|
|
市場調査レポート
商品コード
1660049
分子感染症検査の世界市場:製品・サービス別、タイプ別、検査タイプ別、疾患別、技術別、エンドユーザー別 - 予測(~2029年)Molecular Infectious Disease Testing Market by Product & Service (Kits, Instruments), Type (Singleplex, Multiplex), Test Type (Lab, PoC), Disease (STD, Flu, GI, HAI), Technology (PCR, NGS), End User (Diagnostic Labs, Hospitals) - Global Forecast to 2029 |
||||||
カスタマイズ可能
|
|||||||
| 分子感染症検査の世界市場:製品・サービス別、タイプ別、検査タイプ別、疾患別、技術別、エンドユーザー別 - 予測(~2029年) |
|
出版日: 2025年02月13日
発行: MarketsandMarkets
ページ情報: 英文 449 Pages
納期: 即納可能
|
全表示
- 概要
- 図表
- 目次
世界の分子感染症検査の市場規模は、2024年の推定93億7,000万米ドルから2029年までに177億8,000万米ドルに達すると予測され、予測期間にCAGRで13.7%の成長が見込まれます。
分子診断技術の急速な進歩が市場の主な促進要因です。PCR、次世代シーケンシング、その他の技術の進歩により、検査の精度、完了までのスピード、効率が向上し、低コストでより利用しやすくなっています。さらに、参入企業はワークフローをさらに改善し、診断能力を高めるハイエンド自動診断プラットフォームの開発を続けています。これにより、感染症の正確かつタイムリーな診断、患者の検査結果の改善、新たに登場した病原体の監視の向上がもたらされます。これにより、医療提供者はタイムリーな意思決定が可能となり、感染症検査における分子診断の採用率が高まっています。
| 調査範囲 | |
|---|---|
| 調査対象年 | 2022年~2029年 |
| 基準年 | 2023年 |
| 予測期間 | 2024年~2029年 |
| 単位 | 米ドル |
| セグメント | 製品・サービス、タイプ、検査タイプ、疾患、技術、エンドユーザー |
| 対象地域 | 北米、欧州、アジア太平洋、ラテンアメリカ、中東・アフリカ、GCC諸国 |
「検査タイプ別では、臨床検査セグメントが予測期間に市場でもっとも速い成長率を示見込みです。」
臨床検査は、精度が高く、拡張性があり、あらゆる種類の病原体を扱うことができるため、分子感染症検査市場において検査タイプ別でもっとも急成長しているセグメントです。PCRやシーケンシングベースの診断を含むこれらの検査は、感染症の発見における精度の高さから、病院や診断検査室で広く使用されています。検査室の自動化とハイスループット技術の進歩により、検査にかかる時間は大幅に短縮され、検査室ベース分子検査は非常に効率的なものとなっています。さらに、1回の検査で複数の感染症を検出できる広範な診断パネルへのニーズが、検査室ベースのソリューションへの選好をさらに高めています。信頼性が高く、複雑な診断ニーズに対応できる臨床検査は、医療提供者に好まれるソリューションです。
「タイプ別では、マルチプレックス検査セグメントが2023年に最大の市場シェアを占めました。」
マルチプレックス検査は、1回の検査で複数の病原体を検出・鑑別することができるため、市場をリードしています。この効率性により、診断にかかる時間、コスト、リソースを削減できるため、医療提供者にもっとも好まれる選択肢の1つとなっています。これらの要因に加え、重複感染の増加、徹底的な診断ソリューションの必要性、タイムリーで正確な結果に対する需要が、マルチプレックス検査市場を拡大しています。さらに、新技術の改良には、アッセイ感度の向上や、病院や診断検査室でのマルチプレックス検査の普及を支える使いやすいプラットフォームの導入が含まれます。
「アジア太平洋:もっとも速く成長している分子感染症検査市場」
アジア太平洋は、人口密度の高い国々における感染症の罹患率の増加と、疾患の早期発見に対する意識の高まりにより、先進の診断薬に対する需要が高まり、分子感染症検査市場が大きく成長しています。この地域の政府や医療機関は、医療インフラの改善、診断能力の拡大、研究活動の支援に多額の投資を行っています。さらに、疾病の監視と発生管理が重視されるようになり、医療費も増加していることから、アジア太平洋は市場全体の成長に大きく寄与する地域の1つとなっています。
当レポートでは、世界の分子感染症検査市場について調査分析し、主な促進要因と抑制要因、競合情勢、将来の動向などの情報を提供しています。
目次
第1章 イントロダクション
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 重要考察
- 分子感染症検査市場の概要
- 分子感染症検査市場:製品・サービス別(2024年・2029年)
- 分子感染症検査市場:タイプ別(2024年・2029年)
- 分子感染症検査市場:検査タイプ別(2024年・2029年)
- 分子感染症検査市場:疾患別(2024年・2029年)
- 分子感染症検査市場:技術別(2024年・2029年)
- 分子感染症検査市場:エンドユーザー別(2024年・2029年)
- 分子感染症検査市場:地理的成長機会
第5章 市場の概要
- イントロダクション
- 市場力学
- 促進要因
- 抑制要因
- 機会
- 課題
- 顧客ビジネスに影響を与える動向/混乱
- 価格分析
- 参考販売価格の動向:製品別
- 性感染症検査の参考販売価格の動向:主要企業別
- 消化器疾患検査の参考販売価格の動向:主要企業別
- 参考販売価格の動向:地域別
- バリューチェーン分析
- サプライチェーン分析
- エコシステム分析
- 投資と資金調達のシナリオ
- 技術分析
- 主要技術
- 補完技術
- 隣接技術
- 特許分析
- 貿易分析
- HSコード3822の輸入データ
- HSコード3822の輸出データ
- 主な会議とイベント(2025年~2026年)
- ケーススタディ分析
- 規制情勢
- 規制分析
- 規制機関、政府機関、その他の組織
- ポーターのファイブフォース分析
- 主なステークホルダーと購入基準
- 分子感染症検査市場に対するAIの影響
- イントロダクション
- 分子感染症検査用途におけるAIの市場可能性
- AIのユースケース
- AIを導入している主要企業
- 分子感染症検査市場におけるAIの未来
第6章 分子感染症検査市場:製品・サービス別
- イントロダクション
- 試薬・キット
- 器具
- サービス・ソフトウェア
第7章 分子感染症検査市場:タイプ別
- イントロダクション
- マルチプレックス検査
- シングルプレックス検査
第8章 分子感染症検査市場:検査タイプ別
- イントロダクション
- 臨床検査
- ポイントオブケア検査
第9章 分子感染症検査市場:疾患別
- イントロダクション
- 呼吸器感染症
- 消化器感染症
- 性感染症
- 院内感染
- 膣炎
- 髄膜炎
- 媒介性感染症
- その他の感染症
第10章 分子感染症検査市場:技術別
- イントロダクション
- ポリメラーゼ連鎖反応
- 等温核酸増幅技術
- DNAシーケンシング・次世代シーケンシング
- in situハイブリダイゼーション
- DNAマイクロアレイ
- その他の技術
第11章 分子感染症検査市場:エンドユーザー別
- イントロダクション
- 診断検査室
- 病院・診療所
- その他のエンドユーザー
第12章 分子感染症検査市場:地域別
- イントロダクション
- 北米
- 北米のマクロ経済の見通し
- 米国
- カナダ
- 欧州
- 欧州のマクロ経済の見通し
- ドイツ
- 英国
- フランス
- イタリア
- ロシア
- スペイン
- その他の欧州
- アジア太平洋
- アジア太平洋のマクロ経済の見通し
- 中国
- 日本
- インド
- オーストラリア
- その他のアジア太平洋
- ラテンアメリカ
- ラテンアメリカのマクロ経済の見通し
- ブラジル
- メキシコ
- その他のラテンアメリカ
- 中東・アフリカ
- GCC諸国
第13章 競合情勢
- イントロダクション
- 主要参入企業の戦略/強み
- 収益分析(2021年~2023年)
- 市場シェア分析(2023年)
- 欧州・中東・アフリカの市場シェア分析(2023年)
- 企業の評価と財務指標
- ブランド/製品の比較
- 企業の評価マトリクス:主要企業(2023年)
- 企業の評価マトリクス:スタートアップ/中小企業(2023年)
- 競合シナリオ
第14章 企業プロファイル
- 主要企業
- DANAHER
- F. HOFFMANN-LA ROCHE LTD
- BIOMERIEUX
- HOLOGIC, INC.
- ABBOTT
- THERMO FISHER SCIENTIFIC INC.
- QIAGEN
- REVVITY
- GRIFOLS, S.A.
- SEEGENE INC.
- DIASORIN S.P.A.
- SIEMENS HEALTHINEERS AG
- BD
- その他の企業
- QUIDELORTHO CORPORATION
- BRUKER
- GENETIC SIGNATURES
- CO-DIAGNOSTICS, INC.
- VELA DIAGNOSTICS
- MOLBIO DIAGNOSTICS PVT. LTD.
- SAVYON DIAGNOSTICS
- UNIOGEN OY
- GENEOMBIO TECHNOLOGIES
- ADVANCED MOLECULAR DIAGNOSTICS
- ALTONA DIAGNOSTICS GMBH
- GENEFIRST LIMITED
第15章 付録
List of Tables
- TABLE 1 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: INCLUSIONS AND EXCLUSIONS
- TABLE 2 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: KEY DATA FROM PRIMARY SOURCES
- TABLE 3 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: RISK ANALYSIS
- TABLE 4 GLOBAL HIV DATA, 2020 VS. 2022 VS. 2023
- TABLE 5 INDICATIVE SELLING PRICE TREND OF MOLECULAR INFECTIOUS DISEASE TESTING PRODUCTS, 2022-2024
- TABLE 6 INDICATIVE SELLING PRICE TREND OF SEXUALLY TRANSMITTED DISEASE TESTS, BY KEY PLAYER, 2022-2024
- TABLE 7 INDICATIVE SELLING PRICE TREND OF GASTROINTESTINAL INFECTIOUS DISEASE TESTS, BY KEY PLAYER, 2022-2024
- TABLE 8 INDICATIVE SELLING PRICE TREND OF MOLECULAR INFECTIOUS DISEASE TESTING PRODUCTS, BY REGION, 2022-2024
- TABLE 9 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: ROLE OF COMPANIES IN ECOSYSTEM
- TABLE 10 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: INNOVATIONS AND PATENT REGISTRATIONS, 2022-2023
- TABLE 11 IMPORT DATA FOR HS CODE 3822 (DIAGNOSTIC AND LABORATORY REAGENTS), BY COUNTRY, 2019-2023 (USD MILLION)
- TABLE 12 EXPORT DATA FOR HS CODE 3822 (DIAGNOSTIC AND LABORATORY REAGENTS), BY COUNTRY, 2019-2023 (USD MILLION)
- TABLE 13 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: KEY CONFERENCES AND EVENTS, 2025-2026
- TABLE 14 CLASSIFICATION OF IVD DEVICES IN EUROPE
- TABLE 15 TIME, COST, AND COMPLEXITY OF REGISTRATION PROCESS IN JAPAN
- TABLE 16 CLASSIFICATION OF IVD MEDICAL DEVICES IN AUSTRALIA
- TABLE 17 NORTH AMERICA: KEY REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 18 EUROPE: KEY REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 19 ASIA PACIFIC: KEY REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 20 LATIN AMERICA: KEY REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 21 REST OF THE WORLD: KEY REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 22 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: PORTER'S FIVE FORCES ANALYSIS
- TABLE 23 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS, BY PRODUCT & SERVICE (%)
- TABLE 24 KEY BUYING CRITERIA, BY PRODUCT & SERVICE
- TABLE 25 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 26 KEY REAGENTS & KITS AVAILABLE IN MARKET
- TABLE 27 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR REAGENTS & KITS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 28 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR REAGENTS & KITS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 29 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR REAGENTS & KITS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 30 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR REAGENTS & KITS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 31 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR REAGENTS & KITS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 32 KEY TESTING INSTRUMENTS AVAILABLE IN MARKET
- TABLE 33 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR INSTRUMENTS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 34 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR INSTRUMENTS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 35 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR INSTRUMENTS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 36 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR INSTRUMENTS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 37 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR INSTRUMENTS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 38 KEY SOFTWARE SOLUTIONS AVAILABLE IN MARKET
- TABLE 39 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SERVICES & SOFTWARE, BY REGION, 2022-2029 (USD MILLION)
- TABLE 40 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SERVICES & SOFTWARE, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 41 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SERVICES & SOFTWARE, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 42 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SERVICES & SOFTWARE, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 43 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SERVICES & SOFTWARE, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 44 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 45 KEY MULTIPLEX TESTING PRODUCTS AVAILABLE IN MARKET
- TABLE 46 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR MULTIPLEX TESTING, BY REGION, 2022-2029 (USD MILLION)
- TABLE 47 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR MULTIPLEX TESTING, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 48 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR MULTIPLEX TESTING, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 49 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR MULTIPLEX TESTING, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 50 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR MULTIPLEX TESTING, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 51 KEY SINGLEPLEX TESTING PRODUCTS AVAILABLE IN MARKET
- TABLE 52 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SINGLEPLEX TESTING, BY REGION, 2022-2029 (USD MILLION)
- TABLE 53 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SINGLEPLEX TESTING, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 54 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SINGLEPLEX TESTING, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 55 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SINGLEPLEX TESTING, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 56 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SINGLEPLEX TESTING, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 57 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 58 KEY LABORATORY-BASED TESTS AND DEVICES AVAILABLE IN MARKET
- TABLE 59 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR LABORATORY TESTS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 60 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR LABORATORY TESTS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 61 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR LABORATORY TESTS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 62 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR LABORATORY TESTS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 63 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR LABORATORY TESTS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 64 KEY POINT OF CARE TESTS AND DEVICES AVAILABLE IN MARKET
- TABLE 65 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR POINT OF CARE TESTS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 66 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR POINT OF CARE TESTS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 67 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR POINT OF CARE TESTS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 68 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR POINT OF CARE TESTS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 69 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR POINT OF CARE TESTS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 70 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 71 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 72 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY REGION, 2022-2029 (USD MILLION)
- TABLE 73 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 74 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 75 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 76 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 77 KEY INFLUENZA TESTING PRODUCTS AVAILABLE IN MARKET
- TABLE 78 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR INFLUENZA, BY REGION, 2022-2029 (USD MILLION)
- TABLE 79 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR INFLUENZA, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 80 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR INFLUENZA, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 81 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR INFLUENZA, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 82 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR INFLUENZA, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 83 KEY TUBERCULOSIS TESTING PRODUCTS AVAILABLE IN MARKET
- TABLE 84 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR TUBERCULOSIS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 85 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR TUBERCULOSIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 86 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR TUBERCULOSIS, BY COUNTRY, 2022-2029 (IN THOUSANDS)
- TABLE 87 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR TUBERCULOSIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 88 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR TUBERCULOSIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 89 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR TUBERCULOSIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 90 KEY PHARYNGITIS TESTING PRODUCTS AVAILABLE IN MARKET
- TABLE 91 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR PHARYNGITIS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 92 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR PHARYNGITIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 93 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR PHARYNGITIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 94 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR PHARYNGITIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 95 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR PHARYNGITIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 96 OTHER KEY RESPIRATORY INFECTION DISEASE TESTING PRODUCTS AVAILABLE IN MARKET
- TABLE 97 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER RESPIRATORY INFECTIOUS DISEASES, BY REGION, 2022-2029 (USD MILLION)
- TABLE 98 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER RESPIRATORY INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 99 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER RESPIRATORY INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
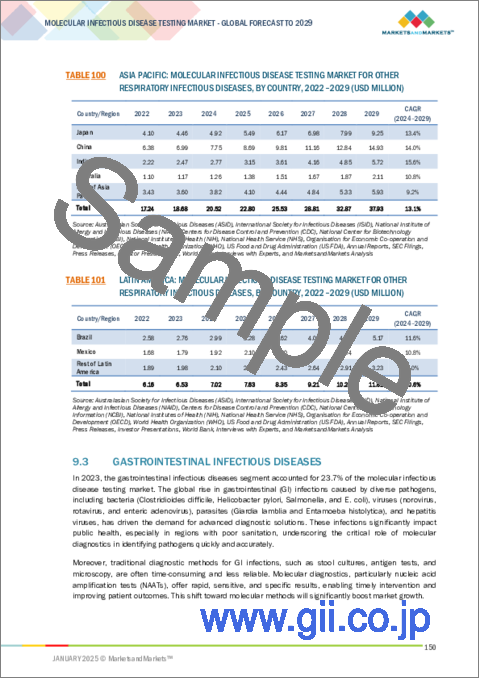
- TABLE 100 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER RESPIRATORY INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 101 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER RESPIRATORY INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 102 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 103 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY REGION, 2022-2029 (USD MILLION)
- TABLE 104 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 105 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 106 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 107 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 108 KEY HEPATITIS TESTING PRODUCTS AVAILABLE IN MARKET
- TABLE 109 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HEPATITIS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 110 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HEPATITIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 111 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HEPATITIS, BY COUNTRY, 2022-2029 (IN THOUSANDS)
- TABLE 112 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HEPATITIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 113 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HEPATITIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 114 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HEPATITIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 115 OTHER KEY GASTROINTESTINAL INFECTIOUS DISEASE TESTING PRODUCTS AVAILABLE IN MARKET
- TABLE 116 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER GASTROINTESTINAL INFECTIOUS DISEASES, BY REGION, 2022-2029 (USD MILLION)
- TABLE 117 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER GASTROINTESTINAL INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 118 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER GASTROINTESTINAL INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 119 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER GASTROINTESTINAL INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 120 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER GASTROINTESTINAL INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 121 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 122 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY REGION, 2022-2029 (USD MILLION)
- TABLE 123 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 124 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 125 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 126 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 127 KEY HIV TESTING PRODUCTS AVAILABLE IN MARKET
- TABLE 128 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HIV, BY REGION, 2022-2029 (USD MILLION)
- TABLE 129 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HIV, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 130 NORTH AMERICA: MOLECULAR INFECTIOUS TESTING MARKET FOR HIV, BY COUNTRY, 2022-2029 (IN THOUSANDS)
- TABLE 131 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HIV, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 132 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HIV, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 133 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HIV, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 134 KEY HUMAN PAPILLOMAVIRUS TESTING PRODUCTS AVAILABLE IN MARKET
- TABLE 135 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HUMAN PAPILLOMAVIRUS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 136 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HUMAN PAPILLOMAVIRUS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 137 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HUMAN PAPILLOMAVIRUS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 138 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HUMAN PAPILLOMAVIRUS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 139 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HUMAN PAPILLOMAVIRUS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 140 KEY CHLAMYDIA TRACHOMATIS TESTING PRODUCTS AVAILABLE IN MARKET
- TABLE 141 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR CHLAMYDIA TRACHOMATIS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 142 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR CHLAMYDIA TRACHOMATIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 143 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR CHLAMYDIA TRACHOMATIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 144 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR CHLAMYDIA TRACHOMATIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 145 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR CHLAMYDIA TRACHOMATIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 146 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR NEISSERIA GONORRHOEAE, BY REGION, 2022-2029 (USD MILLION)
- TABLE 147 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR NEISSERIA GONORRHOEAE, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 148 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR NEISSERIA GONORRHOEAE, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 149 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR NEISSERIA GONORRHOEAE, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 150 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR NEISSERIA GONORRHOEAE, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 151 KEY SYPHILIS TESTING PRODUCTS AVAILABLE IN MARKET
- TABLE 152 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SYPHILIS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 153 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SYPHILIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 154 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SYPHILIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 155 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SYPHILIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 156 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SYPHILIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 157 OTHER KEY SEXUALLY TRANSMITTED DISEASE TESTING PRODUCTS AVAILABLE IN MARKET
- TABLE 158 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER SEXUALLY TRANSMITTED DISEASES, BY REGION, 2022-2029 (USD MILLION)
- TABLE 159 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER SEXUALLY TRANSMITTED DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 160 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER SEXUALLY TRANSMITTED DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 161 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER SEXUALLY TRANSMITTED DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 162 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER SEXUALLY TRANSMITTED DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 163 KEY HOSPITAL-ACQUIRED INFECTION TESTING PRODUCTS AVAILABLE IN MARKET
- TABLE 164 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HOSPITAL-ACQUIRED INFECTIONS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 165 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HOSPITAL-ACQUIRED INFECTIONS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 166 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HOSPITAL-ACQUIRED INFECTIONS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 167 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HOSPITAL-ACQUIRED INFECTIONS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 168 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HOSPITAL-ACQUIRED INFECTIONS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 169 KEY VAGINITIS TESTING PRODUCTS AVAILABLE IN MARKET
- TABLE 170 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR VAGINITIS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 171 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR VAGINITIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 172 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR VAGINITIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 173 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR VAGINITIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 174 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR VAGINITIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 175 KEY MENINGITIS TESTING PRODUCTS AVAILABLE IN MARKET
- TABLE 176 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR MENINGITIS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 177 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR MENINGITIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 178 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR MENINGITIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 179 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR MENINGITIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 180 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR MENINGITIS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 181 KEY VECTOR-BORNE DISEASE TESTING PRODUCTS AVAILABLE IN MARKET
- TABLE 182 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR VECTOR-BORNE DISEASES, BY REGION, 2022-2029 (USD MILLION)
- TABLE 183 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR VECTOR-BORNE DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 184 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR VECTOR-BORNE DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 185 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR VECTOR-BORNE DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 186 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR VECTOR-BORNE DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 187 OTHER KEY INFECTIOUS DISEASE TESTING PRODUCTS AVAILABLE IN MARKET
- TABLE 188 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER INFECTIOUS DISEASES, BY REGION, 2022-2029 (USD MILLION)
- TABLE 189 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 190 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 191 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 192 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER INFECTIOUS DISEASES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 193 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 194 KEY POLYMERASE CHAIN REACTION-BASED TESTS AND DEVICES AVAILABLE IN MARKET
- TABLE 195 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR POLYMERASE CHAIN REACTION, BY REGION, 2022-2029 (USD MILLION)
- TABLE 196 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR POLYMERASE CHAIN REACTION, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 197 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR POLYMERASE CHAIN REACTION, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 198 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR POLYMERASE CHAIN REACTION, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 199 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR POLYMERASE CHAIN REACTION, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 200 KEY ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY-BASED TESTS AND DEVICES AVAILABLE IN MARKET
- TABLE 201 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY, BY REGION, 2022-2029 (USD MILLION)
- TABLE 202 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 203 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 204 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 205 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 206 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR DNA SEQUENCING & NEXT-GENERATION SEQUENCING, BY REGION, 2022-2029 (USD MILLION)
- TABLE 207 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR DNA SEQUENCING & NEXT-GENERATION SEQUENCING, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 208 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR DNA SEQUENCING & NEXT-GENERATION SEQUENCING, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 209 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR DNA SEQUENCING & NEXT-GENERATION SEQUENCING, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 210 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR DNA SEQUENCING & NEXT-GENERATION SEQUENCING, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 211 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR IN SITU HYBRIDIZATION, BY REGION, 2022-2029 (USD MILLION)
- TABLE 212 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR IN SITU HYBRIDIZATION, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 213 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR IN SITU HYBRIDIZATION, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 214 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR IN SITU HYBRIDIZATION, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 215 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR IN SITU HYBRIDIZATION, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 216 KEY MICROARRAY-BASED PRODUCTS AVAILABLE IN MARKET
- TABLE 217 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR DNA MICROARRAYS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 218 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR DNA MICROARRAYS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 219 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR DNA MICROARRAYS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 220 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR DNA MICROARRAYS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 221 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR DNA MICROARRAYS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 222 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER TECHNOLOGIES, BY REGION, 2022-2029 (USD MILLION)
- TABLE 223 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER TECHNOLOGIES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 224 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER TECHNOLOGIES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 225 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER TECHNOLOGIES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 226 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER TECHNOLOGIES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 227 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 228 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR DIAGNOSTIC LABORATORIES, BY REGION, 2022-2029 (USD MILLION)
- TABLE 229 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR DIAGNOSTIC LABORATORIES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 230 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR DIAGNOSTIC LABORATORIES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 231 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR DIAGNOSTIC LABORATORIES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 232 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR DIAGNOSTIC LABORATORIES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 233 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HOSPITALS & CLINICS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 234 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HOSPITALS & CLINICS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 235 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HOSPITALS & CLINICS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 236 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HOSPITALS & CLINICS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 237 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR HOSPITALS & CLINICS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 238 MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER END USERS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 239 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER END USERS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 240 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER END USERS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 241 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER END USERS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 242 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR OTHER END USERS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 243 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY REGION, 2022-2029 (USD MILLION)
- TABLE 244 MACROECONOMIC INDICATORS FOR NORTH AMERICA
- TABLE 245 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 246 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 247 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 248 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 249 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 250 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 251 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 252 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 253 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 254 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 255 US: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 256 US: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 257 US: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 258 US: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 259 US: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 260 US: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 261 US: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 262 US: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 263 US: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 264 CANADA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 265 CANADA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 266 CANADA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 267 CANADA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 268 CANADA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 269 CANADA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 270 CANADA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 271 CANADA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 272 CANADA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 273 MACROECONOMIC INDICATORS FOR EUROPE
- TABLE 274 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 275 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 276 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 277 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 278 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 279 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 280 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 281 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 282 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 283 EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 284 GERMANY: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 285 GERMANY: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 286 GERMANY: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 287 GERMANY: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 288 GERMANY: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 289 GERMANY: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 290 GERMANY: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 291 GERMANY: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 292 GERMANY: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 293 UK: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 294 UK: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 295 UK: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 296 UK: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 297 UK: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 298 UK: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 299 UK: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 300 UK: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 301 UK: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 302 FRANCE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 303 FRANCE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 304 FRANCE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 305 FRANCE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 306 FRANCE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 307 FRANCE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 308 FRANCE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 309 FRANCE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 310 FRANCE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 311 ITALY: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 312 ITALY: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 313 ITALY: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 314 ITALY: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 315 ITALY: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 316 ITALY: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 317 ITALY: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 318 ITALY: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 319 ITALY: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 320 RUSSIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 321 RUSSIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 322 RUSSIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 323 RUSSIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 324 RUSSIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 325 RUSSIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 326 RUSSIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 327 RUSSIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 328 RUSSIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 329 SPAIN: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 330 SPAIN: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 331 SPAIN: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 332 SPAIN: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 333 SPAIN: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 334 SPAIN: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 335 SPAIN: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 336 SPAIN: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 337 SPAIN: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 338 REST OF EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 339 REST OF EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 340 REST OF EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 341 REST OF EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 342 REST OF EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 343 REST OF EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 344 REST OF EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 345 REST OF EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 346 REST OF EUROPE: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 347 MACROECONOMIC INDICATORS FOR ASIA PACIFIC
- TABLE 348 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 349 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 350 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 351 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 352 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 353 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 354 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 355 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 356 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 357 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 358 CHINA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 359 CHINA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 360 CHINA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 361 CHINA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 362 CHINA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 363 CHINA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 364 CHINA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 365 CHINA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 366 CHINA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 367 JAPAN: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 368 JAPAN: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 369 JAPAN: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 370 JAPAN: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 371 JAPAN: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 372 JAPAN: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 373 JAPAN: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 374 JAPAN: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 375 JAPAN: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 376 INDIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 377 INDIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 378 INDIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 379 INDIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 380 INDIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 381 INDIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 382 INDIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 383 INDIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 384 INDIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 385 AUSTRALIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 386 AUSTRALIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 387 AUSTRALIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 388 AUSTRALIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 389 AUSTRALIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 390 AUSTRALIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 391 AUSTRALIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 392 AUSTRALIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 393 AUSTRALIA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 394 REST OF ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 395 REST OF ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 396 REST OF ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 397 REST OF ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 398 REST OF ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 399 REST OF ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 400 REST OF ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 401 REST OF ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 402 REST OF ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 403 MACROECONOMIC INDICATORS FOR LATIN AMERICA
- TABLE 404 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 405 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 406 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 407 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 408 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 409 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 410 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 411 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 412 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 413 LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 414 BRAZIL: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 415 BRAZIL: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 416 BRAZIL: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 417 BRAZIL: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 418 BRAZIL: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 419 BRAZIL: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 420 BRAZIL: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 421 BRAZIL: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 422 BRAZIL: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 423 MEXICO: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 424 MEXICO: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 425 MEXICO: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 426 MEXICO: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 427 MEXICO: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 428 MEXICO: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 429 MEXICO: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 430 MEXICO: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 431 MEXICO: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 432 REST OF LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 433 REST OF LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 434 REST OF LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 435 REST OF LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 436 REST OF LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 437 REST OF LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 438 REST OF LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 439 REST OF LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 440 REST OF LATIN AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 441 MIDDLE EAST & AFRICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 442 MIDDLE EAST & AFRICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 443 MIDDLE EAST & AFRICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 444 MIDDLE EAST & AFRICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 445 MIDDLE EAST & AFRICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 446 MIDDLE EAST & AFRICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 447 MIDDLE EAST & AFRICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 448 MIDDLE EAST & AFRICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 449 MIDDLE EAST & AFRICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 450 MACROECONOMIC INDICATORS FOR MIDDLE EAST & AFRICA
- TABLE 451 GCC COUNTRIES: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2022-2029 (USD MILLION)
- TABLE 452 GCC COUNTRIES: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 453 GCC COUNTRIES: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2022-2029 (USD MILLION)
- TABLE 454 GCC COUNTRIES: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2022-2029 (USD MILLION)
- TABLE 455 GCC COUNTRIES: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR RESPIRATORY INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 456 GCC COUNTRIES: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR GASTROINTESTINAL INFECTIOUS DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 457 GCC COUNTRIES: MOLECULAR INFECTIOUS DISEASE TESTING MARKET FOR SEXUALLY TRANSMITTED DISEASES, BY TYPE, 2022-2029 (USD MILLION)
- TABLE 458 GCC COUNTRIES: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2022-2029 (USD MILLION)
- TABLE 459 GCC COUNTRIES: MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 460 MACROECONOMIC INDICATORS FOR GCC COUNTRIES
- TABLE 461 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS IN MOLECULAR INFECTIOUS DISEASE TESTING MARKET, JANUARY 2021-DECEMBER 2024
- TABLE 462 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: DEGREE OF COMPETITION
- TABLE 463 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: REGION FOOTPRINT
- TABLE 464 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: PRODUCT & SERVICE FOOTPRINT
- TABLE 465 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: TEST TYPE FOOTPRINT
- TABLE 466 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: DISEASE FOOTPRINT
- TABLE 467 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: DETAILED LIST OF KEY STARTUPS/SMES
- TABLE 468 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: COMPETITIVE BENCHMARKING OF STARTUPS/SMES (1/2)
- TABLE 469 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: COMPETITIVE BENCHMARKING OF STARTUPS/SMES (2/2)
- TABLE 470 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2021-DECEMBER 2024
- TABLE 471 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: DEALS, JANUARY 2021-DECEMBER 2024
- TABLE 472 DANAHER: COMPANY OVERVIEW
- TABLE 473 DANAHER: PRODUCTS OFFERED
- TABLE 474 DANAHER: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2021-DECEMBER 2024
- TABLE 475 DANAHER: DEALS, JANUARY 2021-DECEMBER 2024
- TABLE 476 DANAHER: EXPANSIONS, JANUARY 2021-DECEMBER 2024
- TABLE 477 F. HOFFMANN-LA ROCHE LTD: COMPANY OVERVIEW
- TABLE 478 F. HOFFMANN-LA ROCHE LTD: PRODUCTS OFFERED
- TABLE 479 F. HOFFMANN-LA ROCHE LTD: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2021-DECEMBER 2024
- TABLE 480 F. HOFFMANN-LA ROCHE LTD: DEALS, JANUARY 2021-DECEMBER 2024
- TABLE 481 F. HOFFMANN-LA ROCHE LTD: EXPANSIONS, JANUARY 2021-DECEMBER 2024
- TABLE 482 BIOMERIEUX: COMPANY OVERVIEW
- TABLE 483 BIOMERIEUX: PRODUCTS OFFERED
- TABLE 484 BIOMERIEUX: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2021-DECEMBER 2024
- TABLE 485 BIOMERIEUX: DEALS, JANUARY 2021-DECEMBER 2024
- TABLE 486 HOLOGIC, INC.: COMPANY OVERVIEW
- TABLE 487 HOLOGIC, INC.: PRODUCTS OFFERED
- TABLE 488 HOLOGIC, INC.: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2021-DECEMBER 2024
- TABLE 489 HOLOGIC, INC.: DEALS, JANUARY 2021-DECEMBER 2024
- TABLE 490 ABBOTT: COMPANY OVERVIEW
- TABLE 491 ABBOTT: PRODUCTS OFFERED
- TABLE 492 ABBOTT: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2021-DECEMBER 2024
- TABLE 493 ABBOTT: DEALS, JANUARY 2021-DECEMBER 2024
- TABLE 494 THERMO FISHER SCIENTIFIC INC.: COMPANY OVERVIEW
- TABLE 495 THERMO FISHER SCIENTIFIC INC.: PRODUCTS OFFERED
- TABLE 496 THERMO FISHER SCIENTIFIC INC.: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2021-DECEMBER 2024
- TABLE 497 THERMO FISHER SCIENTIFIC INC.: DEALS, JANUARY 2021-DECEMBER 2024
- TABLE 498 QIAGEN: COMPANY OVERVIEW
- TABLE 499 QIAGEN: PRODUCTS OFFERED
- TABLE 500 QIAGEN: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2021-DECEMBER 2024
- TABLE 501 QIAGEN: DEALS, JANUARY 2021-DECEMBER 2024
- TABLE 502 QIAGEN: EXPANSIONS, JANUARY 2021-DECEMBER 2024
- TABLE 503 REVVITY: COMPANY OVERVIEW
- TABLE 504 REVVITY: PRODUCTS OFFERED
- TABLE 505 GRIFOLS, S.A.: COMPANY OVERVIEW
- TABLE 506 GRIFOLS, S.A.: PRODUCTS OFFERED
- TABLE 507 GRIFOLS, S.A.: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2021-DECEMBER 2024
- TABLE 508 GRIFOLS, S.A.: DEALS, JANUARY 2021-DECEMBER 2024
- TABLE 509 SEEGENE INC.: COMPANY OVERVIEW
- TABLE 510 SEEGENE INC.: PRODUCTS OFFERED
- TABLE 511 SEEGENE INC.: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2021-DECEMBER 2024
- TABLE 512 SEEGENE INC.: DEALS, JANUARY 2021-DECEMBER 2024
- TABLE 513 SEEGENE INC.: EXPANSIONS, JANUARY 2021-DECEMBER 2024
- TABLE 514 DIASORIN S.P.A.: COMPANY OVERVIEW
- TABLE 515 DIASORIN S.P.A.: PRODUCTS OFFERED
- TABLE 516 DIASORIN S.P.A.: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2021-DECEMBER 2024
- TABLE 517 DIASORIN S.P.A.: DEALS, JANUARY 2021-DECEMBER 2024
- TABLE 518 SIEMENS HEALTHINEERS AG: COMPANY OVERVIEW
- TABLE 519 SIEMENS HEALTHINEERS AG: PRODUCTS OFFERED
- TABLE 520 SIEMENS HEALTHINEERS AG: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2021-DECEMBER 2024
- TABLE 521 SIEMENS HEALTHINEERS AG: DEALS, JANUARY 2021-DECEMBER 2024
- TABLE 522 SIEMENS HEALTHINEERS AG: EXPANSIONS, JANUARY 2021-DECEMBER 2024
- TABLE 523 BD: COMPANY OVERVIEW
- TABLE 524 BD: PRODUCTS OFFERED
- TABLE 525 BD: PRODUCT LAUNCHES AND APPROVALS, JANUARY 2021-DECEMBER 2024
- TABLE 526 BD: DEALS, JANUARY 2021-DECEMBER 2024
- TABLE 527 BD: EXPANSIONS, JANUARY 2021-DECEMBER 2024
- TABLE 528 QUIDELORTHO CORPORATION: COMPANY OVERVIEW
- TABLE 529 BRUKER: COMPANY OVERVIEW
- TABLE 530 GENETIC SIGNATURES: COMPANY OVERVIEW
- TABLE 531 CO-DIAGNOSTICS, INC.: COMPANY OVERVIEW
- TABLE 532 VELA DIAGNOSTICS: COMPANY OVERVIEW
- TABLE 533 MOLBIO DIAGNOSTICS PVT. LTD.: COMPANY OVERVIEW
- TABLE 534 SAVYON DIAGNOSTICS: COMPANY OVERVIEW
- TABLE 535 UNIOGEN OY: COMPANY OVERVIEW
- TABLE 536 GENEOMBIO TECHNOLOGIES: COMPANY OVERVIEW
- TABLE 537 ADVANCED MOLECULAR DIAGNOSTICS: COMPANY OVERVIEW
- TABLE 538 ALTONA DIAGNOSTICS: COMPANY OVERVIEW
- TABLE 539 GENEFIRST LIMITED: COMPANY OVERVIEW
List of Figures
- FIGURE 1 MOLECULAR INFECTIOUS DISEASE TESTING MARKET SEGMENTATION AND REGIONS COVERED
- FIGURE 2 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: RESEARCH DESIGN
- FIGURE 3 KEY INDUSTRY INSIGHTS
- FIGURE 4 BREAKDOWN OF PRIMARY INTERVIEWS: SUPPLY- AND DEMAND-SIDE PARTICIPANTS
- FIGURE 5 BREAKDOWN OF PRIMARY INTERVIEWS: BY COMPANY TYPE, DESIGNATION, AND REGION
- FIGURE 6 BOTTOM-UP APPROACH: COMPANY REVENUE ESTIMATION APPROACH
- FIGURE 7 CAGR PROJECTIONS: SUPPLY-SIDE ANALYSIS
- FIGURE 8 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: TOP-DOWN APPROACH
- FIGURE 9 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: DATA TRIANGULATION
- FIGURE 10 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: PARAMETRIC ASSUMPTIONS
- FIGURE 11 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2024 VS. 2029 (USD MILLION)
- FIGURE 12 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2024 VS. 2029 (USD MILLION)
- FIGURE 13 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2024 VS. 2029 (USD MILLION)
- FIGURE 14 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2024 VS. 2029 (USD MILLION)
- FIGURE 15 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2024 VS. 2029 (USD MILLION)
- FIGURE 16 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2024 VS. 2029 (USD MILLION)
- FIGURE 17 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY REGION, 2024 VS. 2029 (USD MILLION)
- FIGURE 18 RISING PREVALENCE OF INFECTIOUS DISEASES TO DRIVE MARKET
- FIGURE 19 REAGENTS & KITS SEGMENT TO DOMINATE MARKET IN 2029
- FIGURE 20 MULTIPLEX TESTING SEGMENT TO HAVE LARGEST MARKET SHARE IN 2029
- FIGURE 21 LABORATORY TESTS TO HOLD LARGEST MARKET SHARE IN 2029
- FIGURE 22 SEXUALLY TRANSMITTED DISEASES SEGMENT TO LEAD MARKET IN 2029
- FIGURE 23 POLYMERASE CHAIN REACTION SEGMENT TO HAVE LARGEST MARKET SHARE IN 2029
- FIGURE 24 DIAGNOSTIC LABORATORIES SEGMENT TO COMMAND LARGEST MARKET SHARE IN 2029
- FIGURE 25 ASIA PACIFIC TO REGISTER HIGHEST GROWTH RATE DURING FORECAST PERIOD
- FIGURE 26 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- FIGURE 27 NEW REVENUE POCKETS FOR PLAYERS IN MOLECULAR INFECTIOUS DISEASE TESTING MARKET
- FIGURE 28 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: VALUE CHAIN ANALYSIS
- FIGURE 29 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: SUPPLY CHAIN ANALYSIS
- FIGURE 30 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: ECOSYSTEM ANALYSIS
- FIGURE 31 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: INVESTMENT AND FUNDING SCENARIO, 2021-2024
- FIGURE 32 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: PATENT ANALYSIS, JANUARY 2014-DECEMBER 2023
- FIGURE 33 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: PORTER'S FIVE FORCES ANALYSIS
- FIGURE 34 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS, BY PRODUCT & SERVICE
- FIGURE 35 KEY BUYING CRITERIA, BY PRODUCT & SERVICE
- FIGURE 36 MARKET POTENTIAL OF AI IN MOLECULAR INFECTIOUS DISEASE TESTING APPLICATIONS
- FIGURE 37 REGIONAL INCIDENCE OF HIV, 2023
- FIGURE 38 NORTH AMERICA: MOLECULAR INFECTIOUS DISEASE TESTING MARKET SNAPSHOT
- FIGURE 39 ASIA PACIFIC: MOLECULAR INFECTIOUS DISEASE TESTING MARKET SNAPSHOT
- FIGURE 40 REVENUE ANALYSIS OF KEY PLAYERS IN MOLECULAR INFECTIOUS DISEASE TESTING MARKET, 2021-2023
- FIGURE 41 MARKET SHARE ANALYSIS OF KEY PLAYERS IN MOLECULAR INFECTIOUS DISEASE TESTING MARKET, 2023
- FIGURE 42 MARKET SHARE ANALYSIS OF KEY PLAYERS IN EMEA REGION IN MOLECULAR INFECTIOUS DISEASE TESTING MARKET, 2023
- FIGURE 43 EV/EBITDA OF KEY VENDORS, 2024
- FIGURE 44 YEAR-TO-DATE (YTD) PRICE TOTAL RETURN AND 5-YEAR STOCK BETA OF KEY VENDORS, 2024
- FIGURE 45 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: BRAND/PRODUCT COMPARISON
- FIGURE 46 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: COMPANY EVALUATION MATRIX (KEY PLAYERS), 2023
- FIGURE 47 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: COMPANY FOOTPRINT
- FIGURE 48 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: COMPANY EVALUATION MATRIX (STARTUPS/SMES), 2023
- FIGURE 49 DANAHER: COMPANY SNAPSHOT (2023)
- FIGURE 50 F. HOFFMANN-LA ROCHE LTD: COMPANY SNAPSHOT (2023)
- FIGURE 51 BIOMERIEUX: COMPANY SNAPSHOT (2023)
- FIGURE 52 HOLOGIC, INC.: COMPANY SNAPSHOT (2024)
- FIGURE 53 ABBOTT: COMPANY SNAPSHOT (2023)
- FIGURE 54 THERMO FISHER SCIENTIFIC INC.: COMPANY SNAPSHOT (2023)
- FIGURE 55 QIAGEN: COMPANY SNAPSHOT (2023)
- FIGURE 56 REVVITY: COMPANY SNAPSHOT (2023)
- FIGURE 57 GRIFOLS, S.A.: COMPANY SNAPSHOT (2023)
- FIGURE 58 SEEGENE INC.: COMPANY SNAPSHOT (2023)
- FIGURE 59 DIASORIN S.P.A.: COMPANY SNAPSHOT (2023)
- FIGURE 60 SIEMENS HEALTHINEERS AG: COMPANY SNAPSHOT (2024)
- FIGURE 61 BD: COMPANY SNAPSHOT (2024)
The molecular infectious disease testing market is projected to reach USD 17.78 billion in 2029 from an estimated value of USD 9.37 billion in 2024 at a CAGR of 13.7% during the forecast period. Rapid advancements in molecular diagnostic technologies are the main driving force in the molecular infectious disease testing market. Advancements in PCR, next-generation sequencing, and other technologies enhance test accuracy, speed of completion, and efficiency, making them more accessible at lower costs. Moreover, market players continue to develop high- end automated diagnostic platforms which further improves the workflow and increases diagnostic capabilities. This leads to accurate and timely diagnosis of infectious diseases, improved patient results, and improved surveillance of newly emerging pathogens. Through this, providers can make timely decisions, increasing the adoption rate of molecular diagnostics for infectious disease testing.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2022-2029 |
| Base Year | 2023 |
| Forecast Period | 2024-2029 |
| Units Considered | Value (USD) |
| Segments | Product & Service, Type, Test Type, Disease, Technology, and End User |
| Regions covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa, and the GCC Countries |
"Laboratory tests segment is expected to witness the fastest growth rate in the molecular infectious disease testing market, by test type, during the forecast period."
Based on test type, the molecular infectious disease testing market can be divided into laboratory tests and point of care tests. Laboratory tests are the fastest-growing segment by test type in the molecular infectious disease testing market because of their high accuracy, scalability, and ability to handle all types of pathogens. These tests, including PCR and sequencing-based diagnostics, are widely used in hospitals and diagnostic laboratories for their precision in discovering infections. With the advances in laboratory automation and high-throughput technologies, turnaround times have decreased significantly, thereby making the laboratory-based molecular tests very efficient. Moreover, the need for extensive diagnostic panels that allow detection of multiple infections in one test further enhances the preference for laboratory-based solutions. With high reliability and the capability to address complex diagnostic needs, laboratory tests are the preferred solution for healthcare providers.
"Multiplex testing segment accounted for the largest market share in the molecular infectious disease testing market, by type, in 2023."
Based on type, the molecular infectious disease testing market is bifurcated into singleplex testing and multiplex testing. The multiplex testing segment leads the molecular infectious disease testing market due to the capability of its ability to perform multiple tests for detecting and differentiating several pathogens in a single test. This efficiency cuts down time, costs, and resources for diagnosis, thus becoming one of the most preferred options by healthcare providers. All these factors together with the rise in co-infections, necessity for thorough diagnosis solutions, and demand for timely and accurate results have increased the multiplex testing market. In addition, new technology improvements include greater assay sensitivity and the introduction of user-friendly platforms that support widespread use of multiplex testing in hospitals and diagnostic labs.
"Asia Pacific: The fastest-growing market for molecular infectious disease testing"
The worldwide market for molecular infectious disease testing is categorized into North America, Europe, Asia Pacific, Latin America, Middle East & Africa, and the GCC countries. The Asia Pacific region is experiencing significant growth in the molecular infectious disease testing market driven by the growing incidence of infectious diseases in the densely populated countries, together with growing awareness of the early detection of diseases, which has created a demand for more advanced diagnostics. Governments and healthcare organizations throughout the region are investing heavily to improve healthcare infrastructure, expand diagnostic capabilities, and support research initiatives. Moreover, growing emphasis on diseases surveillance and outbreak management and rising healthcare spending position Asia Pacific as one of the big contributors to growth in the entire market.
The break-up of the profile of primary participants in the molecular infectious disease testing market:
- By Company Type: Tier 1 - 40%, Tier 2 - 30%, and Tier 3 - 30%
- By Designation: C-level - 27%, D-level - 18%, and Others - 55%
- By Region: North America - 51%, Europe - 21%, Asia Pacific - 18%, Latin America - 6%, and Middle East & Africa- 4%
The key players in this market are Danaher (US), F. Hoffmann-La Roche Ltd (Switzerland), bioMerieux (France), Hologic, Inc. (US), Abbott (US), Thermo Fisher Scientific Inc. (US), QIAGEN (Netherands), Revvity (US), Siemens Healthineers AG (Germany), BD (US), Grifols, S.A. (Spain), QuidelOrtho Corporation (US), DiaSorin S.p.A. (Italy), Bruker (US), Seegene Inc. (South Korea), Genetic Signatures (Australia), Co-Diagnostics, Inc. (US), Savyon Diagnostics (Israel), Vela Diagnostics (Singapore), Molbio Diagnostics Pvt. Ltd. (India), Uniogen OY (Finland), geneOmbio Technologies (India), Advanced Molecular Diagnostics (UK), GeneFirst Limited (UK), and altona Diagnostics GmbH (Germany).
Research Coverage:
This research report categorizes the molecular infectious disease testing market by product & service (reagents & kits, instruments, and services & software), by type (singleplex testing and multiplex testing), by test type (laboratory tests and point of car tests), by disease (respiratory infectious diseases (influenza, tuberculosis, pharyngitis, and other respiratory infectious diseases), gastrointestinal infectious diseases (hepatitis and other gastrointestinal infectious diseases), sexually transmitted diseases (human immunodeficiency virus, chlamydia trachomatis, neisseria gonorrhoea, syphilis, human papillomavirus, and other sexually transmitted diseases), vaginitis, meningitis, vector borne diseases, hospital-acquired infections, and other infectious diseases), by technology (polymerase chain reaction, in situ hybridization, isothermal nucleic acid amplification technology, DNA sequencing & next generation sequencing, DNA microarrays, and other technologies), by end user (diagnostic laboratories, hospitals & clinics, and other end users) and region (North America, Europe, Asia Pacific, Latin America, Middle East & Africa, and the GCC countries). The scope of the report covers detailed information regarding the major factors, such as drivers, restraints, opportunities, and challenges influencing the growth of the molecular infectious disease testing market. A detailed analysis of the key industry players has been done to provide insights into their business overview, solutions, key strategies, acquisitions, and agreements. New product & service launches, and recent developments associated with the molecular infectious disease testing market. Competitive analysis of upcoming startups in the molecular infectious disease testing market ecosystem is covered in this report.
Reasons to buy this report:
The report will help the market leaders/new entrants in this market with information on the closest approximations of the revenue numbers for the overall molecular infectious disease testing market and the subsegments. This report will help stakeholders understand the competitive landscape and gain more insights to position their businesses better and plan suitable go-to-market strategies. The report also helps stakeholders understand the pulse of the market and provides them with information on key market drivers, restraints, opportunities, and challenges.
The report provides insights on the following pointers:
- Analysis of key drivers (Rising burden of infectious diseases, rapid technological advancements in molecular diagnostics, growing funding for molecular testing), opportunities (High growth opportunities in emerging economies), restraints (Inconsistent reimbursement policies), and challenges (Changing regulatory landscape, operational barriers and labor shortage) influencing the growth of the molecular infectious disease testing market.
- Product Development/Innovation: Detailed insights on upcoming technologies, research & development activities, and new product launches in the molecular infectious disease testing market.
- Market Development: Comprehensive information about lucrative markets - the report analyses the molecular infectious disease testing market across varied regions.
- Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the molecular infectious disease testing market.
- Competitive Assessment: In-depth assessment of market shares, growth strategies, product offerings of leading players like Danaher (US), F. Hoffmann-La Roche Ltd (Switzerland), bioMerieux (France), Hologic, Inc. (US), and Abbott (US).
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 STUDY SCOPE
- 1.3.1 MARKET SEGMENTATION AND REGIONS COVERED
- 1.3.2 INCLUSIONS AND EXCLUSIONS
- 1.3.3 YEARS CONSIDERED
- 1.4 CURRENCY CONSIDERED
- 1.5 STAKEHOLDERS
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- 2.2 RESEARCH APPROACH
- 2.2.1 SECONDARY RESEARCH
- 2.2.1.1 Key secondary sources
- 2.2.1.2 Key data from secondary sources
- 2.2.1.3 Objectives of secondary research
- 2.2.2 PRIMARY RESEARCH
- 2.2.2.1 Key primary sources
- 2.2.2.2 Key data from primary sources
- 2.2.2.3 Objectives of primary research
- 2.2.2.4 Key industry insights
- 2.2.2.5 Breakdown of primary interviews
- 2.2.1 SECONDARY RESEARCH
- 2.3 MARKET SIZE ESTIMATION
- 2.3.1 BOTTOM-UP APPROACH
- 2.3.1.1 Company revenue estimation approach
- 2.3.1.2 Presentations of companies and primary interviews
- 2.3.1.3 Estimation of total molecular-based tests for HIV in US
- 2.3.1.4 Growth forecast model
- 2.3.1.5 CAGR projections
- 2.3.2 TOP-DOWN APPROACH
- 2.3.1 BOTTOM-UP APPROACH
- 2.4 DATA TRIANGULATION
- 2.5 MARKET SHARE ANALYSIS
- 2.6 RESEARCH ASSUMPTIONS
- 2.6.1 PARAMETRIC ASSUMPTIONS
- 2.6.2 GROWTH RATE ASSUMPTIONS
- 2.7 RESEARCH LIMITATIONS
- 2.8 RISK ANALYSIS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
- 4.1 MOLECULAR INFECTIOUS DISEASE TESTING MARKET OVERVIEW
- 4.2 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE, 2024 VS. 2029
- 4.3 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE, 2024 VS. 2029
- 4.4 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE, 2024 VS. 2029
- 4.5 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE, 2024 VS. 2029
- 4.6 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY, 2024 VS. 2029
- 4.7 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER, 2024 VS. 2029
- 4.8 MOLECULAR INFECTIOUS DISEASE TESTING MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- 5.2.1 DRIVERS
- 5.2.1.1 Rising burden of infectious diseases
- 5.2.1.2 Rapid technological advancements in molecular diagnostics
- 5.2.1.3 Growing funding for molecular testing
- 5.2.2 RESTRAINTS
- 5.2.2.1 Inconsistent reimbursement policies
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 High growth opportunities in emerging economies
- 5.2.4 CHALLENGES
- 5.2.4.1 Changing regulatory landscape
- 5.2.4.2 Operational barriers and labor shortage issues
- 5.2.1 DRIVERS
- 5.3 TRENDS/DISRUPTIONS IMPACTING CUSTOMER BUSINESS
- 5.4 PRICING ANALYSIS
- 5.4.1 INDICATIVE SELLING PRICE TREND, BY PRODUCT
- 5.4.2 INDICATIVE SELLING PRICE TREND OF SEXUALLY TRANSMITTED DISEASE TESTS, BY KEY PLAYER
- 5.4.3 INDICATIVE SELLING PRICE TREND OF GASTROINTESTINAL DISEASE TESTS, BY KEY PLAYER
- 5.4.4 INDICATIVE SELLING PRICE TREND, BY REGION
- 5.5 VALUE CHAIN ANALYSIS
- 5.6 SUPPLY CHAIN ANALYSIS
- 5.7 ECOSYSTEM ANALYSIS
- 5.8 INVESTMENT AND FUNDING SCENARIO
- 5.9 TECHNOLOGY ANALYSIS
- 5.9.1 KEY TECHNOLOGIES
- 5.9.1.1 Polymerase chain reaction
- 5.9.2 COMPLEMENTARY TECHNOLOGIES
- 5.9.2.1 Isolated nucleic acid amplification technology
- 5.9.3 ADJACENT TECHNOLOGIES
- 5.9.3.1 Next-generation sequencing
- 5.9.1 KEY TECHNOLOGIES
- 5.10 PATENT ANALYSIS
- 5.11 TRADE ANALYSIS
- 5.11.1 IMPORT DATA FOR HS CODE 3822
- 5.11.2 EXPORT DATA FOR HS CODE 3822
- 5.12 KEY CONFERENCES AND EVENTS, 2025-2026
- 5.13 CASE STUDY ANALYSIS
- 5.13.1 CASE STUDY 1: IMPROVED PATHOGEN-DIRECTED TREATMENT THROUGH RAPID SYNDROMIC PCR TESTING
- 5.13.2 CASE STUDY 2: CLINICAL IMPACT OF MULTIPLEX MOLECULAR DIAGNOSTIC TESTING IN CHILDREN WITH ACUTE GASTROENTERITIS
- 5.13.3 CASE STUDY 3: IMPACT OF MOLECULAR POINT OF CARE TESTING FOR GROUP A STREPTOCOCCUS PHARYNGITIS
- 5.14 REGULATORY LANDSCAPE
- 5.14.1 REGULATORY ANALYSIS
- 5.14.1.1 NORTH AMERICA
- 5.14.1.1.1 US
- 5.14.1.1.2 Canada
- 5.14.1.2 EUROPE
- 5.14.1.2.1 Germany
- 5.14.1.2.2 UK
- 5.14.1.2.3 France
- 5.14.1.2.4 Italy
- 5.14.1.3 ASIA PACIFIC
- 5.14.1.3.1 China
- 5.14.1.3.2 Japan
- 5.14.1.3.3 India
- 5.14.1.3.4 Australia
- 5.14.1.4 LATIN AMERICA
- 5.14.1.4.1 Brazil
- 5.14.1.4.2 Mexico
- 5.14.1.5 MIDDLE EAST
- 5.14.1.5.1 Africa
- 5.14.1.1 NORTH AMERICA
- 5.14.2 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.14.1 REGULATORY ANALYSIS
- 5.15 PORTER'S FIVE FORCES ANALYSIS
- 5.15.1 BARGAINING POWER OF SUPPLIERS
- 5.15.2 BARGAINING POWER OF BUYERS
- 5.15.3 THREAT OF NEW ENTRANTS
- 5.15.4 THREAT OF SUBSTITUTES
- 5.15.5 INTENSITY OF COMPETITIVE RIVALRY
- 5.16 KEY STAKEHOLDERS AND BUYING CRITERIA
- 5.16.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 5.16.2 BUYING CRITERIA
- 5.17 IMPACT OF AI ON MOLECULAR INFECTIOUS DISEASE TESTING MARKET
- 5.17.1 INTRODUCTION
- 5.17.2 MARKET POTENTIAL OF AI IN MOLECULAR INFECTIOUS DISEASE TESTING APPLICATIONS
- 5.17.3 AI USE CASES
- 5.17.4 KEY COMPANIES IMPLEMENTING AI
- 5.17.5 FUTURE OF AI IN MOLECULAR INFECTIOUS DISEASE TESTING MARKET
6 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY PRODUCT & SERVICE
- 6.1 INTRODUCTION
- 6.2 REAGENTS & KITS
- 6.2.1 ONGOING INNOVATIONS IN MOLECULAR DIAGNOSTICS TO SPUR GROWTH
- 6.3 INSTRUMENTS
- 6.3.1 RISING PREVALENCE OF INFECTIOUS DISEASES TO FOSTER GROWTH
- 6.4 SERVICES & SOFTWARE
- 6.4.1 INCREASING UPTAKE OF ADVANCED INSTRUMENTS TO FACILITATE GROWTH
7 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TYPE
- 7.1 INTRODUCTION
- 7.2 MULTIPLEX TESTING
- 7.2.1 RISING DEMAND FOR FASTER DIAGNOSIS TO PROMOTE GROWTH
- 7.3 SINGLEPLEX TESTING
- 7.3.1 NEED FOR AFFORDABLE AND USER-FRIENDLY TESTING TO AUGMENT GROWTH
8 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TEST TYPE
- 8.1 INTRODUCTION
- 8.2 LABORATORY TESTS
- 8.2.1 INCREASING NEED FOR AUTOMATION TO FAVOR GROWTH
- 8.3 POINT OF CARE TESTS
- 8.3.1 GROWING AWARENESS ABOUT DISEASE SURVEILLANCE, SCREENING, AND OUTBREAK RESPONSE TO DRIVE MARKET
9 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY DISEASE
- 9.1 INTRODUCTION
- 9.2 RESPIRATORY INFECTIOUS DISEASES
- 9.2.1 INFLUENZA
- 9.2.1.1 Growing focus on faster diagnosis and control to propel market
- 9.2.2 TUBERCULOSIS
- 9.2.2.1 Rise in undiagnosed and untreated cases to promote growth
- 9.2.3 PHARYNGITIS
- 9.2.3.1 Need to differentiate between bacterial and viral infections to fuel growth
- 9.2.4 OTHER RESPIRATORY INFECTIOUS DISEASES
- 9.2.1 INFLUENZA
- 9.3 GASTROINTESTINAL INFECTIOUS DISEASES
- 9.3.1 HEPATITIS
- 9.3.1.1 Rising government initiatives to expedite growth
- 9.3.2 OTHER GASTROINTESTINAL INFECTIOUS DISEASES
- 9.3.1 HEPATITIS
- 9.4 SEXUALLY TRANSMITTED DISEASES
- 9.4.1 HUMAN IMMUNODEFICIENCY VIRUS
- 9.4.1.1 Increasing number of blood transfusions and blood donations to augment growth
- 9.4.2 HUMAN PAPILLOMAVIRUS
- 9.4.2.1 Technological advancements for preventing human papillomavirus infections to drive market
- 9.4.3 CHLAMYDIA TRACHOMATIS
- 9.4.3.1 Increasing development of molecular tests to aid growth
- 9.4.4 NEISSERIA GONORRHOEAE
- 9.4.4.1 Increasing development of novel assays to boost market
- 9.4.5 SYPHILIS
- 9.4.5.1 Growing demand for affordable and rapid molecular diagnostic solutions to drive market
- 9.4.6 OTHER SEXUALLY TRANSMITTED DISEASES
- 9.4.1 HUMAN IMMUNODEFICIENCY VIRUS
- 9.5 HOSPITAL-ACQUIRED INFECTIONS
- 9.5.1 GROWING BURDEN OF METHICILLIN-RESISTANT STAPHYLOCOCCUS AUREUS TO FUEL MARKET
- 9.6 VAGINITIS
- 9.6.1 INCREASING AWARENESS ABOUT WOMEN'S HEALTH TO BOLSTER GROWTH
- 9.7 MENINGITIS
- 9.7.1 RISING INCIDENCE OF INVASIVE MENINGOCOCCAL DISEASE TO ASSIST GROWTH
- 9.8 VECTOR-BORNE DISEASES
- 9.8.1 GROWING FOCUS ON DISEASE SURVEILLANCE TO PROPEL MARKET
- 9.9 OTHER INFECTIOUS DISEASES
10 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY TECHNOLOGY
- 10.1 INTRODUCTION
- 10.2 POLYMERASE CHAIN REACTION
- 10.2.1 NEED FOR HIGH PRECISION FOR DISEASE IDENTIFICATION TO SUPPORT GROWTH
- 10.3 ISOTHERMAL NUCLEIC ACID AMPLIFICATION TECHNOLOGY
- 10.3.1 EMERGENCE OF NEW PATHOGENS TO STIMULATE GROWTH
- 10.4 DNA SEQUENCING & NEXT-GENERATION SEQUENCING
- 10.4.1 GROWING APPLICATIONS IN DIAGNOSING BACTERIAL, VIRAL, FUNGAL, AND PARASITIC INFECTIONS TO DRIVE MARKET
- 10.5 IN SITU HYBRIDIZATION
- 10.5.1 NEED FOR SAFER AND MORE PRACTICAL TESTING IN CLINICAL SETTINGS TO FUEL MARKET
- 10.6 DNA MICROARRAYS
- 10.6.1 NEED FOR AFFORDABLE AND SENSITIVE DETECTION TO ENCOURAGE GROWTH
- 10.7 OTHER TECHNOLOGIES
11 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY END USER
- 11.1 INTRODUCTION
- 11.2 DIAGNOSTIC LABORATORIES
- 11.2.1 GROWING EMPHASIS ON QUALITY CONTROL AND STANDARDIZED TESTING PROTOCOLS TO BOOST MARKET
- 11.3 HOSPITALS & CLINICS
- 11.3.1 RISING PATIENT VOLUME TO CONTRIBUTE TO GROWTH
- 11.4 OTHER END USERS
12 MOLECULAR INFECTIOUS DISEASE TESTING MARKET, BY REGION
- 12.1 INTRODUCTION
- 12.2 NORTH AMERICA
- 12.2.1 MACROECONOMIC OUTLOOK FOR NORTH AMERICA
- 12.2.2 US
- 12.2.2.1 Presence of advanced healthcare infrastructure to augment growth
- 12.2.3 CANADA
- 12.2.3.1 Favorable government initiatives to fuel market
- 12.3 EUROPE
- 12.3.1 MACROECONOMIC OUTLOOK FOR EUROPE
- 12.3.2 GERMANY
- 12.3.2.1 Increasing healthcare expenditure to advance growth
- 12.3.3 UK
- 12.3.3.1 Growing number of diagnostic centers to fuel market
- 12.3.4 FRANCE
- 12.3.4.1 Increasing demand for early disease diagnosis to augment growth
- 12.3.5 ITALY
- 12.3.5.1 Growing adoption of advanced diagnostic technologies to fuel market
- 12.3.6 RUSSIA
- 12.3.6.1 Increasing access to quality healthcare to facilitate growth
- 12.3.7 SPAIN
- 12.3.7.1 Growing public health initiatives to boost market
- 12.3.8 REST OF EUROPE
- 12.4 ASIA PACIFIC
- 12.4.1 MACROECONOMIC OUTLOOK FOR ASIA PACIFIC
- 12.4.2 CHINA
- 12.4.2.1 Rising public access to modern healthcare to spur growth
- 12.4.3 JAPAN
- 12.4.3.1 Universal healthcare reimbursement policy to foster growth
- 12.4.4 INDIA
- 12.4.4.1 Increased private and public investments to bolster growth
- 12.4.5 AUSTRALIA
- 12.4.5.1 Growing investments for testing centers and treatment facilities to drive market
- 12.4.6 REST OF ASIA PACIFIC
- 12.5 LATIN AMERICA
- 12.5.1 MACROECONOMIC OUTLOOK FOR LATIN AMERICA
- 12.5.2 BRAZIL
- 12.5.2.1 Growing focus on improving healthcare infrastructure to fuel market
- 12.5.3 MEXICO
- 12.5.3.1 Increasing accessibility and affordability of healthcare services to foster growth
- 12.5.4 REST OF LATIN AMERICA
- 12.6 MIDDLE EAST & AFRICA
- 12.6.1 BOOMING HEALTHCARE INVESTMENTS TO AID GROWTH
- 12.6.2 MACROECONOMIC OUTLOOK FOR MIDDLE EAST & AFRICA
- 12.7 GCC COUNTRIES
- 12.7.1 GROWING PUBLIC HEALTHCARE FUNDING TO DRIVE MARKET
- 12.7.2 MACROECONOMIC OUTLOOK FOR GCC COUNTRIES
13 COMPETITIVE LANDSCAPE
- 13.1 INTRODUCTION
- 13.2 KEY PLAYER STRATEGIES/RIGHT TO WIN
- 13.2.1 OVERVIEW OF STRATEGIES ADOPTED BY PLAYERS IN MOLECULAR INFECTIOUS DISEASE TESTING MARKET
- 13.3 REVENUE ANALYSIS, 2021-2023
- 13.4 MARKET SHARE ANALYSIS, 2023
- 13.5 MARKET SHARE ANALYSIS OF EMEA REGION, 2023
- 13.6 COMPANY VALUATION AND FINANCIAL METRICS
- 13.7 BRAND/PRODUCT COMPARISON
- 13.8 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2023
- 13.8.1 STARS
- 13.8.2 EMERGING LEADERS
- 13.8.3 PERVASIVE PLAYERS
- 13.8.4 PARTICIPANTS
- 13.8.5 COMPANY FOOTPRINT, KEY PLAYERS, 2023
- 13.8.5.1 Company footprint
- 13.8.5.2 Region footprint
- 13.8.5.3 Product & service footprint
- 13.8.5.4 Test type footprint
- 13.8.5.5 Disease footprint
- 13.9 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2023
- 13.9.1 PROGRESSIVE COMPANIES
- 13.9.2 RESPONSIVE COMPANIES
- 13.9.3 DYNAMIC COMPANIES
- 13.9.4 STARTING BLOCKS
- 13.9.5 COMPETITIVE BENCHMARKING OF STARTUPS/SMES, 2023
- 13.9.5.1 Detailed list of key startups/SMEs
- 13.9.5.2 Competitive benchmarking of startups/SMEs
- 13.10 COMPETITIVE SCENARIO
- 13.10.1 PRODUCT LAUNCHES AND APPROVALS
- 13.10.2 DEALS
14 COMPANY PROFILES
- 14.1 KEY PLAYERS
- 14.1.1 DANAHER
- 14.1.1.1 Business overview
- 14.1.1.2 Products offered
- 14.1.1.3 Recent developments
- 14.1.1.3.1 Product launches and approvals
- 14.1.1.3.2 Deals
- 14.1.1.3.3 Expansions
- 14.1.1.4 MnM view
- 14.1.1.4.1 Right to win
- 14.1.1.4.2 Strategic choices
- 14.1.1.4.3 Weaknesses and competitive threats
- 14.1.2 F. HOFFMANN-LA ROCHE LTD
- 14.1.2.1 Business overview
- 14.1.2.2 Products offered
- 14.1.2.3 Recent developments
- 14.1.2.3.1 Product launches and approvals
- 14.1.2.3.2 Deals
- 14.1.2.3.3 Expansions
- 14.1.2.4 MnM view
- 14.1.2.4.1 Right to win
- 14.1.2.4.2 Strategic choices
- 14.1.2.4.3 Weaknesses and competitive threats
- 14.1.3 BIOMERIEUX
- 14.1.3.1 Business overview
- 14.1.3.2 Products offered
- 14.1.3.3 Recent developments
- 14.1.3.3.1 Product launches and approvals
- 14.1.3.3.2 Deals
- 14.1.3.4 MnM view
- 14.1.3.4.1 Right to win
- 14.1.3.4.2 Strategic choices
- 14.1.3.4.3 Weaknesses and competitive threats
- 14.1.4 HOLOGIC, INC.
- 14.1.4.1 Business overview
- 14.1.4.2 Products offered
- 14.1.4.3 Recent developments
- 14.1.4.3.1 Product launches and approvals
- 14.1.4.3.2 Deals
- 14.1.4.4 MnM view
- 14.1.4.4.1 Right to win
- 14.1.4.4.2 Strategic choices
- 14.1.4.4.3 Weaknesses and competitive threats
- 14.1.5 ABBOTT
- 14.1.5.1 Business overview
- 14.1.5.2 Products offered
- 14.1.5.3 Recent developments
- 14.1.5.3.1 Product launches and approvals
- 14.1.5.3.2 Deals
- 14.1.5.4 MnM view
- 14.1.5.4.1 Right to win
- 14.1.5.4.2 Strategic choices
- 14.1.5.4.3 Weaknesses and competitive threats
- 14.1.6 THERMO FISHER SCIENTIFIC INC.
- 14.1.6.1 Business overview
- 14.1.6.2 Products offered
- 14.1.6.3 Recent developments
- 14.1.6.3.1 Product launches and approvals
- 14.1.6.3.2 Deals
- 14.1.7 QIAGEN
- 14.1.7.1 Business overview
- 14.1.7.2 Products offered
- 14.1.7.3 Recent developments
- 14.1.7.3.1 Product launches and approvals
- 14.1.7.3.2 Deals
- 14.1.7.3.3 Expansions
- 14.1.8 REVVITY
- 14.1.8.1 Business overview
- 14.1.8.2 Products offered
- 14.1.9 GRIFOLS, S.A.
- 14.1.9.1 Business overview
- 14.1.9.2 Products offered
- 14.1.9.3 Recent developments
- 14.1.9.3.1 Product launches and approvals
- 14.1.9.3.2 Deals
- 14.1.10 SEEGENE INC.
- 14.1.10.1 Business overview
- 14.1.10.2 Products offered
- 14.1.10.3 Recent developments
- 14.1.10.3.1 Product launches and approvals
- 14.1.10.3.2 Deals
- 14.1.10.3.3 Expansions
- 14.1.11 DIASORIN S.P.A.
- 14.1.11.1 Business overview
- 14.1.11.2 Products offered
- 14.1.11.3 Recent developments
- 14.1.11.3.1 Product launches and approvals
- 14.1.11.3.2 Deals
- 14.1.12 SIEMENS HEALTHINEERS AG
- 14.1.12.1 Business overview
- 14.1.12.2 Products offered
- 14.1.12.3 Recent developments
- 14.1.12.3.1 Product launches and approvals
- 14.1.12.3.2 Deals
- 14.1.12.3.3 Expansions
- 14.1.13 BD
- 14.1.13.1 Business overview
- 14.1.13.2 Products offered
- 14.1.13.3 Recent developments
- 14.1.13.3.1 Product launches and approvals
- 14.1.13.3.2 Deals
- 14.1.13.3.3 Expansions
- 14.1.1 DANAHER
- 14.2 OTHER PLAYERS
- 14.2.1 QUIDELORTHO CORPORATION
- 14.2.2 BRUKER
- 14.2.3 GENETIC SIGNATURES
- 14.2.4 CO-DIAGNOSTICS, INC.
- 14.2.5 VELA DIAGNOSTICS
- 14.2.6 MOLBIO DIAGNOSTICS PVT. LTD.
- 14.2.7 SAVYON DIAGNOSTICS
- 14.2.8 UNIOGEN OY
- 14.2.9 GENEOMBIO TECHNOLOGIES
- 14.2.10 ADVANCED MOLECULAR DIAGNOSTICS
- 14.2.11 ALTONA DIAGNOSTICS GMBH
- 14.2.12 GENEFIRST LIMITED
15 APPENDIX
- 15.1 DISCUSSION GUIDE
- 15.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 15.3 CUSTOMIZATION OPTIONS
- 15.4 RELATED REPORTS
- 15.5 AUTHOR DETAILS