
|
市場調査レポート
商品コード
1869572
mRNA合成・製造市場:2035年までの業界動向と世界の予測 - 製品別、事業規模別、合成・製造活動別、適応症タイプ別、治療領域別、応用分野別、地域別mRNA Synthesis and Manufacturing Market: Industry Trends and Global Forecasts, Till 2035 - Distribution by Product, Scale of Operation, Synthesis and Manufacturing Activity, Indication Type, Therapeutic Area, Application Area and Geographical Regions |
||||||
カスタマイズ可能
|
|||||||
| mRNA合成・製造市場:2035年までの業界動向と世界の予測 - 製品別、事業規模別、合成・製造活動別、適応症タイプ別、治療領域別、応用分野別、地域別 |
|
出版日: 2025年10月24日
発行: Roots Analysis
ページ情報: 英文 341 Pages
納期: 即日から翌営業日
|
概要
mRNA合成・製造市場:概要
Rootsの分析によると、mRNA合成・製造の市場規模は、2028年~2035年に20%のCAGRで拡大し、現在の11億5,000万米ドルから2035年までに13億5,000万米ドルに成長すると予測されています。
mRNA合成・製造市場
市場規模および機会分析は、以下のパラメータに基づいてセグメント化されています。
製品タイプ
- 医薬品有効成分
- 完成剤形
事業規模
- 前臨床および臨床規模
- 商業規模
合成・製造活動
- 受託製造
- 自社製造
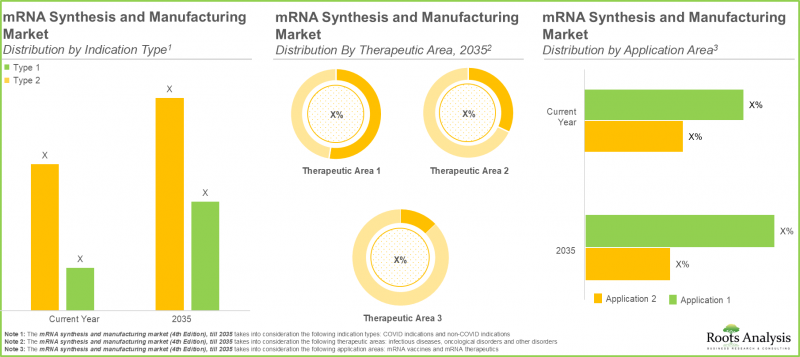
適応症タイプ
- COVID関連適応症
- 非COVID適応症
治療領域
- 感染症
- 腫瘍学領域
- その他
応用分野
- mRNAワクチン
- mRNA治療薬
地域
- 北米
- 欧州
- アジア太平洋
- ラテンアメリカ
- 中東・北アフリカ
mRNA合成・製造市場:成長と動向
リボ核酸(RNA)は、あらゆる生物の細胞内に存在する分子であり、遺伝情報を様々なタンパク質へと変換し、細胞の多様な機能を担っています。重要な点として、RNAはメッセンジャーRNA(mRNA)、トランスファーRNA(tRNA)、リボソームRNA(rRNA)など、様々な形態で存在します。このうち、mRNAは現代の医療産業において極めて重要な治療アプローチとなっています。
mRNAは、DNAに存在する遺伝情報をタンパク質合成へと伝達する一本鎖RNA分子です。mRNA上の遺伝情報はアミノ酸へと翻訳され、その後機能的な産物(タンパク質)へと加工されます。特にCOVID-19パンデミック以降、mRNAワクチンおよび治療薬の生産は、COVID-19感染症治療のための次世代医薬品として注目を浴びています。さらに、mRNA療法は様々な腫瘍性疾患、感染症、遺伝性疾患の管理においても評価が進められています。この多様性と拡張性により、mRNA治療薬は採用が拡大し、個別化mRNA医療生産とワクチン開発の基盤として位置づけられています。加えて、挿入変異誘発リスクが低いため、患者様にとって安全性プロファイルが向上すると期待されています。
この成功により、mRNA合成・生産の需要は大幅に増加し、mRNA治療薬/ワクチン開発企業は現在の需要を満たす代替手段を積極的に模索しています。この点において、mRNA治療薬/ワクチン開発に携わる企業にとって、アウトソーシングは収益性の高い選択肢となり、mRNA治療薬およびワクチン開発に向けた効果的かつ拡張性のあるソリューションを提供しています。mRNA合成・製造分野における現在の動向と予測される機会を考慮すると、この分野は近い将来、一桁台の市場成長が見込まれると予測されます。
mRNA合成・製造市場:主要な知見
当レポートは、mRNA合成・製造市場の現状を詳細に分析し、業界内の潜在的な成長機会を特定しています。主な調査結果は以下の通りです:
現在の市場情勢では、世界中でmRNA合成のカスタマイズサービスを提供すると主張する約55社のプロバイダーが存在しており、その60%は北米に本社を置いています。
2. mRNAカスタム合成サービス提供企業の大半(約95%)は、mRNAの5'末端修飾を提供しています。これは主に、mRNAの安定性や翻訳効率の向上、免疫原性のリスク低減に寄与するためです。
3.利害関係者は、自社の製品ポートフォリオを強化し、mRNAカスタム合成分野で活動する他社に対する競争優位性を獲得するため、既存の能力を積極的に向上させています。
4. mRNAワクチン需要の高まりと、mRNA合成・製造技術の進歩に伴い、約45社がmRNA受託製造サービスを積極的に提供しています。
5.約60%の企業が原薬と製剤の両方に対するmRNA受託製造サービスを提供しており、約90%の事業者が臨床規模でのmRNA受託製造サービスを提供しています。
6. mRNA受託製造サービスプロバイダーは、サービスポートフォリオの強化と競争力のある市場基盤の確立に向け、mRNA製造能力の積極的な拡充と多様化を進めています。
7.現在、mRNA合成用キットは市場に約115種類が流通しており、分子生物学、タンパク質生産、ワクチン開発など幅広い用途に対応するよう設計されています。
8. mRNA合成キットは、お客様の特定のニーズに応えるため、様々な構成要素で構成されています。特に、T7ポリメラーゼは、95%以上のmRNA合成キットで提供される最も好まれる酵素タイプとして台頭しています。
9.現在、mRNA合成用のキットは市場に約115種類が流通しています。これらのキットは、分子生物学、タンパク質生産、ワクチン開発など、幅広い用途に対応できるよう設計されています。
10. mRNA合成キットは、お客様の特定のニーズに応えるため、様々な構成要素で構成されています。特に、T7ポリメラーゼは、95%以上のmRNA合成キットで提供される最も好まれる酵素タイプとして浮上しています。
11.この市場への関心の高まりは、近年さまざまな利害関係者間で結ばれた多様な提携関係にも反映されています。実際、取引の約50%は過去3年間に締結されました。
12.世界中の様々なmRNAベースの治療薬/ワクチン開発企業は、自社医薬品ポートフォリオをさらに拡充するため、mRNA合成・製造サービスプロバイダーとの戦略的提携を構築することが予想されます。
13.この分野における大手製薬企業の大半(約30%)の活動はサノフィ社が主導しており、その中でも大半の取り組み(50%)は、この分野で活動する様々な利害関係者との提携や共同研究の事例でした。
14.現在、mRNA合成・製造市場シェアの大部分は北米が占めています。これは先進的な医療インフラが整備されており、関係企業が広範な調査を実施できる環境が整っているためと考えられます。
15.感染症サブセグメントは、mRNA治療薬およびワクチンが当該疾患の治療に有効であることから、今年度は市場全体の大部分を占めると予測されます。
16.北米はmRNA合成・製造市場全体において重要なシェアを獲得すると予想され、この傾向は今後も変化しない見込みです。
17.米国における主要企業別mRNAベースのワクチンおよび治療薬の急速な成功に牽引され、mRNA合成・製造市場はCAGR6.5%で成長すると予測されます。
mRNA合成・製造市場:主要セグメント
医薬品原薬がmRNA合成・製造市場で最大のシェアを占める
当社の推計によれば、本年度において、医薬品原薬サブセグメントがmRNA合成・製造市場シェアの大部分(60%超)を占める見込みです。これは、医薬品原薬があらゆる治療薬/ワクチンの基本成分であり、製品の全体的な有効性と安全性に影響を与えるという事実に起因します。
商業規模がmRNA合成・製造市場で最大のシェアを占める
本年度、商業規模サブセグメントは、多数のmRNAベース治療薬・ワクチンの商業化により、最高シェア(85%超)を占めています。さらに、前臨床・臨床規模市場は予測期間中に緩やかな増加が見込まれます。この分野における重要な研究開発が前臨床および臨床領域の成長を促進しており、mRNAベース製品の開発増加につながっています。加えて、各社は異なる規模での操業強化に向け、mRNA生産施設の拡充に注力しています。
mRNA合成・製造市場では受託製造が支配的と予測
本年、受託製造サブセグメントが最大の市場シェア(約60%)を占めています。この優位性は主に、専門的なノウハウを必要とし、しばしば第三者のサービスプロバイダーに依存するmRNA生産の複雑性に起因しています。さらに、予測期間中に社内製造サブセグメントのシェアが大幅に増加し、CAGR 7.5%で成長する見込みです。
非COVID適応症は予測期間中に顕著なCAGRで成長する見込み
新型コロナウイルス感染症(COVID-19)関連適応症サブセグメントは、今年度において最大の市場シェア(95%)を占めています。これは、mRNAベースの製品が細胞に特定の病原体タンパク質を産生するよう指示でき、実際の病原体に曝露することなく強力かつ標的を絞った免疫応答を引き起こすことに起因しています。さらに、非COVID-19適応症の市場シェアは大幅に増加し、予測期間中に37.5%のCAGRで成長する見込みです。
感染症向けmRNA療法への注目
本年、感染症サブセグメントが最大の市場シェア(約95%)を占めています。これは、mRNA治療薬およびワクチンがCOVID-19パンデミック対策において極めて高い成功を収めた事実によるものです。さらに、がん疾患の増加傾向と、腫瘍学的疾患を効率的に治療するための新規治療法開発の必要性から、腫瘍学セグメントにおいても著しい市場成長が見込まれます。
mRNAワクチンがmRNA合成・製造市場で最大のシェアを占める
mRNA合成・製造市場の予測によれば、本年はmRNAワクチンサブセグメントが市場を牽引しています。その確かな臨床的成功、幅広い予防的応用可能性、柔軟な特性が背景にあります。さらに、mRNA治療薬のシェアは予測期間中にCAGR 61.1%で成長し、大幅な増加が見込まれます。これは、腫瘍学、遺伝性疾患、自己免疫疾患、心血管疾患などの治療領域における治療用途が急速に拡大しているためであり、mRNA治療薬に対する強力な臨床的勢いを生み出しています。
今後数年間、北米がmRNA合成・製造分野を牽引
北米は市場の大半(40%超)を占めると予想されます。加えて、北米市場は予測期間中に6.6%のCAGRで成長すると見込まれています。これは、北米の先進的な医療インフラが、業界の複数のプレイヤーによる広範な研究の実施と、多様な治療適応症に向けた新規mRNAモダリティの開発を可能にしているためです。
mRNA合成・製造市場における代表的参入企業例
- Aldevron
- APExBIO
- Aurigene Pharmaceutical Services
- BioCell
- Biomay
- BioNTech
- Biorbyt
- CELLSCRIPT
- Creative Biogene
- Curia
- Eurogentec
- GenScript
- eJena Bioscience
- Lonza
- Merck
- Porton Advanced
- Revolution Biotechnology
- Samsung Biologics
- ST Pharm
- Thermo Fisher Scientific
- TriLink BioTechnologies
- Vernal Biosciences
- WuXi Biologics
- YXgene
目次
第1章 背景
第2章 調査手法
第3章 市場力学
第4章 マクロ経済指標
- 章の概要
- 市場力学
- 結論
第5章 エグゼクティブサマリー
第6章 イントロダクション
- mRNAの概要
- mRNAの構造
- mRNAワクチンの進化
- mRNA合成プロセスの種類
- mRNAの応用
- mRNA合成に伴う課題
- 一般的にアウトソーシングされるmRNA製造業務
- 製造業務のアウトソーシングのメリット
- 結論
第7章 mRNAカスタム合成サービスプロバイダー:市場情勢
- mRNAカスタム合成サービスプロバイダーの概要
第8章 企業競争力分析:mRNAカスタム合成サービスプロバイダー
- 調査手法と主要なパラメータ
- 採点基準
- ピアグループの概要
- 企業競争力分析
第9章 mRNA受託製造サービスプロバイダー:市場情勢
- mRNA契約製造サービスプロバイダーの概要
第10章 企業競争力分析:mRNA受託製造サービスプロバイダー
- 調査手法と主要なパラメータ
- 採点基準
- ピアグループの概要
- 企業競争力分析
第11章 mRNA合成キット:市場情勢
- mRNA合成キットの概要
- mRNA合成キット:開発者の情勢
第12章 製品競争力分析:mRNA合成キット
- 調査手法と主要なパラメータ
- 採点基準
- 主要なmRNA合成キット:2Dドットプロット
第13章 企業プロファイル:mRNAカスタム合成サービスプロバイダー
- 方法
- Aldevron
- Thermo Fischer Scientific
- Revolution Biomanufacturing
- BioNTech
- Eurogentec
- Biocell
- YXgene
- Aurigene Pharmaceutical Services
- Biomay
- GenScript
- Trilink Biotechnologies
第14章 企業プロファイル:mRNA受託製造サービスプロバイダー
- 方法
- Curia
- Lonza
- Merck
- Porton Advanced
- Samsung Biologics
- ST Pharm
- Vernal Biosciences
- Wuxi Biologics
第15章 企業プロファイル:mRNA合成キット
- 方法
- APExBIO
- Biorbyt
- Cell Script
- Creative Biogene
- Jena Bioscience
- Thermo Fischer Scientific
第16章 パートナーシップとコラボレーション
- パートナーシップモデル
- mRNA合成・製造市場:パートナーシップとコラボレーション
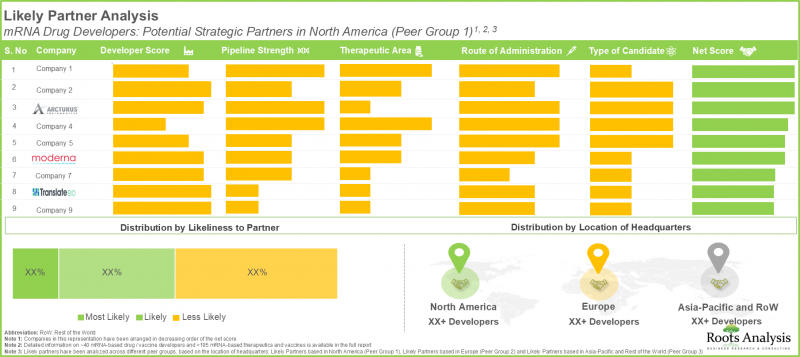
第17章 パートナー候補の分析
- mRNA合成・製造市場:パートナー候補分析
- 調査手法と主要なパラメータ
- 採点基準
- mRNA医薬品開発企業:北米を拠点とするパートナー候補
- mRNA医薬品開発企業:欧州を拠点とするパートナー候補
- mRNA医薬品開発者:アジア太平洋およびその他の地域に拠点を置くパートナー候補
第18章 大手企業の取り組み
- 調査手法
- 主要な取り組みの分析
- mRNA合成・製造市場:大手企業の取り組み
第19章 市場影響分析
- 市場の促進要因
- 市場の抑制要因
- 市場の機会
- 市場の課題


