
|
市場調査レポート
商品コード
1850027
がんワクチン:市場シェア分析、産業動向、統計、成長予測(2025年~2030年)Cancer Vaccines - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
カスタマイズ可能
適宜更新あり
|
|||||||
| がんワクチン:市場シェア分析、産業動向、統計、成長予測(2025年~2030年) |
|
出版日: 2025年06月16日
発行: Mordor Intelligence
ページ情報: 英文 115 Pages
納期: 2~3営業日
|
概要
がんワクチン市場は2025年に106億7,000万米ドルに達し、2030年には174億2,000万米ドルに達すると予測され、2025-2030年のCAGRは10.30%です。
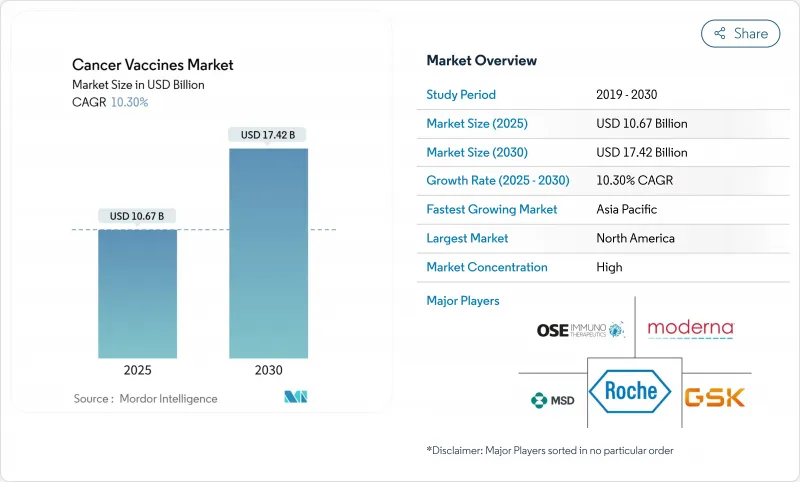
成長の加速は、従来の予防から、人工知能による抗原予測やスケールアップサイクルを短縮するモジュール式マイクロファクトリー製造に支えられた、患者特異的新抗原をコード化した個別化mRNAベース免疫療法への軸足を反映しています。FDAのブレークスルー指定やEMAのPRIME承認に見られるように、規制のハーモナイゼーションは国境を越えた臨床試験の摩擦を軽減し、パートナーシップを重視したビジネスモデルは単体の製品よりもプラットフォームの差別化に資本を振り向ける。北米がリーダーシップを維持する一方で、アジア太平洋地域は、中国の開発者がmRNAワクチンを欧米レベルより99%低いコストで提供するため、最も急速に普及が進んでいます。
世界がんワクチン市場の動向と洞察
世界的ながん罹患率の増加
がんの診断件数は2020年から2040年の間に47%増加すると予測され、総合的な腫瘍学インフラが整っていない地域で最も急増します。高齢化社会はより高い変異負荷をもたらす一方、早期診断の実施により、オーダーメイド免疫療法の対象となる患者層が拡大します。外来患者向けのワクチンレジメンは、入院による腫瘍治療からの移行に合致しており、高所得市場では患者一人当たり15万米ドルを超えることもあるシステムコストを削減することができます。そのため、支払者は、長期にわたる全身療法と比較した場合、ワクチンをコスト抑制の手段と見なしています。
研究開発投資と政府資金の増加
官民パートナーシップの構造は、従来の助成金に取って代わりつつあり、リスクを共有し、スケジュールを短縮しています。CEPIのCMCフレームワークは現在、がんワクチン製造の品質基準の指針となり、多地域での申請をスムーズにしています。がん技術に関する欧州の特許出願件数は70%以上増加し、大学が出願する割合が増加しており、共同イノベーションの勢いを示しています。英国のBioNTechプログラムは、2030年までに1万人の患者に個別化ワクチンを提供することを約束しており、国の医療制度が商業化経路に直接投資していることを示しています。ベンチャーキャピタルの流れは依然としてがん領域に偏っており、政府系ファンドがそのギャップを埋めつつあります。
厳しい規制のタイムラインと複雑さ
個別化されたバッチリリースプロトコールとAIアルゴリズムの検証は、標準的な生物製剤よりも承認サイクルを18~24ヶ月引き延ばします。EMAのPRIMEは、臨床データが成熟すれば早期承認が可能であるにもかかわらず、グローバルな規制チームを持たない中小企業は不釣り合いな負担に直面します。AIモデルの透明性に関する共通の基準がないため、審査プロセスはさらに不透明となり、コンプライアンスコストが追加され、利幅が損なわれます。
セグメント分析
2024年がんワクチン市場では、遺伝子組換え型プラットフォームが43.33%のシェアを維持した。mRNA/ネオアンチゲンワクチンは、開発者が抗原の多重コード化と迅速なカスタマイズを優先しているため、2030年までのCAGRは11.21%と加速しています。自己増幅型コンストラクトは投与量を10分の1に減らし、コールドチェーンのストレスを軽減するため、資源に制約のある環境での経済性を向上させる。ウイルスベクターやDNAを用いた方法は、特に新興市場において、耐熱性が最重要とされるニッチな集団に対応し続けています。膠芽腫を対象としたディアコノス・オンコロジーの2,000万米ドルの資金調達は、投資家の関心を裏付けています。
技術スペクトルは、ファーストムーバーにとって重要な差別化である、数週間以内の抗原交換を可能にするプラットフォーム・エコシステムへと収束しつつあります。シェアード・ネオアンチゲン・ライブラリーは、特注品を超えて対応可能な集団を拡大し、患者一人当たりのコストを削減し、規制当局の審査を短縮します。その結果、mRNAコンストラクトに起因するがんワクチン市場規模は、特に常温製剤が後期臨床試験に入るとリードを広げると予測されます。
子宮頸がんは、2024年がんワクチン市場規模の72.21%を占めました。一方、メラノーマワクチンは、強固なバイオマーカーが患者との正確なマッチングを容易にし、規制当局が画期的な指定を与えるため、CAGR 11.02%で進展しています。前立腺がんや神経膠芽腫のプログラムは樹状細胞プラットフォームを基盤としており、共有ネオアンチゲン戦略は大腸がんや胃がんに門戸を開いています。黒色腫の良好な結果は、隣接する固形がんのリスク認識を低下させ、複数のがんプラットフォーム試験に資本を引き寄せる。
単一腫瘍のサクセスストーリーからプラットフォームベースの多がんソリューションへの移行により、子宮頸がんの優位性は時間の経過とともに薄れ、2030年までにがんワクチン市場シェアは適応症間でより均等に分配されると予想されます。
地域分析
北米の2024年のシェア46.21%は、成熟した規制経路、広範な試験ネットワーク、国立がん研究所の250万米ドルのトランスレーショナルグラントのような安定した公的資金に起因します。USMCAは国境を越えた研究を合理化し、カナダとメキシコの利害関係者を共同製造ベンチャーに引き込みます。ベンチャー投資文化がリスクの高い研究開発を支え、コスト圧力の高まりにもかかわらず、この地域がんワクチン市場の成長は世界平均を大きく上回っています。
2030年までに1万人の患者を目標とする英国ーバイオNTechパートナーシップは、国の医療制度がイノベーションを促進するために購買力をどのように活用しているかを例証しています。EMA PRIMEは後期審査を加速し、ドイツ、フランス、イタリアは学術的専門知識とGMP能力を提供します。患者中心の成果を重視する償還の枠組みは、個別化されたソリューションの採用に有利であり、欧州の競争力を維持しています。
アジア太平洋地域のCAGRが11.38%と最も速いのは、国が支援するバイオテクノロジー・プログラムと、欧米の価格優位性を侵食する低コスト製造によるものです。中国はモジュール式マイクロ工場とHPV駆動装置の無償提供に資金を提供し、日本と韓国は高度なプロセス技術を輸出しています。インドは、製造受託の厚みと患者基盤の広さから、極めて重要な臨床試験のハブとなっています。オーストラリアはICH基準に沿った規制を採用しているため、太平洋横断的な商業化の架け橋となる市場です。
その他の特典:
- エクセル形式の市場予測(ME)シート
- 3ヶ月のアナリストサポート
よくあるご質問
目次
第1章 イントロダクション
- 調査の前提条件と市場の定義
- 調査範囲
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 市場情勢
- 市場概要
- 市場促進要因
- 世界のがん発症率の増加
- 研究開発投資と政府資金の増加
- mRNAおよびネオアンチゲンプラットフォームの進歩
- AIによる抗原予測でコストを削減
- モジュラーマイクロファクトリー製造ハブ
- CPIと併用療法によるリスク軽減試験(報告不足)
- 市場抑制要因
- 厳格な規制のタイムラインと複雑さ
- 代替免疫療法の利用可能性
- パーソナライズされた物流のためのコールドチェーンのギャップ
- ネオアンチゲンIPクラスタリングによる参入制限
- 規制情勢
- テクノロジーの展望
- ポーターのファイブフォース分析
- 買い手の交渉力
- 供給企業の交渉力
- 新規参入業者の脅威
- 代替品の脅威
- 競争企業間の敵対関係
第5章 市場規模と成長予測
- 技術別
- 組み換えワクチン
- ウイルスベクターワクチンとDNAワクチン
- mRNA/ネオアンチゲン個別化ワクチン
- 全細胞ワクチンと樹状細胞ワクチン
- その他の技術
- 治療方法別
- 予防ワクチン
- 治療ワクチン
- がんタイプ別
- 子宮頸がん(HPV)
- 前立腺がん
- メラノーマ
- その他のがん
- デリバリールート別
- 筋肉内
- 皮内/皮下
- 静脈内
- 地域別
- 北米
- 米国
- カナダ
- メキシコ
- 欧州
- ドイツ
- 英国
- フランス
- イタリア
- スペイン
- ロシア
- その他欧州地域
- アジア太平洋地域
- 中国
- 日本
- インド
- オーストラリア
- 韓国
- その他アジア太平洋地域
- 中東・アフリカ
- GCC
- 南アフリカ
- その他中東・アフリカ地域
- 南米
- ブラジル
- アルゼンチン
- その他南米
- 北米
第6章 競合情勢
- 市場集中度
- 市場シェア分析
- 企業プロファイル
- Merck & Co., Inc.
- GlaxoSmithKline plc
- Moderna Inc.
- Bristol Myers Squibb Co.
- AstraZeneca plc
- F. Hoffmann-La Roche AG(Genentech)
- BioNTech SE
- Gritstone bio, Inc.
- Vaccitech plc
- OSE Immunotherapeutics SA
- Anixa Biosciences Inc.
- Dendreon Pharmaceuticals LLC
- Providence Therapeutics Holdings
- eTheRNA Immunotherapies NV
- Imugene Ltd.
- Transgene SA
- OncoSec Medical Incorporated
- NantKwest Inc.
- Ultimovacs ASA
- ISA Pharmaceuticals BV


