
|
市場調査レポート
商品コード
1687170
世界の血糖モニタリング-市場シェア分析、産業動向・統計、成長予測(2025~2030年)Global Blood Glucose Monitoring - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
カスタマイズ可能
適宜更新あり
|
|||||||
| 世界の血糖モニタリング-市場シェア分析、産業動向・統計、成長予測(2025~2030年) |
|
出版日: 2025年03月18日
発行: Mordor Intelligence
ページ情報: 英文 236 Pages
納期: 2~3営業日
|
全表示
- 概要
- 目次
世界の血糖モニタリング市場規模は、2025年に245億6,000万米ドルと推定され、予測期間(2025~2030年)のCAGRは7.82%で、2030年には357億9,000万米ドルに達すると予測されます。
COVID-19パンデミックは、血糖モニタリングシステム市場の成長にプラスの影響を与えました。SARS-CoV-2に感染した糖尿病患者は、さらなるストレスを経験し、グルココルチコイドやカテコールアミンなどの高血糖ホルモンの分泌が増加し、血糖上昇、異常なグルコース変動、糖尿病合併症を引き起こしました。悪化を避けるために、COVID-19患者の入院中は患者の血糖モニタリングを考慮しなければならず、血糖モニタリング装置の重要性が強調されました。米国食品医薬品局は、パンデミックの間、病院内での個人用血糖測定器と持続血糖測定器の使用を許可しました。パンデミックは、患者と医療提供者による遠隔診療を増加させ、多くの長年の規制障壁を取り除いた。
WHOによると、糖尿病は高血糖を特徴とする長期にわたる代謝障害であり、その結果、心臓、血管、目、腎臓、神経に長期にわたり深刻な障害をもたらします。最も一般的なのは2型糖尿病で、一般的に成人に発症し、体がインスリンに対して抵抗性を持つか、インスリンを十分に分泌できなくなります。過去30年間で、2型糖尿病の罹患率は所得水準の異なる国々で著しく増加しています。1型糖尿病は、以前は若年性糖尿病またはインスリン依存性糖尿病と呼ばれ、膵臓からのインスリンの分泌が不十分、または全くない慢性疾患です。糖尿病患者にとって、インスリンを含む治療を安価に受けられることは、生存に不可欠です。2025年までに糖尿病と肥満の増加を食い止めるという世界の目標が設定されています。
Lancetが2023年に発表した最近の推定によると、2050年までに全世界で1億人以上が糖尿病に罹患する可能性があると予測されています。この驚異的な数字は、人生を左右する健康合併症を引き起こすだけでなく、死亡率の上昇や他の様々な病気を悪化させる原因となる病気とともに生活している世界人口のかなりの部分に相当します。予想される糖尿病有病率の増加は、主に2型糖尿病の罹患率の上昇によってもたらされます。この背景には、肥満の蔓延と人口動態の変化があります。現在のところ、2型糖尿病は糖尿病患者全体の90%を占めています。この負担の大部分は、高BMI、食事のリスク、環境や職業上の危険、タバコやアルコールの使用、運動量の少なさなどの社会的危険因子に起因しています。これらの危険因子は、私たちを取り巻く環境の肥満化しやすい性質や、資源や社会組織の不公平な分配によって繁栄しています。
血糖モニタリングは、血液中のグルコース濃度(血糖値)を検査します。糖尿病管理において特に重要であり、血糖値検査は通常、血糖値の変動を判定し、それに応じて薬を服用したり変更したりするために行われます。血糖値を頻繁にモニタリングしなければ、最適な血糖値を達成することは非常に困難です。
SMBGデータを安全なクラウドベースのデータベースに自動的にアップロードする携帯電話接続機器など、血糖測定器の技術的進歩により、SMBGデータの共有とモニタリングが改善されています。SMBGデータのリアルタイムモニタリングは、SMBGの異常記録に対応する患者にタイムリーなサポートを提供する機会を記載しています。このような糖尿病遠隔モニタリングプログラムは、コントロール不良の糖尿病患者に対して、重要な転帰を改善するために必要な追加サポートを提供することができ、それによって今後数年間の市場展望を向上させることができます。
血糖モニタリング市場動向
自己血糖モニタリングセグメントが今年度最高市場シェアを獲得
自己血糖モニタリングセグメントは、今年度の血糖モニタリング市場で約66.09%の主要市場シェアを占めました。
血糖自己測定は、糖尿病患者がグルコメーター、テストストリップ、ランセットを使用して、血糖値を自分で測定するために使用されるアプローチです。測定値に基づいて、患者は治療効果を調整したり確認したりすることができます。CGMは糖尿病患者にとって、グルコース測定値をリアルタイムで確認できる先進的な方法であるが、SMBGはCGMに比べて経済的に手頃であり、使用方法も洗練されていないため、患者に最も好まれている機器です。
国際的なガイドラインでは、効果的な糖尿病管理と治療のために自己血糖測定(SMBG)を定期的に使用することが推奨されています。グルコメーターによる測定値と実際の血糖値との間には不一致があるかもしれないが、グルコメーターによって得られる即時の結果は、時間のかかる検査室での検査によって得られる結果よりも便利です。その結果、SMBG機器は、患者の自宅から病院の救急室まで、医療現場で広く使用されるようになりました。
検査ストリップの市場数量とシェアの伸びは、使用事例の頻度の違いから、グルコース測定器よりも高くなると予想されます。グルコースメーターの平均使用期間は6ヵ月から3年であるのに対し、テストストリップは1回限りの使用です。ユーザーに与える苦痛が少ない新種のランセットデバイスの導入は、予測期間中に受容率が上昇する可能性が高いため、市場の成長に貢献すると予想されます。
個々の血糖測定値を表示と要約し、インスリン投与量、食事/間食、身体活動などの追加関連データを組み込んだデジタルヘルスアプリなどの革新的技術は、疾病負担を軽減し、糖尿病ケア全体に利益をもたらしながら、自己管理をさらに支援することができます。血糖モニタリングとインスリン計算機、自動インスリン滴定ソフトウェア、遠隔コーチングとの統合は、コントロール不良の糖尿病患者に、重要な転帰を改善するために必要な追加サポートを提供するさらなる開発であり、それによって今後数年間の市場展望を向上させています。
市場参入企業は、市場シェアを拡大するために、提携、パートナーシップ、合併、買収、製品開拓、事業拡大など様々な戦略を採用しています。例えば、2023年1月、ライフスキャン社は、専門誌「Journal of Diabetes Science and Technology」に、Bluetooth接続血糖測定器とモバイル糖尿病アプリを用いた血糖コントロールの改善(Improved Glycemic Control Using a Bluetooth Connected Blood Glucose Meter and a Mobile Diabetes App)が掲載されたと発表した「Real-World Evidence From Over 144,000 People With Diabetes」には、血糖測定器とモバイル糖尿病アプリを組み合わせたデータセットとしては過去最大規模となる14万4,000人以上の糖尿病患者から得られた実世界のデータをレトロスペクティブに分析した結果が詳述されています。
このように、上記の要因が予測期間中の同セグメントの成長を促進すると予想されます。
北米が血糖モニタリング市場を独占する見込み
今年、北米、特に米国は、大規模な患者プールと先進技術の幅広い受け入れにより、血糖モニタリング市場で約54.5%の最大シェアを占め、次いで欧州が緩やかな成長を示しました。
米国疾病予防管理センター(CDC)の全国糖尿病統計報告書2022では、米国では1億3,000万人以上の成人が糖尿病または糖尿病予備軍であると推定されています。2型糖尿病は、有色人種、農村部に住む人々、教育水準が低く、所得が低く、ヘルスリテラシーが低い人々の間でより一般的であり、糖尿病はより深刻です。
米国糖尿病学会(ADA)、内分泌学会、米国医師会、米国小児科学会、一般内科学会、米国医療アカデミーが声明を発表しました。個人、組織、施策レベルで健康の社会的決定要因(SDOH)に対処するための行動を呼びかけた。2021年、ADAはまた、社会経済的地位、ヘルスリテラシー、食環境、食の不安、近隣環境と物理的環境などに焦点を当て、SDOHと糖尿病リスクと転帰との関連を記述した科学的レビューを発表しました。
さらに、現在の持続血糖測定装置は、データをダウンロードすることによって血糖値の動向を遡及的に表示するか、受信機のディスプレイによって血糖値をリアルタイムで表示することができます。最新のCGMモデルであるAbbott Freestyle Libre 3とDexcom G7は、多くの技術的障壁を克服しました。Eversense E3 CGMのような技術の進歩は、医療提供者の糖尿病治療能力と患者の血糖値管理能力を大幅に向上させました。
受益者とより大きな医療システムのためにCGMをカバーし、CGMへのアクセスを増やすことができるメディケイド機関のための医療戦略センターによると、インスリンポンプまたは1日複数回のインスリン注射でインスリン治療を受けているすべての人に対するCGM使用の利点を支持する強力な証拠があり、基礎インスリンを使用している患者におけるCGMの利点を示す新たな証拠も出てきています。
したがって、前述の要因によって、調査された市場の成長は北米地域で予測されます。
血糖モニタリング産業概要
血糖モニタリング市場は適度にセグメント化されています。Dexcom、Medtronic、Abbott、Senseonicsなどの企業がCGM機器市場を独占しています。SMBG機器市場は、Roche、Sinocare、LifeScan、Arkray、Ascensiaなどの大手企業と、その他のジェネリック企業で構成されています。最近の参入企業間の合併や買収は、各社が市場での存在感を強めるのに役立っています。
その他の特典
- エクセル形式の市場予測(ME)シート
- 3ヶ月間のアナリストサポート
目次
第1章 イントロダクション
- 調査の前提条件と市場定義
- 調査範囲
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 市場力学
- 市場概要
- 市場促進要因
- 市場抑制要因
- 産業の魅力-ポーターのファイブフォース分析
- 供給企業の交渉力
- 消費者の交渉力
- 新規参入業者の脅威
- 代替品の脅威
- 競争企業間の敵対関係の強さ
第5章 市場セグメンテーション
- デバイス別(売上高と数量)
- 自己血糖測定装置
- グルコメーター機器
- 検査ストリップ
- ランセット
- 持続グルコースモニタリング
- センサ
- 耐久品(レシーバーとトランスミッター)
- 自己血糖測定装置
- エンドユーザー別(売上高と数量)
- 病院/クリニックでの使用
- 自己/自宅使用
- 指標別(売上高と数量)
- 1型糖尿病人口
- 2型糖尿病人口
- 地域別(売上高と数量)
- 北米
- デバイス別、エンドユーザー別、指標別
- 国別
- 米国
- カナダ
- その他の北米
- 欧州
- デバイス別、エンドユーザー別、指標別
- 国別
- フランス
- ドイツ
- イタリア
- スペイン
- 英国
- ロシア
- その他の欧州
- ラテンアメリカ
- デバイス別、エンドユーザー別、指標別
- 国別
- メキシコ
- ブラジル
- その他のラテンアメリカ
- アジア太平洋
- デバイス別、エンドユーザー別、指標別
- 国別
- 日本
- 韓国
- 中国
- インド
- オーストラリア
- ベトナム
- マレーシア
- インドネシア
- フィリピン
- タイ
- その他のアジア太平洋
- 中東・アフリカ
- デバイス別、エンドユーザー別、指標別
- 国別
- サウジアラビア
- イラン
- エジプト
- オマーン
- 南アフリカ
- その他の中東・アフリカ
- 北米
第6章 市場指標
- 1型糖尿病人口
- 2型糖尿病人口
第7章 競合情勢
- 企業プロファイル
- Abbott Diabetes Care
- Roche Holding AG
- LifeScan
- Dexcom Inc.
- Medtronic PLC
- Arkray Inc.
- Ascensia Diabetes Care
- Agamatrix Inc.
- Bionime Corporation
- Acon Laboratories Inc.
- Medisana AG
- Trivida Functional Medicine
- Rossmax International Ltd
- Sinocare
- Senseonics
- 企業シェア分析
- 自己血糖モニタリング
- Abbott Diabetes Care
- Roche Holding AG
- LifeScan
- その他
- 連続血糖モニタリング装置
- Abbott Diabetes Care
- Dexcom Inc.
- Medtronic PLC
- その他
- 自己血糖モニタリング
第8章 市場機会と今後の動向
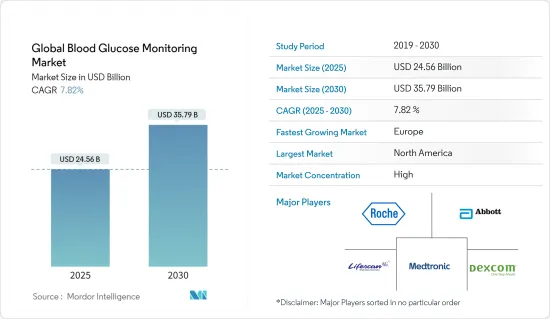
The Global Blood Glucose Monitoring Market size is estimated at USD 24.56 billion in 2025, and is expected to reach USD 35.79 billion by 2030, at a CAGR of 7.82% during the forecast period (2025-2030).

The COVID-19 pandemic positively impacted the blood glucose monitoring system market growth. Patients with diabetes who were infected with SARS-CoV-2 experienced additional stress and increased secretion of hyperglycemic hormones such as glucocorticoid and catecholamines, which resulted in elevated blood glucose, abnormal glucose variability, and diabetic complications. To avoid aggravation, a patient's blood glucose monitoring had to be considered during the COVID-19 patient's hospitalization, which underlined the importance of blood glucose monitoring devices. The United States Food and Drug Administration also allowed personal blood glucose meters and continuous glucose monitoring devices in hospitals during the pandemic. The pandemic increased remote care from patients and providers and removed many long-standing regulatory barriers.
According to WHO, diabetes is a long-lasting metabolic disorder characterized by high blood glucose (or blood sugar) levels, resulting in severe damage to the heart, blood vessels, eyes, kidneys, and nerves over time. The most prevalent form is Type 2 diabetes, typically occurring in adults, where the body becomes resistant to insulin or fails to produce enough insulin. In the past three decades, the incidence of Type 2 diabetes has significantly increased across countries with varying income levels. Type 1 diabetes, previously referred to as juvenile diabetes or insulin-dependent diabetes, is a chronic condition in which the pancreas produces insufficient or no insulin on its own. For individuals living with diabetes, affordable access to treatment, including insulin, is crucial for their survival. A global target has been established to halt the escalation of diabetes and obesity by 2025.
According to recent estimates published in 2023 by The Lancet, it is projected that over 1*31 billion individuals worldwide could be affected by diabetes by the year 2050. This staggering number represents a significant portion of the global population living with a disease that not only leads to life-altering health complications but also contributes to higher mortality rates and exacerbates various other illnesses. The anticipated increase in diabetes prevalence is primarily driven by the rising incidence of type 2 diabetes. This, in turn, can be attributed to the growing prevalence of obesity and demographic shifts. In the current year, type 2 diabetes accounted for 90% of all diabetes cases. The majority of this burden can be attributed to social risk factors, including high BMI, dietary risks, environmental and occupational hazards, tobacco and alcohol use, and low levels of physical activity. These risk factors thrive due to the obesogenic nature of our environments and the inequitable distribution of resources and societal organization.
Blood glucose monitoring tests the glucose concentration in the blood (glycemia). Particularly important in diabetes management, a blood glucose test is typically performed to determine the fluctuation in blood glucose level to take or alter the medication accordingly, especially for insulin users. Achieving optimum glycemic results can be very difficult without frequent monitoring of blood glucose levels.
Technological advancements in blood glucose meters, including cellular-connected devices that automatically upload SMBG data to secure cloud-based databases, allow for improved sharing and monitoring of SMBG data. Real-time monitoring of SMBG data presents opportunities to provide timely support to patients responding to abnormal SMBG recordings. Such diabetes remote monitoring programs can provide patients with poorly controlled diabetes additional support needed to improve critical outcomes, thereby enhancing the market prospects in the years to come.
Blood Glucose Monitoring Market Trends
The Self-blood Glucose Monitoring Segment held the Highest Market Share in the Current Year
The self-blood glucose monitoring segment held a major market share of about 66.09% in the blood glucose monitoring market in the current year.
Self-monitoring of blood glucose is an approach used by diabetic patients to measure their blood sugar level themselves, using a glucometer, test strips, and lancets. Based on the readings, patients can adjust or check the effect of their treatment. Although CGM is an advanced way for people living with diabetes to check glucose readings in real-time, SMBG is the most preferred device by patients due to its economic affordability and less sophisticated usage when compared to CGM.
The international guidelines suggest that self-monitoring blood glucose (SMBG) should be used regularly for effective diabetes management and treatment. Although there may be discrepancies between glucometer readings and actual blood glucose levels, the immediate results provided by glucometers are more convenient than those obtained through laboratory testing, which can be time-consuming. As a result, SMBG devices have become widely used in medical settings, from patients' homes to hospital emergency rooms.
The growth in market volume and share of test strips are expected to be higher than that of glucose meters because of the difference in use-case frequency. The average efficiency of the glucose meter ranges between six months to three years, whereas test strips are for one-time use. Introducing new kinds of lancet devices that induce lesser pain to the users is expected to help the market's growth, as the acceptance rate is likely to rise during the forecast period.
Innovative technologies, such as digital health apps that display and summarize individual blood glucose measurements and incorporate additional relevant data, such as insulin doses, meals/snacks, and physical activity, can further support self-management while decreasing disease burden and benefitting overall diabetes care. Integration of Blood Glucose Monitoring with insulin calculators, automated insulin titration software, and remote coaching are further developments that provide patients with poorly controlled diabetes additional support needed to improve critical outcomes, thereby enhancing the market prospects in the years to come.
The market players are adopting various strategies such as collaborations, partnerships, mergers, acquisitions, product development, and expansions to increase market share. For instance, in January 2023, LifeScan announced that the peer-reviewed Journal of Diabetes Science and Technology published Improved Glycemic Control Using a Bluetooth Connected Blood Glucose Meter and a Mobile Diabetes App: Real-World Evidence From Over 144,000 People With Diabetes, detailing results from a retrospective analysis of real-world data from over 144,000 people with diabetes one of the largest combined blood glucose meter and mobile diabetes app datasets ever published.
Thus, the above-mentioned factors are expected to drive the segment growth over the forecast period.
North America Expected to Dominate the Blood Glucose Monitoring Market
In the current year, North America, especially the United States, held the largest share of about 54.5% in the blood glucose monitoring market due to the large patient pool and wide acceptance of advanced technologies, followed by Europe, with moderate growth.
The Centers for Disease Control and Prevention (CDC) National Diabetes Statistics Report 2022 estimated that more than 130 million adults are living with diabetes or prediabetes in the United States. Type 2 diabetes is more common, and diabetes is more consequential among communities of color, those who live in rural areas, and those with less education, lower incomes, and lower health literacy.
The American Diabetes Association (ADA), the Endocrine Society, the American College of Physicians, the American Academy of Pediatrics, the Society of General Internal Medicine, and the National Academy of Medicine published statements. It issued calls to action to address social determinants of health (SDOH) at the individual, organizational, and policy levels. In 2021, the ADA also published a scientific review describing the associations between SDOH and diabetes risk and outcomes, focusing on socioeconomic status, health literacy, the food environment, food insecurity, and neighborhood and physical environments, among other topics.
Furthermore, the current continuous glucose monitoring devices can either retrospectively display the trends in blood glucose levels by downloading the data or give a real-time picture of glucose levels through receiver displays. The newest CGM models, the Abbott Freestyle Libre 3 and the Dexcom G7, overcame many technical barriers. Technological advances such as Eversense E3 CGMs have significantly improved providers' ability to treat diabetes and patients' ability to manage their blood glucose levels.
According to the Centre for Health Care Strategies for Medicaid agencies that can cover and increase access to CGMs for their beneficiaries and the larger healthcare system, there is strong evidence that supports the benefits of CGM use for all people who are insulin-treated with an insulin pump or multiple daily insulin injections, and emerging evidence is showing the benefit of CGMs in patients on basal insulin.
Therefore, owing to the aforementioned factors, the growth of the studied market is anticipated in the North American region.
Blood Glucose Monitoring Industry Overview
The blood glucose monitoring market is moderately fragmented. Companies like Dexcom, Medtronic, Abbott, and Senseonics dominate the CGM devices market. The market for SMBG devices comprises major players like Roche, Sinocare, LifeScan, Arkray, Ascensia, etc., and other generic players. Mergers and acquisitions among the players in the recent past have helped the companies strengthen their market presence.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.3 Market Restraints
- 4.4 Industry Attractiveness - Porter's Five Forces Analysis
- 4.4.1 Bargaining Power of Suppliers
- 4.4.2 Bargaining Power of Consumers
- 4.4.3 Threat of New Entrants
- 4.4.4 Threat of Substitute Products and Services
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION
- 5.1 By Device (Revenue and Volume)
- 5.1.1 Self-Monitoring Blood Glucose
- 5.1.1.1 Glucometer Devices
- 5.1.1.2 Test Strips
- 5.1.1.3 Lancets
- 5.1.2 Continuous Glucose Monitoring
- 5.1.2.1 Sensors
- 5.1.2.2 Durables (Receivers and Transmitters)
- 5.1.1 Self-Monitoring Blood Glucose
- 5.2 By End User (Revenue and Volume)
- 5.2.1 Hospital/Clinics Usage
- 5.2.2 Self/Home Usage
- 5.3 By Indicators (Revenue and Volume)
- 5.3.1 Type-1 Diabetes Population
- 5.3.2 Type-2 Diabetes Population
- 5.4 Geography (Revenue and Volume)
- 5.4.1 North America
- 5.4.1.1 By Device, By End User, By Indicators
- 5.4.1.2 By Country
- 5.4.1.2.1 United States
- 5.4.1.2.2 Canada
- 5.4.1.2.3 Rest of North America
- 5.4.2 Europe
- 5.4.2.1 By Device, By End User, By Indicators
- 5.4.2.2 By Country
- 5.4.2.2.1 France
- 5.4.2.2.2 Germany
- 5.4.2.2.3 Italy
- 5.4.2.2.4 Spain
- 5.4.2.2.5 United Kingdom
- 5.4.2.2.6 Russia
- 5.4.2.2.7 Rest of Europe
- 5.4.3 Latin America
- 5.4.3.1 By Device, By End User, By Indicators
- 5.4.3.2 By Country
- 5.4.3.2.1 Mexico
- 5.4.3.2.2 Brazil
- 5.4.3.2.3 Rest of Latin America
- 5.4.4 Asia-Pacific
- 5.4.4.1 By Device, By End User, By Indicators
- 5.4.4.2 By Country
- 5.4.4.2.1 Japan
- 5.4.4.2.2 South Korea
- 5.4.4.2.3 China
- 5.4.4.2.4 India
- 5.4.4.2.5 Australia
- 5.4.4.2.6 Vietnam
- 5.4.4.2.7 Malaysia
- 5.4.4.2.8 Indonesia
- 5.4.4.2.9 Philippines
- 5.4.4.2.10 Thailand
- 5.4.4.2.11 Rest of Asia-Pacific
- 5.4.5 Middle East and Africa
- 5.4.5.1 By Device, By End User, By Indicators
- 5.4.5.2 By Country
- 5.4.5.2.1 Saudi Arabia
- 5.4.5.2.2 Iran
- 5.4.5.2.3 Egypt
- 5.4.5.2.4 Oman
- 5.4.5.2.5 South Africa
- 5.4.5.2.6 Rest of Middle East and Africa
- 5.4.1 North America
6 MARKET INDICATORS
- 6.1 Type-1 Diabetes population
- 6.2 Type-2 Diabetes population
7 COMPETITIVE LANDSCAPE
- 7.1 COMPANY PROFILES
- 7.1.1 Abbott Diabetes Care
- 7.1.2 Roche Holding AG
- 7.1.3 LifeScan
- 7.1.4 Dexcom Inc.
- 7.1.5 Medtronic PLC
- 7.1.6 Arkray Inc.
- 7.1.7 Ascensia Diabetes Care
- 7.1.8 Agamatrix Inc.
- 7.1.9 Bionime Corporation
- 7.1.10 Acon Laboratories Inc.
- 7.1.11 Medisana AG
- 7.1.12 Trivida Functional Medicine
- 7.1.13 Rossmax International Ltd
- 7.1.14 Sinocare
- 7.1.15 Senseonics
- 7.2 COMPANY SHARE ANALYSIS
- 7.2.1 Self-monitoring Blood Glucose
- 7.2.1.1 Abbott Diabetes Care
- 7.2.1.2 Roche Holding AG
- 7.2.1.3 LifeScan
- 7.2.1.4 Others
- 7.2.2 Continuous Glucose Monitoring Devices
- 7.2.2.1 Abbott Diabetes Care
- 7.2.2.2 Dexcom Inc.
- 7.2.2.3 Medtronic PLC
- 7.2.2.4 Others
- 7.2.1 Self-monitoring Blood Glucose


