till 2035
- 11.2.6. Molecular Diagnostics Market for Mass Spectrometry, till 2035
- 11.2.7. Molecular Diagnostics Market for Other Technologies, till 2035
12. MOLECULAR DIAGNOSTICS MARKET, BY THERAPEUTIC AREA
- 12.1. Market Movement Analysis
- 12.2. Molecular Diagnostics Market: Distribution by Therapeutic Area
- 12.2.1. Molecular Diagnostics Market for Infectious Diseases, till 2035
- 12.2.1.1. Molecular Diagnostics Market for COVID-19, till 2035
- 12.2.1.2. Molecular Diagnostics Market for Respiratory Infections (Excluding COVID-19), till 2035
- 12.2.1.3. Molecular Diagnostics Market for Healthcare-associated Infections, till 2035
- 12.2.1.4. Molecular Diagnostics Market for Hepatitis, till 2035
- 12.2.1.5. Molecular Diagnostics Market for HIV, till 2035
- 12.2.1.6. Molecular Diagnostics Market for Sexually Transmitted Diseases, till 2035
- 12.2.1.7. Molecular Diagnostics Market for Other Infectious Diseases, till 2035
- 12.2.2. Molecular Diagnostics Market for Oncological Disorders, till 2035
- 12.2.2.1. Molecular Diagnostics Market for Lung Cancer, till 2035
- 12.2.2.2. Molecular Diagnostics Market for Breast Cancer, till 2035
- 12.2.2.3. Molecular Diagnostics Market for Colorectal Cancer, till 2035
- 12.2.2.4. Molecular Diagnostics Market for Prostate Cancer, till 2035
- 12.2.2.5. Molecular Diagnostics Market for Gastric Cancer, till 2035
- 12.2.2.6. Molecular Diagnostics Market for Other Oncological Disorders, till 2035
- 12.2.3. Molecular Diagnostics Market for Cardiovascular Diseases, till 2035
- 12.2.4. Molecular Diagnostics Market for Neurological Diseases, till 2035
- 12.2.5. Molecular Diagnostics Market for Genetic Diseases, till 2035
- 12.2.6. Molecular Diagnostics Market for Other Therapeutic Areas, till 2035
- 12.2.1. Molecular Diagnostics Market for Infectious Diseases, till 2035
13. MOLECULAR DIAGNOSTICS MARKET, BY END USERS
- 13.1. Market Movement Analysis
- 13.2. Molecular Diagnostics Market: Distribution by End Users
- 13.2.1. Molecular Diagnostics Market for Laboratories, till 2035
- 13.2.1.1. Molecular Diagnostics Market for Large Laboratories, till 2035
- 13.2.1.2. Molecular Diagnostics Market for Small and Medium-sized Laboratories, till 2035
- 13.2.2. Molecular Diagnostics Market for Hospitals, till 2035
- 13.2.3. Molecular Diagnostics Market for Other End Users, till 2035
- 13.2.1. Molecular Diagnostics Market for Laboratories, till 2035
14. MOLECULAR DIAGNOSTICS MARKET, BY GEOGRAPHICAL REGIONS
- 14.1. Market Movement Analysis
- 14.2. Molecular Diagnostics Market: Distribution by Geographical Regions
- 14.2.1. Molecular Diagnostics Market in North America, till 2035
- 14.2.1.1. Molecular Diagnostics Market in the US, till 2035
- 14.2.1.2. Molecular Diagnostics Market in Canada, till 2035
- 14.2.2. Molecular Diagnostics Market in Europe, till 2035
- 14.2.2.1. Molecular Diagnostics Market in Austria, till 2035
- 14.2.2.2. Molecular Diagnostics Market in Belgium, till 2035
- 14.2.2.3. Molecular Diagnostics Market in France, till 2035
- 14.2.2.4. Molecular Diagnostics Market in Germany, till 2035
- 14.2.2.5. Molecular Diagnostics Market in Italy, till 2035
- 14.2.2.6. Molecular Diagnostics Market in the Netherlands, till 2035
- 14.2.2.7. Molecular Diagnostics Market in Poland, till 2035
- 14.2.2.8. Molecular Diagnostics Market in Spain, till 2035
- 14.2.2.9. Molecular Diagnostics Market in Switzerland, till 2035
- 14.2.2.10. Molecular Diagnostics Market in the UK, till 2035
- 14.2.2.11. Molecular Diagnostics Market in the Rest of Europe, till 2035
- 14.2.3. Molecular Diagnostics Market in Asia, till 2035
- 14.2.3.1. Molecular Diagnostics Market in China, till 2035
- 14.2.3.2. Molecular Diagnostics Market in India, till 2035
- 14.2.3.3. Molecular Diagnostics Market in Indonesia, till 2035
- 14.2.3.4. Molecular Diagnostics Market in Japan, till 2035
- 14.2.3.5. Molecular Diagnostics Market in Singapore, till 2035
- 14.2.3.6. Molecular Diagnostics Market in South Korea, till 2035
- 14.2.3.7. Molecular Diagnostics Market in Thailand, till 2035
- 14.2.3.8. Molecular Diagnostics Market in Rest of Asia, till 2035
- 14.2.4. Molecular Diagnostics Market in Latin America, till 2035
- 14.2.4.1. Molecular Diagnostics Market in Brazil, till 2035
- 14.2.4.2. Molecular Diagnostics Market in Argentina, till 2035
- 14.2.4.3. Molecular Diagnostics Market in Mexico, till 2035
- 14.2.4.4. Molecular Diagnostics Market in Rest of Latin America, till 2035
- 14.2.5. Molecular Diagnostics Market in Middle East and North Africa, till 2035
- 14.2.5.1. Molecular Diagnostics Market in Egypt, till 2035
- 14.2.5.2. Molecular Diagnostics Market in Israel, till 2035
- 14.2.5.3. Molecular Diagnostics Market in Saudi Arabia, till 2035
- 14.2.5.4. Molecular Diagnostics Market in the Rest of Middle East and North Africa, till 2035
- 14.2.6. Molecular Diagnostics Market in Rest of the World, till 2035
- 14.2.6.1. Molecular Diagnostics Market in Australia, till 2035
- 14.2.6.2. Molecular Diagnostics Market in New Zealand, till 2035
- 14.2.1. Molecular Diagnostics Market in North America, till 2035
15. MOLECULAR DIAGNOSTICS MARKET, BY LEADING PLAYERS
- 15.1. Molecular Diagnostics Market: Distribution of Leading Players by Annual Revenues
16. MARKET OVERVIEW: LEADING MOLECULAR DIAGNOSTIC SOLUTION PROVIDERS
- 16.1. Molecular Diagnostic Solution: Overall Market Landscape
- 16.1.1. Analysis by Type of Technology
- 16.1.2. Analysis by Diagnostic Applications
- 16.1.3. Analysis by Type of Technology and Diagnostic Applications
- 16.2. Molecular Diagnostics: Solution Providers Landscape
- 16.2.1. Analysis by Year of Establishment
- 16.2.2. Analysis by Company Size
- 16.2.3. Analysis by Location of Headquarters
- 16.2.4. Analysis by Company Ownership
17. COMPANY COMPETITIVENESS ANALYSIS: MOLECULAR DIAGNOSTIC SOLUTION PROVIDERS
- 17.1. Methodology and Key Parameters Assessed
- 17.2. Molecular Diagnostic Solution Providers: Competitiveness Analysis of Very Large Players
- 17.3. Molecular Diagnostic Solution Providers: Competitiveness Analysis of Large Players
- 17.4. Benchmarking Analysis: Leading Molecular Diagnostics Solution Providers
- 17.4.1. Benchmarking of Companies
- 17.4.1.1. Roche: Benchmarking Analysis
- 17.4.1.2. Abbott: Benchmarking Analysis
- 17.4.1.3. Thermo Fisher Scientific: Benchmarking Analysis
- 17.4.1.4. Qiagen: Benchmarking Analysis
- 17.4.1.5. bioMerieux: Benchmarking Analysis
- 17.4.1.6. DiaSorin: Benchmarking Analysis
- 17.4.1.7. Illumina: Benchmarking Analysis
- 17.4.1.8. Sysmex: Benchmarking Analysis
- 17.4.1.9. Perkin Elmer: Benchmarking Analysis
- 17.4.1.10. Bio-Rad: Benchmarking Analysis
- 17.4.2. Benchmarking of Parameters
- 17.4.2.1. Leading Molecular Diagnostics Solution Providers: Benchmarking by Competitiveness
- 17.4.2.2. Leading Molecular Diagnostic Solution Providers: Benchmarking by Type of Technology Score
- 17.4.2.3. Leading Molecular Diagnostic Solution Providers: Benchmarking by Diagnostic Applications Score
- 17.4.1. Benchmarking of Companies
18. COMPANY PROFILES: MOLECULAR DIAGNOSTICS SOLUTION PROVIDERS BASED IN NORTH AMERICA
- 18.1. Detailed Company Profiles
- 18.1.1. Abbott
- 18.1.1.1. Company Overview
- 18.1.1.2. Product Portfolio
- 18.1.1.3. Financial Information
- 18.1.1.4. Recent Developments and Future Outlook
- 18.1.2. Agilent Technologies
- 18.1.3. BD
- 18.1.4. Danaher
- 18.1.5. Thermo Fisher Scientific
- 18.1.1. Abbott
- 18.2. Short Company Profiles
- 18.2.1. Bio-Rad
- 18.2.1.1. Company Overview
- 18.2.1.2. Product Portfolio
- 18.2.2. Illumina
- 18.2.3. Hologic
- 18.2.4. PerkinElmer
- 18.2.5. QuidelOrtho
- 18.2.1. Bio-Rad
19. COMPANY PROFILES: MOLECULAR DIAGNOSTICS SOLUTION PROVIDERS BASED IN EUROPE
- 19.1. Detailed Company Profiles
- 19.1.1. bioMerieux
- 19.1.1.1. Company Overview
- 19.1.1.2. Product Portfolio
- 19.1.1.3. Financial Information
- 19.1.1.4. Recent Developments and Future Outlook
- 19.1.2. Grifols
- 19.1.3. Roche
- 19.1.4. Siemens Healthineers
- 19.1.1. bioMerieux
- 19.2. Brief Company Profiles
- 19.2.1. DiaSorin
- 19.2.1.1. Company Overview
- 19.2.1.2. Product Portfolio
- 19.2.2. Qiagen
- 19.2.1. DiaSorin
20. COMPANY PROFILES: MOLECULAR DIAGNOSTICS SOLUTION PROVIDERS BASED IN ASIA
- 20.1. Detailed Company Profiles
- 20.1.1. Sysmex
- 20.1.1.1. Company Overview
- 20.1.1.2. Product Portfolio
- 20.1.1.3. Financial Information
- 20.1.1.4. Recent Developments and Future Outlook
- 20.1.1. Sysmex
- 20.2. Brief Company Profiles
- 20.2.1. BGI Genomics
- 20.2.1.1. Company Overview
- 20.2.1.2. Product Portfolio
- 20.2.2. Sansure
- 20.2.3. Seegene
- 20.2.1. BGI Genomics
21. PORTER'S FIVE FORCES ANALYSIS
- 21.1. Methodology and Assumptions
- 21.2. Key Parameters
- 21.2.1. Threats of New Entrants
- 21.2.2. Bargaining Power of Buyers
- 21.2.3. Bargaining Power of Suppliers
- 21.2.4. Threats of Substitute Products
- 21.2.5. Rivalry among Existing Competitors
- 21.3. Porter's Five Force Analysis: Harvey Ball Analysis
- 21.4. Concluding Remarks


