
|
市場調査レポート
商品コード
1437924
医療機器検査・認証:市場シェア分析、業界動向と統計、成長予測(2024~2029年)Global Medical Device Testing and Certification - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
カスタマイズ可能
適宜更新あり
|
|||||||
| 医療機器検査・認証:市場シェア分析、業界動向と統計、成長予測(2024~2029年) |
|
出版日: 2024年02月15日
発行: Mordor Intelligence
ページ情報: 英文 121 Pages
納期: 2~3営業日
|
- 全表示
- 概要
- 目次
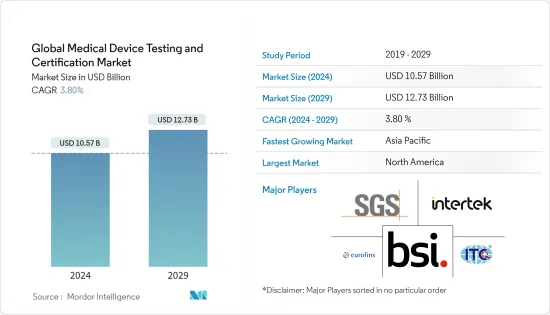
世界の医医療機器検査・認証市場規模は、2024年に105億7,000万米ドルと推定され、2029年までに127億3,000万米ドルに達すると予測されており、予測期間(2024年から2029年)中に3.80%のCAGRで成長します。
2020年のCOVID-19の流行中、病気の感染を防ぐためにロックダウン措置や国家間の輸出入活動の制限などのいくつかの措置が講じられたが、これによりサプライチェーンが混乱し、調査対象となった市場全体に悪影響を及ぼしました。
さらに、COVID-19による国際サプライチェーンの混乱により、世界中で重要な医療機器が不足しています。したがって、多くの国は、医療機器の国内製造など、機器を輸入することで不足を緩和するための明確な措置を講じています。さらに、必須の医療機器の国内製造は貿易障壁を克服し、同時に製品の品質と市場の安定性を確保することが期待されています。
英国規格協会(BSI)によると、同社は2020年 2月に、COVID-19のパンデミックの影響を考慮して、プロセスを見直し、英国規格協会(BSI)の顧客や同僚へのリスクを最小限に抑えながら、環境を維持するためのプログラムを計画しました。認定要件を満たし、潜在的な世界貿易リスクを軽減します。
医療機器の有効性と安全性を確保することは非常に重要であるため、医療機器は厳格な規制プロトコルに準拠する必要があります。したがって、すべてのデバイスは市場に投入される前に国内および国際標準に準拠することが義務付けられています。医療機器の標準ガイドラインは国によって異なり、すべてのメーカーは、その国で製品をマーケティングまたは販売する際にこれらのガイドラインに従うことが義務付けられています。たとえば、米国は食品医薬品局(FDA)のガイドラインに従い、欧州はConformite Europeenne(CE)の承認を検討し、カナダはカナダ保健省登録が必要で、インドは中央医薬品標準管理機構(CDSCO)の承認が必要です。この多様な規制環境が試験および認証市場を推進しています。
規制は国ごとに異なるため、各医療機器メーカーがその特定の国の規制ガイドラインを登録または受け取ることが重要です。これにより、認可された第三者が自社の機器を登録する必要性が示されます。各国の規制当局は、その特定の国で製品を販売するメーカーが標準ガイドラインに準拠し、第三者認証システムによるチェックを受けることを望んでいます。
これにより、試験および認証市場が促進され、市場へのアクセスが容易になる可能性があります。医療機器の検証と検証(V&V)の必要性の高まりなどの他の要因も、医療機器の試験と認証市場を推進しています。ただし、規制の多様性などの要因により、予測期間中の市場の成長が妨げられると予想されます。
医療機器検査市場の動向
テストサービス部門は予測期間中に急速な成長が見込まれる
世界的に、医療機器はさまざまな規制当局とコンプライアンスによって規制されています。これは主に、これらの機器のエンドユーザーがこれらの医療機器に優れた性能、有効性、安全性を期待しているためです。したがって、製造業者は医療機器のテスト戦略を適切に定義して実施することが必須であり、これにより機器の効果が高まり、品質の確認により生産が容易になります。
2020年4月、COVID-19感染症のパンデミックにより、欧州委員会(EC)は、COVID-19のパンデミックにより特定の医療機器の需要が増加したため、医療機器規制(MDR)の適用日を1年間延期する提案を採択しました。これは、そのようなデバイスの潜在的な不足によるリスクや困難を回避するために重要でした。さらに、COVID-19のパンデミックにより臨床試験が遅れ、医療機器のプロセスが混乱しました。
一部の医療機器は、2020年のCOVID-19のパンデミック中に需要が急増しました。たとえば、人工呼吸器は重篤な状態にある患者を生かし続ける病院の重要なツールであるため、COVID-19患者にとって需要が高かったのです。たとえば、2020年3月、メドトロニックPLCは、世界中の患者とヘルスケアシステムの緊急ニーズに応えて、これまでに生産を40%以上増加させ、人工呼吸器の製造および供給能力を2倍以上に拡大する予定であると発表しました。
2020年 6月、Intertek Group PLCは、国立労働安全衛生研究所(NIOSH)が定めた要件に合わせたN95マスクのテストを含む個人用保護具サービスの拡大を発表しました。この拡大に伴い、同社は、COVID-19のパンデミック中に顧客と国際社会をサポートするためのソリューションとリソースも拡大しました。
効果的な医療機器テスト戦略には、いくつかのテスト要件が必要です。一連の要件は、コンポーネントの選択から医療機器の最終組み立てに至るまで、完全な製造プロセスのさまざまな段階で継続的にテストが実行されるため、テストの実施をスムーズにするために必要です。各段階には、満たすべき異なる要件と異なるパラメータがあります。したがって、医療機器の増加により、これらの検査サービスも増加する可能性があり、市場全体の成長を促進すると予想されます。
北米が市場を独占しており、予測期間中も同様に推移すると予想される
北米地域の市場成長を促進する要因には、医療機器の品質への注目の高まり、医療機器業界にサービスを提供する多数の企業の存在、およびよく発達したヘルスケアの存在が含まれます。
2020年5月に発表されたA.チャンディマル・ニコラス氏の調査論文によると、2020年のCOVID-19パンデミック中にカナダで保健大臣はCOVID-19に関連して使用する医療機器の輸入および販売に関する暫定命令に署名しました。これにより、ヘルスケア提供者が使用するCOVID-19の医療機器への迅速なアクセスが可能になりました。さらに、カナダ保健省によると、この暫定命令は、COVID-19医療機器の輸入と販売の迅速な承認に役立ちました。
さらに、2020年のCOVID-19のパンデミック中、米国では食品医薬品局(FDA)が、COVID-19を診断するための医療機器と、患者と接するヘルスケア提供者を保護するために必要な個人用保護具に対して緊急使用許可(EUA)を発行しました。
米国では、医療機器は食品医薬品局(FDA)によって管理され、機器の安全性と有効性が保証されています。 Center for Devices and Radiological Health(CDRH)は、食品医薬品局(FDA)の部門です。クラス IIデバイスには、「ラベル付け、ガイダンス、追跡、計画、性能基準、および市販後の観察」に関して顕著な管理が必要であり、ほとんどの場合、合法的に市販されたデバイスとの実質的な同等性を評価するために市販前通知 510(k)が必要です。
メディケアおよびメディケイドサービスセンターによると、2018年の米国のヘルスケア支出は4.6%増加し、3兆6,000億米ドル、つまり1人当たり11,172米ドルに達しました。国内総生産に占める医療支出の割合は17.7%でした。さらに、この地域での医療機器の承認の増加に伴い、医療機器検査サービスの需要の高まりが市場の成長を促進する可能性があります。したがって、上記の要因を考慮すると、予測期間中に北米地域の市場の成長を促進すると予想されます。
医療機器検査業界の概要
医療機器検査・認証市場は高度に統合されており、検査・認証サービスを提供する企業はほとんどありません。医療機器市場の成長に伴い、今後さらに多くの企業が市場に参入すると予想されています。今後数年間で、中小企業がかなりの市場シェアを獲得する可能性があります。市場参加者には、BSI Group、Intertek Group PLC、Institute for Testing and Certification Inc.、Eurofins Scientific、SGS SAなどが含まれます。
その他の特典
- エクセル形式の市場予測(ME)シート
- 3か月のアナリストサポート
目次
第1章 イントロダクション
- 調査の前提条件と市場の定義
- 調査範囲
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 市場力学
- 市場概要
- 市場促進要因
- 医療機器の検証と検証(V&V)のニーズの高まり
- 規格の遵守
- 市場抑制要因
- 規制の多様性
- 業界の魅力- ポーターのファイブフォース分析
- 買い手の交渉力
- 供給企業の交渉力
- 新規参入業者の脅威
- 代替製品の脅威
- 競争企業間の敵対関係の激しさ
第5章 市場セグメンテーション
- サービスの種類別
- テストサービス
- 検査サービス
- 認証サービス
- 他のサービス
- 調達タイプ別
- 社内
- 外部委託
- デバイスクラス別
- クラスI
- クラス II
- クラスIII
- テクノロジー別
- アクティブインプラント医療機器
- アクティブ医療機器
- 非アクティブ医療機器
- 体外診断用医療機器
- 眼科用医療機器
- 整形外科および歯科用医療機器
- その他の技術
- 地域別
- 北米
- 米国
- カナダ
- メキシコ
- 欧州
- ドイツ
- 英国
- フランス
- イタリア
- スペイン
- その他欧州
- アジア太平洋
- 中国
- 日本
- インド
- オーストラリア
- 韓国
- その他アジア太平洋地域
- 中東とアフリカ
- GCC
- 南アフリカ
- その他中東とアフリカ
- 南米
- ブラジル
- アルゼンチン
- その他南米
- 北米
第6章 競合情勢
- 企業プロファイル
- BSI Group
- Dekra Testing and Certification GmbH
- Eurofins Scientific
- Institute for testing and Certification Inc.
- Intertek Group PLC
- SGS SA
- TUV Rheinland
- UL LLC
- Bureau Veritas
- Element Materials Technology
- Avomeen
- Gateway Analytical LLC
- Medistri SA
- Pace Analytical Services LLC
- WuXi AppTec
第7章 市場機会と将来の動向
The Global Medical Device Testing and Certification Market size is estimated at USD 10.57 billion in 2024, and is expected to reach USD 12.73 billion by 2029, growing at a CAGR of 3.80% during the forecast period (2024-2029).

During the COVID-19 outbreak in 2020, several measures were taken to prevent the transmission of diseases, such as lockdown measurements and restricting import-export activities between the countries, which disrupted the supply chain, thus, negatively impacting the overall market studied.
Moreover, the disruption caused due to COVID-19 in international supply chains has led to shortages of critical medical devices across the world. Therefore, many countries have taken definite measures to ease the shortages by importing equipment, such as domestic manufacturing of medical devices. Additionally, domestic manufacturing of essential medical devices is expected overcome trade barriers, at the same time, ensure product quality and market stability.
As per British Standards Institution (BSI), in February 2020, by taking into consideration the effects of the COVID-19 pandemic, the company reviewed processes and planned a program to minimize the risk to British Standards Institution (BSI) clients and colleagues while maintaining accreditation requirements and mitigating potential global trade risks.
The medical devices are subjected to comply with strict regulatory protocols, as it is vital to ensure the efficacy and safety of medical devices. Therefore, it is compulsory for every device to comply with national and international standards before entering a market. The standard guidelines of medical devices vary from country to country, and it is mandatory for every manufacturer to follow these guidelines for marketing or selling their products in a country. For instance, the United States follows Food and Drug Administration (FDA) guidelines, Europe considers Conformite Europeenne (CE) approval, Canada needs Health Canada Registration, and India requires approval from Central Drugs Standard Control Organisation (CDSCO). This diverse range of regulatory landscapes drives the testing and certification market.
Since regulations are different in every country, it is crucial for each medical device manufacturer to register or receive regulatory guidelines of that specific country, which, in turn, indicates the need for authorized third parties to register their devices. National regulatory authorities of every country prefer that the manufacturers selling their products in that particular country should comply with standard guidelines and get it checked by a third-party certification system.
This may result in propelling the testing and certification market, as well as increase easy market access. The other factors, such as the increasing need for validation and verification (V&V) for medical devices, are driving the medical device testing and certification market. However, a factor such as diversity in regulation is expected to impede market growth over the forecast period.
Medical Device Testing Market Trends
Testing Services Segment is Expected to Witness Rapid Growth During the Forecast Period
Globally, medical devices are regulated by various regulatory authorities and compliances. This is mainly because the end-users of these devices expect outstanding performance, effectiveness, and safety from these medical devices. Therefore, it is mandatory for the manufacturers to properly define and implement a medical device testing strategy, which makes the device effective and production becomes easier due to the confirmation of quality.
In April 2020, due to the COVID-19 pandemic, the European Commission (EC) adopted a proposal to postpone the application date of the Medical Device Regulation (MDR) for one year because the COVID-19 pandemic increased demand for certain medical devices, which were crucial to avoid risks or difficulties of potential shortages of such devices. Moreover, the COVID-19 pandemic has delayed clinical trials and disrupted processes for medical devices.
Some of the medical devices experienced a sudden surge in demand during the COVID-19 pandemic 2020. For instance, ventilators were in high demand for COVID-19 patients, as they are an important tool in hospitals that can keep the patients in critical conditions alive. For instance, in March 2020, Medtronic PLC announced that it had increased production by more than 40% to date and was on track to more than double its capacity to manufacture and supply ventilators in response to the urgent needs of patients and healthcare systems across the world confronting COVID-19.
In June 2020, Intertek Group PLC announced the expansion of personal protective equipment services to include testing of N95 respirators to requirements set by the National Institute for Occupational Safety and Health (NIOSH). With this expansion, the company also expanded upon its solutions and resources to support customers and the global community during the COVID-19 pandemic.
An effective medical device testing strategy needs several sets of test requirements. The sets of requirements are required to smoothen test implementation as tests are carried out continuously at different stages of the complete manufacturing process, from component selection to a final assembly of a medical device. Each stage has different requirements and different parameters to be satisfied. Thus, increasing medical devices may also increase these testing services, which is expected to augment the overall growth of the market.
North America Dominates the Market and is Expected to do the Same over the Forecast Period
Some of the factors driving the market growth in the North American region include increased focus on the quality of the medical devices and the presence of a large number of companies that serve the medical device industry, along with the presence of well-developed healthcare and the presence of top multinational medical device companies.
According to a research article by A. Chandimal Nicholas published in May 2020, in Canada, during the COVID-19 pandemic 2020, the Minister of Health signed the Interim Order Respecting the Importation and Sale of Medical Devices for Use in relation to COVID-19, which allowed expedited access to COVID-19 medical devices for use by healthcare providers. Moreover, as per Health Canada, the Interim Order helped for quick approval of the importation and sale of COVID-19 medical devices.
Additionally, during the COVID-19 pandemic 2020, in the United States, the Food and Drug Administration (FDA) issued Emergency Use Authorizations (EUA) for medical devices to diagnose COVID-19 and personal protective equipment needed to protect healthcare providers interacting with patients.
In the United States, medical devices are managed by the Food and Drug Administration (FDA) to guarantee the safety and effectiveness of devices. The Center for Devices and Radiological Health (CDRH) is a Food and Drug Administration (FDA) segment. Class II devices require remarkable controls for 'labeling, guidance, tracking, plan, performance standards, and post-market observation,' and most require premarket notification 510(k) to appraise substantial equivalence to a lawfully marketed device.
According to the Center for Medicare & Medicaid Services, the United States healthcare spending grew by 4.6% in 2018, reaching USD 3.6 trillion or USD 11,172 per person. As a share of the nation's Gross Domestic Product, health spending accounted for 17.7%. Moreover, with increasing approval of medical devices in the region, rising demand for medical device testing services may boost the market growth. Thus, considering the above-mentioned factors, it is expected to fuel the market growth in the North American region over the forecast period.
Medical Device Testing Industry Overview
The medical device testing and certification market is highly consolidated, and few companies provide testing and certification services. It has been observed that with the growing medical device market, more companies are expected to enter the market in the future. Substantial market share may be gained by the small to mid-sized companies in the coming years. Some market players include BSI Group, Intertek Group PLC, Institute for Testing and Certification Inc., Eurofins Scientific, and SGS SA, among others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Need of Validation and Verification (V&V) for Medical Devices
- 4.2.2 Compliance of Standards
- 4.3 Market Restraints
- 4.3.1 Diversity in Regulation
- 4.4 Industry Attractiveness - Porter's Five Forces Analysis
- 4.4.1 Bargaining Power of Buyers/Consumers
- 4.4.2 Bargaining Power of Suppliers
- 4.4.3 Threat of New Entrants
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION
- 5.1 By Service Type
- 5.1.1 Testing Services
- 5.1.2 Inspection Services
- 5.1.3 Certification Services
- 5.1.4 Other Services
- 5.2 By Sourcing Type
- 5.2.1 In-house
- 5.2.2 Outsourced
- 5.3 By Device Class
- 5.3.1 Class I
- 5.3.2 Class II
- 5.3.3 Class III
- 5.4 By Technology
- 5.4.1 Active Implant Medical Device
- 5.4.2 Active Medical Device
- 5.4.3 Non-active Medical Device
- 5.4.4 In Vitro Diagnostic Medical Device
- 5.4.5 Ophthalmic Medical Device
- 5.4.6 Orthopedic and Dental Medical Device
- 5.4.7 Other Technologies
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East & Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East & Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 BSI Group
- 6.1.2 Dekra Testing and Certification GmbH
- 6.1.3 Eurofins Scientific
- 6.1.4 Institute for testing and Certification Inc.
- 6.1.5 Intertek Group PLC
- 6.1.6 SGS SA
- 6.1.7 TUV Rheinland
- 6.1.8 UL LLC
- 6.1.9 Bureau Veritas
- 6.1.10 Element Materials Technology
- 6.1.11 Avomeen
- 6.1.12 Gateway Analytical LLC
- 6.1.13 Medistri SA
- 6.1.14 Pace Analytical Services LLC
- 6.1.15 WuXi AppTec


