
|
市場調査レポート
商品コード
1444218
末梢血管デバイス:世界市場シェア分析、業界動向と統計、成長予測(2024~2029年)Global Peripheral Vascular Devices - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029) |
||||||
カスタマイズ可能
適宜更新あり
|
|||||||
| 末梢血管デバイス:世界市場シェア分析、業界動向と統計、成長予測(2024~2029年) |
|
出版日: 2024年02月15日
発行: Mordor Intelligence
ページ情報: 英文 115 Pages
納期: 2~3営業日
|
全表示
- 概要
- 目次
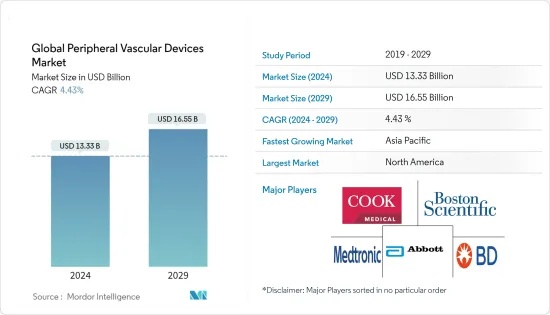
世界の末梢血管デバイス市場規模は、2024年に133億3,000万米ドルと推定され、2029年までに165億5,000万米ドルに達すると予測されており、予測期間(2024年から2029年)中に4.43%のCAGRで成長します。
COVID-19のパンデミックは、診断や治療の手順だけでなく、この分野の研究開発活動にも影響を与えたため、調査対象の市場に重大な影響を与えたCOVID-19以外の治療や診断の手順に悪影響を及ぼしました。さらに、多くの研究では、心疾患を持つ人々はCOVID-19による大きなリスクにさらされていることが示唆されており、これがさらに病院や診断センターへの入場者数の減少につながっています。たとえば、国立バイオテクノロジー情報センターが2021年5月に発表した「英国における心臓処置活動と関連する30日死亡率に対するCOVID-19の影響」というタイトルの調査研究によると、COVID-19パンデミックのさなか、心臓処置活動は英国では手術件数が劇的に減少し、約4万5000件の手術が不足したが、パンデミック中に行われたほとんどの心臓手術では死亡リスクの増加はありません。この研究は、COVID-19が循環器科サービスに悪影響を及ぼしていることを示しています。さらに、この研究は英国で行われました。
しかし、COVID-19の感染者数が減少し、ロックダウンが解除されると、市場は勢いを増し始めました。たとえば、オーストラリア保健福祉研究所の2022年 5月によると、1,180万人の入院のうち、7.0%の入院は集中治療室への滞在を伴い、3.8%の入院は心臓血管疾患が関係していました。このような救急および救命救急治療への入院の増加により、動脈閉塞治療の利用可能性の必要性が生じ、これが分析期間中に調査された末梢血管デバイス市場の成長を促進すると予想されます。
それに加えて、低侵襲手術に対する需要の高まりと末梢動脈疾患(PAD)の発生率の増加が、調査対象市場の成長に積極的な影響を与えています。
米国心臓協会の2021年報告書によると、末梢動脈疾患(PAD)は世界中で2億人以上が罹患しており、高い死亡率と罹患率に関連しています。世界人口の高齢化に伴い、将来的にはPADがさらに一般的になる可能性があります。したがって、統計は、PADの数がより速いペースで増加しており、それが最終的に末梢血管デバイスの市場を牽引していることを示しています。
2021年8月に米国心臓協会が発表した「下肢末梢動脈疾患:現代の疫学、管理ギャップ、および将来の方向性」と題された科学的研究では、下肢末梢動脈疾患(PAD)は世界中で2億3,000万人以上が罹患しており、関連性があると述べられています。多くの好ましくない臨床転帰(冠状動脈性心疾患や脳卒中などの心血管疾患、切断状態などの四肢転帰を含む)のリスクが上昇します。 PADの発生率の増加は、最終的に予測期間中に末梢血管デバイス市場を押し上げるでしょう。
したがって、前述の要因により、調査対象の市場は分析期間中に成長すると予想されます。ただし、設置とメンテナンスのコストが高いため、市場の成長が妨げられる可能性があります。
末梢血管デバイスの市場動向
末梢血管ステントは予測期間中に成長すると予想される
心疾患の増加により、末梢血管ステントの需要が世界的に増加しています。米国心臓協会(AHA)の心臓病および脳卒中統計-2022年最新データによると、心血管疾患(CVD)は、2020年に世界中で1,905万人が死亡する主要な基礎的死因としてリストされています。同じ情報源によると、2020年には世界中で約708万人が脳血管疾患が原因で死亡したとされています。この国の人口における心臓病の有病率が非常に高いため、末梢血管ステントの需要が高まりました。
テクノロジーの進歩と製品承認の増加、主要企業によるパートナーシップやコラボレーションが市場の成長に貢献しています。たとえば、世界の心血管技術企業であるコルディスは、2022年 3月に、橈骨末梢手術用に特別に設計された自己拡張型ステントであるSMART RADIANZ血管ステントシステムに対する米国食品医薬品局(FDA)の承認を発表しました。このシステムは、最近承認された血管ステントシステム、BRITE TIP RADIANZガイディングシース、およびSABERX RADIANZ PTAカテーテルによって完成されます。このシステムは、放射状アクセスを最適化し、顕著な結果を生み出し、患者の満足度を高めるために特別に作成されました。
さらに、2021年10月、ボストン・サイエンティフィック・コーポレーションは、ラスベガスで開催されたVascular InterVentional Advances(VIVA)会議での臨床試験プレゼンテーションで、Eluvia薬剤溶出血管ステントシステムの良好な臨床試験結果を発表しました。発表されたEMINENT試験のデータによると、Eluviaステントは、長さ210mmまでの末梢動脈疾患(PAD)および浅大腿動脈(SFA)病変患者の治療において、自己拡張型ベアメタルステント(BMS)よりも優れた性能を発揮しました。この研究には775人の患者が参加し、PAD治療に関するこれまでで最大の薬剤溶出ステント無作為化試験となった。このような開発により、末梢血管ステントの使用が促進されると予想されます。
したがって、調査対象市場の末梢血管ステントセグメントは成長しています。
北米が末梢血管デバイス市場を独占
北米は、心血管疾患の発生率の上昇、高齢者人口の増加、この地域における業界関係者の強い存在感、医療インフラの改善、利用可能なテクノロジーに対する人々とヘルスケア業界の利害関係者の意識、米国には市場参加者が集中しています。
2020年9月に発表された疾病管理予防センター(CDC)の「心臓病の事実」というタイトルの記事によると、心臓病は米国の主な死因となっています。同情報源は、毎年約80万5,000人のアメリカ人が心臓発作を起こしているとも報告しています。心臓病による死亡者数が増加しているため、末梢血管デバイスは動脈閉塞や動脈狭窄の治療に役立つため、心臓病の適切な治療が引き続き必要とされており、予測期間中に成長が見込まれるためです。
特に米国での製品の発売、提携、買収の増加により、市場の成長が加速しています。たとえば、2022年 3月に、Siemens Healthineersは、次世代のACUSON AcuNav Volume 4D ICEカテーテルを米国で発売しました。同社によると、AcuNav Volume ICEカテーテルは、これまで構造的心臓処置を受けることができなかった患者の治療を可能にすることで、医療提供を変革します。
さらに、2021年9月にアボットは、末梢血栓を除去するために設計された低侵襲の機械的吸引血栓除去システムを備えた医療機器会社であるウォーク・バスキュラーLLCの買収を発表しました。 Walk Vascularの末梢血栓除去システムは、Abbottの既存の血管内製品ポートフォリオに組み込まれる予定です。この提携により、アボットは末梢血管サービスの範囲を拡大することができました。
したがって、上記の要因により、調査対象市場は北米地域での成長が予想されます。
末梢血管デバイス業界の概要
末梢血管デバイス市場は、世界的にも地域的にも事業を展開している数社の存在により、本質的には若干統合されています。競合情勢には、市場シェアを保持し、よく知られている数社の国際企業と地元企業の分析が含まれています。
その他の特典
- エクセル形式の市場予測(ME)シート
- 3か月のアナリストサポート
目次
第1章 イントロダクション
- 調査の前提条件と市場の定義
- 調査範囲
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 市場力学
- 市場概要
- 市場促進要因
- 低侵襲手術に対する需要の高まり
- 末梢動脈疾患(PAD)の発生率の増加
- 市場抑制要因
- 末梢血管デバイスに関する厳しい規制
- ポーターのファイブフォース分析
- 新規参入業者の脅威
- 買い手の交渉力
- 供給企業の交渉力
- 代替製品の脅威
- 競争企業間の敵対関係の激しさ
第5章 市場セグメンテーション
- デバイスの種類別
- 末梢血管ステント
- PTAバルーンカテーテル
- PTAガイドワイヤー
- アテレクトミーデバイス
- 塞栓防止デバイス
- 下大静脈フィルター
- その他
- 地域
- 北米
- 米国
- カナダ
- メキシコ
- 欧州
- ドイツ
- 英国
- フランス
- イタリア
- スペイン
- その他欧州
- アジア太平洋
- 中国
- 日本
- インド
- オーストラリア
- 韓国
- その他アジア太平洋
- 中東とアフリカ
- GCC
- 南アフリカ
- その他中東とアフリカ
- 南米
- ブラジル
- アルゼンチン
- その他南米
- 北米
第6章 競合情勢
- 企業プロファイル
- Abbott Laboratories
- Boston Scientific Corporation
- Becton, Dickinson and Company
- Cook
- Cordis Corporation
- Edward Lifesciences
- Medtronic
- Volcano Corporation
第7章 市場機会と将来の動向
The Global Peripheral Vascular Devices Market size is estimated at USD 13.33 billion in 2024, and is expected to reach USD 16.55 billion by 2029, growing at a CAGR of 4.43% during the forecast period (2024-2029).

The COVID-19 pandemic negatively affected the treatment and diagnostics procedures other than COVID-19 which had a significant impact on the studied market as it not only affected diagnostic and treatment procedures but also research and development activities in the area. Further, many studies suggested that people with cardiac diseases were at major risk from COVID-19, which further led to the reduction in footfall in hospitals and diagnostic centers. For instance, according to the research study titled 'Impact of COVID-19 on cardiac procedure activity in England and associated 30-day mortality' published by the National Center for Biotechnology Information in May 2021, during the COVID-19 pandemic, cardiac procedural activity in England decreased dramatically, with a deficit of about 45,000 procedures, with no increase in the risk of mortality for most cardiac procedures conducted during the pandemic. This study shows the negative impact of COVID-19 on cardiology services. Further, the study was conducted in England.
However the market started to gain traction as the COVID-19 cases declined and the lockdowns were taken off. For instance, according to the Australian Institute of Health and Welfare May 2022, out of 11.8 million admissions, 7.0% of hospitalizations involved a stay in the intensive care unit, and 3.8% of hospitalizations involved Cardio vascular diseases. Such increasing admission in emergency and critical care created the need for the availability of arterial blockage treatment and this is expected to drive the growth of the peripheral vascular devices market studied over the analysis period.
In addition to it, rising demand for minimally-invasive procedures and an increase in the incidence of peripheral arterial disease (PAD) are actively affecting the growth of the studied market.
According to the American Heart Association 2021 report, Peripheral artery disease (PAD) affects more than 200 million people worldwide and is associated with high mortality and morbidity. With the aging global population, it is likely that PAD may be increasingly common in the future. Hence, the statistics show that the number of PAD is increasing at a faster pace, which is ultimately driving the market for peripheral vascular devices.
A Scientific study titled 'Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions' published by the American Heart Association in August 2021 stated that lower extremity peripheral artery disease (PAD) affects more than 230 million persons worldwide and is linked to an elevated risk of a number of unfavorable clinical outcomes (including cardiovascular diseases like coronary heart disease and stroke and limb outcomes like amputee status). The increased incidence of PAD is ultimately boost the peripheral vascular devices market over the forecast period.
Therefore, owing to the aforementioned factors the studied market is anticipated to witness growth over the analysis period. However, the high cost of installation and maintenance is likely to impede the market growth.
Peripheral Vascular Devices Market Trends
Peripheral Vascular Stents are expected to witness growth over the forecast period
Due to the increase in the number of cardiac disorders there is an increased demand for peripheral vascular stents globally. The American Heart Association (AHA), Heart Disease and Stroke Statistics - 2022 Update data shows that cardiovascular disease (CVD) is listed as the primary underlying cause of death accounting for 19.05 million deaths all over the world in the year 2020. As per the same source, around 7.08 million deaths worldwide were attributed to cerebrovascular diseases in the year 2020. Such a high prevalence of cardiac disease among the population in the country reaped the demand for peripheral vascular stents.
The advancements in technology and increasing product approvals, along with partnerships and collaborations by key players are helping in the market growth. For instance, in March 2022, Cordis, a global cardiovascular technology company, announced the United States Food and Drug Administration (FDA) approval for the S.M.A.R.T. RADIANZ Vascular Stent System, a self-expanding stent specifically designed for radial peripheral procedures The RADIANZ Radial Peripheral System is completed by the recently approved vascular stent system, BRITE TIP RADIANZ Guiding Sheath, and SABERX RADIANZ PTA Catheter. This system was created specially to optimize radial access, produce remarkable results, and increase patient satisfaction.
Furthermore, in October 2021, Boston Scientific Corporation presented favorable clinical trial results for the Eluvia Drug-Eluting Vascular Stent System during a clinical trial presentation at the Vascular InterVentional Advances (VIVA) meeting in Las Vegas. The Eluvia stent outperformed self-expanding bare metal stents (BMS) for the treatment of patients with peripheral arterial disease (PAD) and superficial femoral artery (SFA) lesions up to 210 mm in length, according to data from the EMINENT trial that were presented. The study included 775 patients, making it the biggest drug-eluting stent randomized trial for PAD treatment to date. Such developments are anticipated to fuel the usage of peripheral vascular stents.
Therefore, there is a growth in the Peripheral vascular stents segment of the studied market.
North America Dominates the Peripheral Vascular Devices Market
North America is expected to dominate the market owing to factors such as the rising incidence of cardiovascular diseases, growing geriatric population, the strong presence of industry players in the region, better healthcare infrastructure, awareness among people and healthcare industry stakeholders about available technologies, and the high concentration of market players in the United States.
According to the Centers for Disease Control and Prevention (CDC)'s article titled 'Heart Disease Facts' published in September 2020, heart disease is the leading cause of death in the United States. The same source also reports that every year about 805,000 Americans have a heart attack. As the number of deaths due to heart diseases is increasing there is a continuous need for the proper treatment of cardiac diseases since peripheral vascular devices provides helps in the treatment of artery blockages and narrowing and hence are expected to show growth over the forecast period.
The increasing product launches, partnerships, and acquisitions particularly in the United States are leading to an increase in market growth. For instance, in March 2022, Siemens Healthineers launched next-generation ACUSON AcuNav Volume 4D ICE Catheter in the United States. As per the company, AcuNav Volume ICE catheter transforms care delivery by enabling the treatment of patients who were previously not able to undergo Structural Heart procedures.
Additionally, in September 2021, Abbott announced the acquisition of Walk Vascular, LLC, a medical device company with a minimally invasive mechanical aspiration thrombectomy system designed to remove peripheral blood clots. Walk Vascular's peripheral thrombectomy systems will be incorporated into Abbott's existing endovascular product portfolio. The collaboration enabled Abbott to increase the range of its peripheral vascular services.
Therefore, owing to the above-mentioned factors, the growth of the studied market is anticipated in the North America Region.
Peripheral Vascular Devices Industry Overview
The peripheral vascular devices market is slightly consolidated in nature due to the presence of a few companies operating globally as well as regionally. The competitive landscape includes an analysis of a few international as well as local companies which hold the market shares and are well known. Abbott Laboratories, Boston Scientific Corporation, Becton, Dickinson and Company, Cook, Medtronic, Cordis Corporation, Edward Lifesciences, and Volcano Corporation among others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rising Demand for Minimally-invasive Procedures
- 4.2.2 Increase in Incidence of Peripheral Arterial Disease (PAD)
- 4.3 Market Restraints
- 4.3.1 Stringent Regulation Related to Peripheral Vascular Devices
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION
- 5.1 By Device Type
- 5.1.1 Peripheral Vascular Stents
- 5.1.2 Peripheral Transluminal Angioplasty (PTA) Balloon Catheters
- 5.1.3 Peripheral Transluminal Angioplasty (PTA) Guidewires
- 5.1.4 Atherectomy Devices
- 5.1.5 Embolic Protection Devices
- 5.1.6 Inferior Vena Cava Filters
- 5.1.7 Other Device Types
- 5.2 Geography
- 5.2.1 North America
- 5.2.1.1 United States
- 5.2.1.2 Canada
- 5.2.1.3 Mexico
- 5.2.2 Europe
- 5.2.2.1 Germany
- 5.2.2.2 United Kingdom
- 5.2.2.3 France
- 5.2.2.4 Italy
- 5.2.2.5 Spain
- 5.2.2.6 Rest of Europe
- 5.2.3 Asia-Pacific
- 5.2.3.1 China
- 5.2.3.2 Japan
- 5.2.3.3 India
- 5.2.3.4 Australia
- 5.2.3.5 South Korea
- 5.2.3.6 Rest of Asia-Pacific
- 5.2.4 Middle-East and Africa
- 5.2.4.1 GCC
- 5.2.4.2 South Africa
- 5.2.4.3 Rest of Middle-East and Africa
- 5.2.5 South America
- 5.2.5.1 Brazil
- 5.2.5.2 Argentina
- 5.2.5.3 Rest of South America
- 5.2.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Abbott Laboratories
- 6.1.2 Boston Scientific Corporation
- 6.1.3 Becton, Dickinson and Company
- 6.1.4 Cook
- 6.1.5 Cordis Corporation
- 6.1.6 Edward Lifesciences
- 6.1.7 Medtronic
- 6.1.8 Volcano Corporation


