
|
市場調査レポート
商品コード
1849938
In Vitro診断:市場シェア分析、産業動向、統計、成長予測(2025年~2030年)In Vitro Diagnostic - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030) |
||||||
カスタマイズ可能
適宜更新あり
|
|||||||
| In Vitro診断:市場シェア分析、産業動向、統計、成長予測(2025年~2030年) |
|
出版日: 2025年06月13日
発行: Mordor Intelligence
ページ情報: 英文 186 Pages
納期: 2~3営業日
|
概要
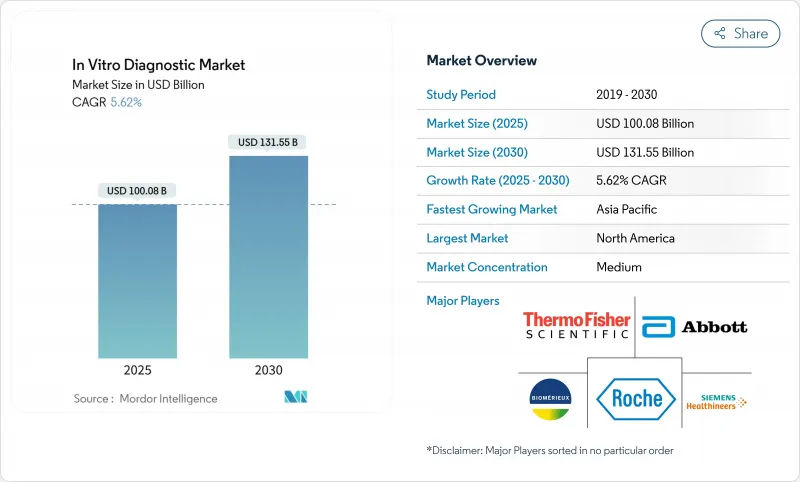
In Vitro診断市場は、2025年に1,000億8,000万米ドルとなり、2030年にはCAGR 5.62%で成長し、1,315億5,000万米ドルに達すると予測されています。
AIを活用した病理検査、プラットフォームの自動化、迅速なポイントオブケア(POC)技術により、所要時間が短縮され、価値の高い検査へのアクセスが拡大します。慢性疾患の負担の増大、高齢化、早期発見に対する支払者の支援により、検査件数は着実に増加し、ソフトウェア中心のイノベーションによりデータ主導の臨床判断が可能になります。酵素不足からハリケーンによる輸液の途絶まで、サプライチェーンの脆弱性が調達戦略を形作る経営リスクを浮き彫りにしています。FDAの臨床検査室開発試験ルールは、欧州のIVDR移行延長が標準化の圧力を除去するのではなく、遅延させるものであるにもかかわらず、コンプライアンス・コストを引き上げています。
世界のIn Vitro診断市場の動向と洞察
慢性疾患の高い有病率
米国成人の76%が2024年に少なくとも1つの慢性疾患を有すると報告しており、糖尿病、心血管、腫瘍、腎臓の各疾患における持続的な診断需要が高まっています。頻繁なモニタリングは、下流の治療コストを削減する早期介入を求める支払者のインセンティブに合致します。検査施設はNGAL、シスタチンC、KIM-1のアッセイを導入することでこれに対応し、症状が現れる前に腎臓障害のフラグを立てる。AIモデルは現在、長期的な結果パターンをトリアージして増悪を早期に予測し、一方、自動化は労働力不足の中で処理能力を維持します。このような力学により、体外診断市場は慢性期医療に不可欠な柱となっています。
POC診断の採用拡大
POCプラットフォームは、単一分析項目のストリップから、15分以内に検査室レベルの結果をもたらすマルチプレックス分子システムへと進化しています。ロシュの12ターゲット呼吸器PCRやドラゴンフライの携帯型mpox検査は、この飛躍を象徴するもので、95%以上の感度と現場での携帯性を兼ね備えています。小売クリニックでは、このようなツールが主流となっている:CVSは現在、1,600の施設で3-in-1インフルエンザ-COVIDパネルを提供しており、病院の負担を軽減しながらアクセスを広げています。支払い側にとっては、急性感染症管理の時間を1分でも節約できれば、感染リスクや高額な入院を抑制できるため、POCの経済性が強化されます。その結果、アジア太平洋地域のプライマリーケアチェーンへの浸透が、In Vitro診断市場全体の成長を加速します。
厳しい複数地域の規制承認スケジュール
FDAの臨床検査室開発試験ルールは、最大35億6,000万米ドルの新たなコンプライアンスコストを課し、中小イノベーターの予算を圧迫し、製品上市を遅らせています。欧州のIVDR延長は時間稼ぎにはなるが、それでもメーカーは品質システムをアップグレードし、ノーティファイドボディの枠を確保しなければならないです。管轄区域をまたぐ二重申請は戦略を断片化し、研究開発費を新しいアッセイの探索から逸脱させる。過去の前例がないAIベースのツールにとってこの足かせは深刻で、提出書類の準備や審査官のトレーニングが複雑になるため、In Vitro診断市場全体の軌道を緩めることになります。
セグメント分析
免疫診断は2024年にIn Vitro診断市場シェアの29.05%を占め、感染症や代謝検査に不可欠なタンパク質アッセイがその柱となっています。しかし分子診断は、次世代シーケンシング、マルチプレックスPCR、等温増幅法が精密医療の主役となるにつれて、このセグメントのIn Vitro診断市場規模を引き上げ、年間6.59%の成長が予測されています。ロシュの温度トリガー式12病原体PCRはランタイムをさらに短縮し、かつて分散型環境での分子導入を制限していたスループットの制約を取り除きます。
ハイブリッド・プラットフォームは、核酸と抗原を同じカートリッジで検出し、分子特異性と免疫測定の容易さを融合させる。AIアルゴリズムは、遺伝子発現の結果を血清学的マーカーと結び付け、腫瘍学や抗菌薬スチュワードシップにおける予後精度を向上させる。こうして検査室は、従来の免疫測定法から低コストで高プレックスの核酸測定法へと業務量を移行し、2030年まで分子測定法の性能を維持します。
2024年の体外診断用医薬品市場規模に占める試薬の割合は55.35%で、消耗品の繰り返し購入による経済性を反映しています。しかし、ソフトウェアとサービスは、検査室がワークフローの効率化と予測的洞察を引き出すAIアナリティクスに投資しているため、CAGR 9.35%で成長しています。フィリップスとアイベックスは、デジタルパソロジーAIが病理医のレビュー前にスライドをトリアージすることで、生産性が37%向上したと報告しています。
サブスクリプション・モデルは、一本調子の分析装置の売上を経常収益に変え、ベンダーのインセンティブを成果ベースの業績に合わせる。クラウドネイティブプラットフォームは、一晩でアルゴリズムのアップグレードを可能にし、継続的な学習のための複数サイトのデータプールを可能にします。その結果、試薬大手はアルゴリズムライセンスをキット販売にバンドルし、体外診断市場全体で顧客の囲い込みを強化しています。
地域分析
北米は、堅調な償還、定着した研究開発、先駆的なAI導入を背景に、2024年のIn Vitro診断市場シェアの38.08%を維持した。FDAのLDTルールはコストがかかるもの、品質基準を引き上げ、各州で調和されれば全国的なデータ相互運用性を促進する可能性があります。CVSとウォルグリーンはPOC分子パネルを展開し、診断の旅を短縮し、新たなボリュームストリームを創出します。一方、BDの培養液不足やバクスターの輸液障害といったサプライチェーン・ショックは、国内製造の回復力への再投資を促しています。
アジア太平洋地域はCAGR 6.85%で最も急成長している地域であり、日本、中国、インドにおける人口動態の高齢化と政府資金の拡大に後押しされています。LabcorpのBMLジャパンとの提携拡大や、Sansureの敗血症アッセイに関する中国合弁事業は、プレシジョン・メディシンの導入を加速させる国境を越えた技術移転を物語っています。中国国内企業は、現地での膨大なスクリーニングの取り組みから得た経験を活用し、輸出向けにコスト効率の高い分析装置の規模を拡大しています。このような動きにより、地域の体外診断用医薬品市場のキャパシティが拡大し、世界的な価格競争圧力が高まる。
欧州はIVDRの混乱にもかかわらず堅調な伸びを示します。移行期の遅れは当面の検査不足を防ぐが、長期的な整合化は避けられず、中小メーカーは戦略的パートナーを探すか撤退を余儀なくされます。フィリップス・イベックスのAIエッジやディアグネクシアの前立腺AIパートナーシップによる病理検査ターンアラウンドの短縮など、空間オミクスとデジタル病理学でリーダーシップを発揮しています。中東・アフリカ、ラテンアメリカは、主にPOCと薬剤スクリーニングプログラムを通じて能力を構築し、インテリジェントバイオとIVYダイアグノスティックスの提携は、規制や文化のギャップを埋める販売網を拡大しています。
その他の特典:
- エクセル形式の市場予測(ME)シート
- 3ヶ月間のアナリストサポート
よくあるご質問
目次
第1章 イントロダクション
- 調査の前提条件と市場の定義
- 調査範囲
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 市場情勢
- 市場概要
- 市場促進要因
- 慢性疾患の有病率の高さ
- ポイントオブケア(POC)診断の導入拡大
- 継続的なプラットフォームイノベーション(AI、自動化、多重化)
- パーソナライズ診断/コンパニオン診断の普及
- 小売店・クリニック・自宅でのサンプリングエコシステム
- 空間オミクスとIVDワークフローの融合
- 市場抑制要因
- 厳格な複数地域の規制承認タイムライン
- 新たな試験クラスにおける償還の不確実性
- コネクテッドIVDにおけるサイバーセキュリティとデータ相互運用性のギャップ
- 酵素/試薬サプライチェーンの地政学的輸出規制へのエクスポージャー
- サプライチェーン分析
- テクノロジーの展望
- ポーターのファイブフォース
- 新規参入業者の脅威
- 買い手の交渉力
- 供給企業の交渉力
- 代替品の脅威
- 競争企業間の敵対関係
第5章 市場規模と成長予測
- 検査タイプ別
- 臨床化学
- 免疫診断
- 分子診断
- 血液学
- 凝固
- 微生物学
- その他の検査タイプ
- 製品別
- 機器
- 試薬とキット
- ソフトウェアとサービス
- ユーザビリティ別
- 使い捨て体外診断用機器
- 再利用可能な機器
- 用途別
- 感染症
- 糖尿病
- 腫瘍学
- 心臓病学
- 自己免疫疾患
- 腎臓学
- その他の用途
- エンドユーザー別
- 独立型ラボ
- 病院併設の検査室
- ポイントオブケア環境
- 在宅ケアと自己検査のユーザー
- 地域別
- 北米
- 米国
- カナダ
- メキシコ
- 欧州
- ドイツ
- 英国
- フランス
- イタリア
- スペイン
- その他欧州地域
- アジア太平洋地域
- 中国
- 日本
- インド
- 韓国
- オーストラリア
- その他アジア太平洋地域
- 中東・アフリカ
- GCC
- 南アフリカ
- その他中東・アフリカ地域
- 南米
- ブラジル
- アルゼンチン
- その他南米
- 北米
第6章 競合情勢
- 市場集中度
- 市場シェア分析
- 企業プロファイル
- F. Hoffmann-La Roche Ltd
- Abbott Laboratories
- Siemens Healthineers AG
- Danaher Corp(Beckman Coulter, Cepheid)
- Thermo Fisher Scientific Inc.
- Sysmex Corp
- bioMerieux SA
- Becton, Dickinson and Company
- Bio-Rad Laboratories Inc.
- Qiagen NV
- DiaSorin SpA
- Grifols SA
- Agilent Technologies Inc.
- Ortho Clinical Diagnostics/QuidelOrtho
- Hologic Inc.
- Illumina Inc.
- PerkinElmer Inc.
- Randox Laboratories Ltd
- Meril Diagnostics Pvt Ltd


