
|
市場調査レポート
商品コード
1851592
インフルエンザ診断薬:市場シェア分析、産業動向、統計、成長予測(2025年~2030年)Influenza Diagnostics - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
カスタマイズ可能
適宜更新あり
|
|||||||
| インフルエンザ診断薬:市場シェア分析、産業動向、統計、成長予測(2025年~2030年) |
|
出版日: 2025年07月31日
発行: Mordor Intelligence
ページ情報: 英文 110 Pages
納期: 2~3営業日
|
概要
インフルエンザ診断薬市場規模は、2025年に18億2,000万米ドル、2030年には24億1,000万米ドルに達すると予測され、この期間を通じてCAGR 5.8%で成長します。
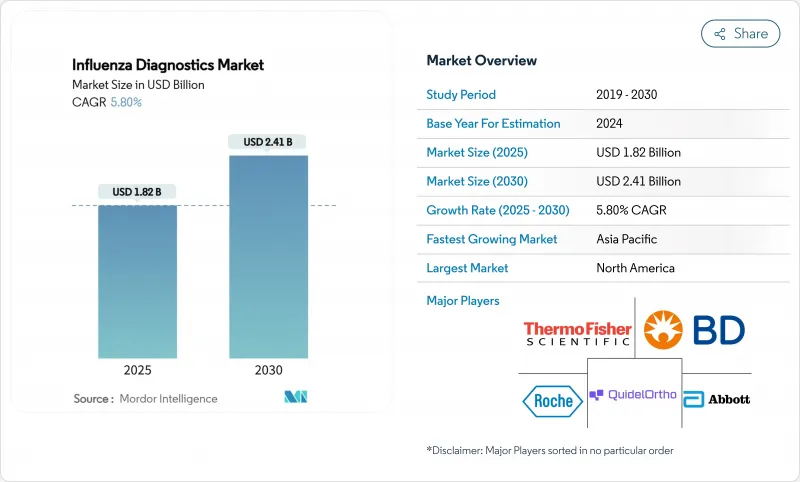
この健全な軌道は、市場がパンデミック時代の不安定な状態から、日常的な技術主導の呼吸器疾患管理へと移行していることを示しています。成長を支えているのは、従来の迅速抗原検査よりも高い精度を提供する分子プラットフォームの幅広い採用、監視インフラに対する政府の着実な資金援助、家庭用およびポイントオブケア(POC)ソリューションに対する消費者の需要の高まりです。ベンダーは、分子精度と患者に近いスピードの両立を目指して統合を進めており、AI対応ソフトウェアは検査室のターンアラウンドタイムを短縮し、品質管理を向上させています。地域ダイナミックスが需要をさらに形成している:北米は設置ベースと償還の明確さでリードしているのに対し、アジア太平洋は公衆衛生検査室への継続的な投資により、最も早い普及を記録しています。
世界のインフルエンザ診断薬市場の動向と洞察
季節性インフルエンザおよび人獣共通感染症アウトブレイクの有病率と重症度の上昇
CDCは、2024-2025年のシーズン中に3万9,053件の検査で入院が確認されたと記録しており、これは2010-2011年以来の高率です。同時に、カリフォルニア州では高病原性H5N1が発生し、酪農労働者の間で38件のヒト感染例が発生したため、家畜のサーベイランスが拡大した。シンガポールのSteadfast assayのような新しいキットは、3時間以内に高病原性株と低病原性株を区別し、アウトブレイクへの対応を強化します。このような出来事により、医療システムは緊急レベルの検査能力を通年で維持する必要に迫られ、高精度の分子プラットフォームの調達が維持されています。
外来患者における迅速POC検査の採用拡大
サウサンプトン大学の病院での研究によると、POCインフルエンザ検査では結果が出るまでの時間が1時間未満に短縮され、より迅速な抗ウイルス剤の投与開始と患者の入院期間の短縮が可能になりました。現在では、96.3%の感度で13分でインフルエンザA/Bの結果を返すアボット社のID NOWのような分子オプションもプラットフォームに含まれています。AIを搭載したリーダーは、解釈時間をさらに2分に短縮します。輸送コストの削減と訪問治療により、診療所や小売店でのPOC普及の経済的論拠となります。
変動するRIDTの感度と偽陰性率
多くのRIDTは、ウイルス量が少ない初期の感染を見逃しています。ある市販のキットでは、偽陰性率が30%を超えるという調査結果もあります。Panbio COVID-19/Flu A&Bパネルのインフルエンザに対する感度はわずか80.8%でした。WHOの2024年ガイダンスでは、現在、重症または高リスクの症例に対して核酸検査を推奨しています。診療所では、RIDTのスピードの利点を消し去り、セグメントの拡大を抑制する確認PCRワークフローを導入しています。
セグメント分析
Rapid形式は41.6%の売上シェアで優位を保ったが、臨床医が感度と多重化を優先するにつれ、インフルエンザ診断薬市場は再配置されつつあります。CRISPRアッセイは、15分でサブタイプを判別するBroad InstituteのSHINEテストに牽引され、2030年までにCAGR 9.7%を示します。インフルエンザA/B、RSV、SARS-CoV-2をバンドルした分子パネルが救急部門の業務効率化を実現。CRISPRプラットフォームのインフルエンザ診断薬市場規模は、ワークフローの簡素化と装置の設置面積の縮小により、すべてのモダリティの中で最も急速に拡大すると予測されます。直接蛍光抗体検査とウイルス培養検査は、菌株タイピングや抗ウイルス感受性が必要とされるニッチな研究分野で引き続き利用されているが、主流となる購買決定にはもはや影響を与えていないです。
RT-PCRや等温形式を含む分子診断法は、AIツールが結果の解釈を合理化するため、普及が加速しています。マルチプレックスCRISPR-Cas13aストリップは、増幅ステップを削除しながら、RT-qPCRと100%の一致を達成しました。病院では、1つのサンプルで重複する呼吸器症状を鑑別するシンドロミック・パネルが好まれ、一方、小売クリニックでは、迅速なウォークインエンカウンターのためにCLIA免責の分子カートリッジが採用されています。精度とスピードのこの収束は、資本の障壁が軽減されるにつれて、分子ソリューションがRIDTのリーダーシップを侵食していくことを意味しています。
地域分析
北米のリーダーシップは、包括的なサーベイランスシステムと成熟した償還モデルに起因します。CDCは125カ国にまたがる8つの地域サーベイランス・ハブを調整しているが、国内では最大の検査拠点を維持しています。北米のインフルエンザ診断薬市場規模は、サーモフィッシャーサイエンティフィックが20億米ドルを投じて国内生産を拡大し、サプライチェーンの安定化を図っていることが寄与しています。小売薬局ではCLIA免除の分子カートリッジが訪問診療に使用される一方、医療保険会社による在宅採取キットへの払い戻しが増加し、消費者のアクセスが拡大しています。
アジア太平洋地域のCAGRは8.1%と最速であるが、これは検査室の急速な増設と政府の資金援助によるものです。WHOは、東南アジア全域で11の国立インフルエンザセンターを完全に稼働させました。日本は品質管理規則をISO 13485:2016と整合させるために更新し、外国のアッセイ開発者の承認経路をスムーズにしました。中国とインドは、ワクチン関連のmRNAへの投資を診断薬に振り向け、CRISPRカートリッジの地域流通を促進します。
欧州は体外診断用医薬品規制(IVDR)を通じて影響力を維持しており、適合性評価要件をアッセイの15%から90%近くまで引き上げています。2024年に移行期間が延長され、当面の供給不足は回避されるが、コンプライアンスコストが上昇し、研究開発がより少ない、より価値の高い検査にシフトする可能性があります。中東・アフリカと南米は、多国間援助と官民パートナーシップによる能力向上を追求しています。OECDは、パンデミック時に経験した物流上のショックを緩和するため、調達先の多様化を促しています。このように、準備態勢にばらつきがあるため、採用曲線も異なっているが、呼吸器サーベイランスに重点を置いていることは共通しており、堅牢な検査法に対する世界的な需要は維持されています。
その他の特典:
- エクセル形式の市場予測(ME)シート
- 3ヶ月のアナリストサポート
よくあるご質問
目次
第1章 イントロダクション
- 調査の前提条件と市場の定義
- 調査範囲
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 市場情勢
- 市場概要
- 市場促進要因
- 季節性および人獣共通感染症インフルエンザの流行率と重症度の上昇
- 外来患者における迅速POC検査の採用拡大
- 政府資金による監視プログラムとパンデミック対策予算
- AIを活用した結果解釈ソフトウェアが分子ワークフローのスループットを高める
- コンボSARS-CoV-2/インフルエンザマルチプレックスパネルの商業化でインストールベースが拡大
- 在宅インフルエンザ検査キットとテレヘルスの統合が進む
- 市場抑制要因
- RIDTの可変感度と偽陰性率
- 分子診断プラットフォームの高い資本コストとランニングコスト
- CRISPRベースのインフルエンザアッセイに対する規制の不確実性
- PCRアッセイの重要試薬に影響を与えるサプライチェーンの混乱
- サプライチェーン分析
- 規制情勢
- テクノロジーの展望
- ポーターのファイブフォース分析
- 供給企業の交渉力
- 買い手の交渉力
- 新規参入業者の脅威
- 代替品の脅威
- 競争企業間の敵対関係
第5章 市場規模と成長予測
- 検査タイプ別
- 従来の診断検査
- 迅速インフルエンザ診断検査(RIDTs)
- 直接蛍光抗体(DFA)検査
- ウイルス文化
- 迅速な細胞培養
- 分子診断検査
- 逆転写酵素PCR(RT-PCR)
- ループ媒介等温増幅法(LAMP)
- ニッキング酵素増幅反応(NEAR)
- CRISPRベースアッセイ
- シンドロミックマルチプレックスPCRパネル
- 従来の診断検査
- エンドユーザー別
- 病院および臨床検査室
- 独立診断研究所
- ポイントオブケア設定
- その他
- 地域別
- 北米
- 米国
- カナダ
- メキシコ
- 欧州
- ドイツ
- 英国
- フランス
- イタリア
- スペイン
- その他欧州地域
- アジア太平洋地域
- 中国
- 日本
- インド
- 韓国
- オーストラリア
- その他アジア太平洋地域
- 中東・アフリカ
- GCC
- 南アフリカ
- その他中東・アフリカ
- 南米
- ブラジル
- アルゼンチン
- その他南米
- 北米
第6章 競合情勢
- 市場集中度
- 市場シェア分析
- 企業プロファイル
- F. Hoffmann-La Roche AG
- Abbott Laboratories(incl. ID NOW)
- QuidelOrtho Corporation
- Thermo Fisher Scientific(Cepheid, Mesa Bio)
- Becton, Dickinson & Co.
- bioMerieux SA
- Hologic Inc.
- Siemens Healthineers
- Danaher Corp.(Cepheid)
- Sekisui Diagnostics
- GenMark Diagnostics(Roche)
- Meridian Bioscience
- Luminex Corp.(DiaSorin)
- QIAGEN NV
- Bio-Rad Laboratories
- Fujirebio
- Cue Health
- Ellume
- Genetic Signatures
- Lucira Health


