|
|
市場調査レポート
商品コード
1565909
再生医療の世界市場:市場規模、シェア、予測、動向分析 - 製品別、用途別、エンドユーザー別、予測(~2031年)Regenerative Medicine Market Size, Share, Forecast, & Trends Analysis by Product Application End User - Global Forecast to 2031 |
||||||
カスタマイズ可能
|
|||||||
| 再生医療の世界市場:市場規模、シェア、予測、動向分析 - 製品別、用途別、エンドユーザー別、予測(~2031年) |
|
出版日: 2024年10月03日
発行: Meticulous Research
ページ情報: 英文 248 Pages
納期: 即納可能
|
全表示
- 概要
- 図表
- 目次
世界の再生医療の市場規模は、2024~2031年にかけてCAGR 21.5%で成長し、2031年までに372億7,000万米ドルに達すると予測されています。
本レポートは、広範な2次調査と1次調査、市場シナリオの詳細な分析を経て、主要な産業促進要因、抑制要因、課題、機会の分析から構成されています。
世界の再生医療市場の成長は、新しい再生医療の承認の増加、再生医療の進歩、慢性疾患の罹患率の上昇、再生医療開発のための資金調達の増加、新しい治療領域における再生医療の応用の拡大が原動力となっています。しかし、高い治療費と幹細胞に関連する倫理的問題がこの市場の成長を抑制しています。
さらに、個別化医療に対する需要の高まり、臓器移植手術件数の増加、再生医療のための強力な製品パイプラインは、市場成長の機会を生み出すと期待されています。しかし、不利な償還政策、再生医療の製造における複雑さ、再生医療の安全性と有効性に関する標準化された枠組みの欠如は、世界の再生医療市場における主要な課題です。
本レポートは、製品ポートフォリオの提供、地域情勢、過去3~4年間(2020~2024年)に産業の主要市場プレーヤーが採用した主な戦略的発展の広範な評価に基づく競合情勢を提供しています。再生医療市場に参入している主要企業は、Novartis AG(スイス)、Biogen Inc.(米国)、Kite Pharma, Inc.(米国)、Spark Therapeutics, Inc.(米国)、Integra LifeSciences Corporation(米国)、Sarepta Therapeutics, Inc.(米国)、Takeda Pharmaceutical Company Limited(日本)、Amgen Inc.(米国)、CORESTEMCHEMON Inc.(韓国)、Smith & Nephew plc(英国)、Vertex Pharmaceuticals Incorporated(米国)、CSL Behring, LLC(米国)、Janssen Global Services, LLC(米国)、Medtronic plc(アイルランド)、AbbVie Inc.(米国)、Bristol-Myers Squibb Company(米国)、Ferring Pharmaceuticals A/S(スウェーデン)、Pfizer Inc.(米国)、bluebird bio Inc.(米国)、Vericel Corporation(米国)が挙げられます。
本レポートで調査した全製品の中で、細胞治療セグメントは予測期間中に25.0%の最も高いCAGRを記録すると予測されています。細胞療法分野はさらに、幹細胞療法、細胞ベースの免疫療法、血小板を豊富に含む血漿療法に区分されます。細胞ベースの免疫療法は、予測期間中に最も高いCAGRを記録すると予測されています。この分野の急成長は、がん罹患率の増加、CAR T細胞療法の使用に関する認知度の上昇、細胞ベースの免疫療法の承認・臨床試験件数の増加に起因しています。また、細胞ベースの免疫療法の開発資金も増加しており、市場の成長をさらに後押ししています。例えば、2024年5月、ドイツがん支援はライプニッツ免疫療法研究所(LIT)とレーゲンスブルク大学病院(UKR)(ドイツ)に280万米ドル(260万ユーロ)の助成金を提供しました。この助成金は、幹細胞様CAR T細胞を用いて進行性リンパ腫患者を治療することに焦点を当てた臨床研究にあてられました。
本レポートで調査したすべての用途の中で、がん分野は予測期間中に26.5%という最も高いCAGRを記録すると予測されています。再生医療は、幹細胞治療、遺伝子治療、組織工学など、がん治療のためのさまざまなアプローチを提供します。これらのアプローチは、個別化された治療オプションを提供し、従来のがん治療に伴う副作用のリスクを最小限に抑えるのに役立ちます。さらに、がん治療研究のための資金が増加していることも、このセグメントの成長に寄与しています。例えば、2024年2月、BioNTech SE(ドイツ)はAutolus Therapeutics(英国)に2億米ドルを投資し、がん治療パイプラインを支援するための戦略的協力関係を確立しました。
本レポートで調査したすべてのエンドユーザーの中で、病院・診療所セグメントは予測期間中に最も高いCAGRを記録すると予測されています。病院・クリニックはインフラが整備され、高度な技術を持つ医療従事者がおり、アクセスが容易で、様々な治療を含む高度な治療を提供しています。それゆえ、患者は病院&クリニックを訪れる傾向が強いです。また、多くの病院が再生医療に特化したユニットを立ち上げており、市場の成長を後押ししています。例えば、2023年4月、ジャスロック病院(インド)は複雑な疾患の治療を目的とした修復再生医療部門を開設しました。この部門は、骨や関節の障害に対する様々な細胞ベースの治療のための整形生物学的製剤や、整形外科、血管、美容への応用のための血小板を豊富に含む血漿療法を提供しています。
世界の再生医療市場の地域別シナリオの詳細分析では、5つの主要地域(北米、欧州、アジア太平洋、ラテンアメリカ、中東・アフリカ)の詳細な質的・量的洞察と、各地域の主要国のカバレッジを提供しています。アジア太平洋は、予測期間中に25.0%という最高のCAGRを記録すると予測されています。2024年には、アジア太平洋の再生医療市場で日本が最大のシェアを占めると予想されています。日本の市場シェアが大きいのは、65歳以上の人口が多いこと、再生医療導入に向けた政府の取り組み、再生医療の承認に向けた支持的な規制の枠組みなどが背景にあります。例えば、日本は再生医療の条件付き承認制度を導入しており、企業は新しい治療法をより迅速に市場に投入することができます。また、国のヘルスケア制度もこれらの新しい治療法をカバーしており、外資系企業にとって日本での製品導入のさらなるインセンティブとなっています。
調査範囲:
再生医療市場評価:製品別
- 遺伝子治療
- 細胞療法
- 幹細胞療法
- 自家療法
- 同種療法
- 多血小板血漿療法
- 細胞ベースの免疫療法
- 組織工学
再生医療市場評価:用途別
- 循環器
- 眼科
- 腫瘍学
- 神経学
- 免疫・炎症
- 筋骨格系
- 皮膚科学
- その他の用途
注:その他の用途には創傷治療、整形外科、血液疾患が含まれます。
再生医療市場評価:エンドユーザー別
- 病院および診療所
- 外来手術センター
再生医療市場評価:地域別
- 北米
- 米国
- カナダ
- 欧州
- ドイツ
- フランス
- 英国
- イタリア
- スペイン
- スイス
- オランダ
- スウェーデン
- その他の欧州
- アジア太平洋
- 中国
- 日本
- インド
- 韓国
- オーストラリア
- その他のアジア太平洋
- ラテンアメリカ
- ブラジル
- メキシコ
- その他のラテンアメリカ
- 中東・アフリカ
目次
第1章 イントロダクション
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 市場洞察
- 市場概要
- 市場成長に影響を与える要因
- 促進要因
- 新規再生医療の承認
- 再生医療開発資金の増加
- 新しい治療領域における再生医療の応用拡大
- 抑制要因
- 幹細胞に関する倫理的問題
- 機会
- 個別化医療に対する需要の高まり
- 課題
- 再生医療の製造における複雑さ
- 要因分析
- 促進要因
- 動向
- 再生医療における3Dバイオプリンティングの活用
- 再生医療開発におけるAIツールの統合
- 人工多能性幹細胞
- 規制分析
- 北米
- 米国
- カナダ
- アジア太平洋
- 日本
- 中国
- インド
- 欧州
- 中東・アフリカ
- PRP資格基準
- 北米
- ポーターのファイブフォース分析
- 投資と資金調達のシナリオ
- パイプライン分析
第5章 再生医療市場評価:製品別
- 概要
- 細胞療法
- 細胞ベースの免疫療法
- 幹細胞療法
- 同種療法
- 自家療法
- 多血小板血漿療法
- 遺伝子治療
- 組織工学製品
第6章 再生医療市場評価:用途別
- 概要
- 筋骨格系
- 腫瘍学
- 眼科
- 免疫・炎症
- 神経学
- 皮膚科学
- 心臓病学
- その他の用途
第7章 再生医療市場評価:エンドユーザー別
- イントロダクション
- 病院・クリニック
- 外来手術センター
第8章 再生医療市場評価:地域別
- 概要
- 北米
- 米国
- カナダ
- 欧州
- ドイツ
- 英国
- フランス
- スイス
- イタリア
- スペイン
- オランダ
- スウェーデン
- その他の欧州
- アジア太平洋
- 日本
- 中国
- オーストラリア
- インド
- 韓国
- その他のアジア太平洋
- ラテンアメリカ
- ブラジル
- メキシコ
- その他のラテンアメリカ
- 中東・アフリカ
第9章 競合分析
- 概要
- 主要成長戦略
- 競合ベンチマーキング
- 競合ダッシュボード
- 産業リーダー
- 市場差別化要因
- 先行企業
- 新興企業
- 市場シェア分析(2023年)
- Novartis AG(Switzerland)
- Kite Pharma, Inc.(U.S.)
- Biogen, Inc.(U.S.)
- Bristol-Myers Squibb Company(U.S.)
- Janssen Global Services, LLC(U.S.)
第10章 企業プロファイル
- Novartis AG
- Kite Pharma, Inc.(A Subsidiary of Gilead Sciences, Inc.)
- Janssen Global Services, LLC(A Subsidiary of Johnson & Johnson)
- Bristol-Myers Squibb Company
- Biogen Inc.
- Smith & Nephew Plc
- Amgen Inc.
- Vericel Corporation
- Integra Lifesciences Holdings Corporation
- Pfizer Inc.
- AbbVie Inc.
- Sarepta Therapeutics, Inc.
- Spark Therapeutics, Inc.(A Subsidiary of F. Hoffmann-La Roche Ag)
- CORESTEMCHEMON, Inc.
- CSL Behring, LLC(A Subsidiary of CSL Limited)
- Takeda Pharmaceutical Company Limited
- Ferring Pharmaceuticals
- Medtronic plc
- Bluebird Bio, Inc.
- Vertex Pharmaceuticals Incorporated
(注:上位5社のSWOT分析を掲載)
第11章 付録
LIST OF TABLES
- Table 1 U.S. FDA-Approved Cell & Gene Therapy Products (As of August 2024)
- Table 2 Estimated Number of New Cancer Cases, by Region, 2022-2030 (Thousand)
- Table 3 Comparison of Breakthrough Therapy Designation and Regenerative Medicine Advanced Therapy Designation
- Table 4 Risk Classification of Regenerative Medicines, by Type and Class
- Table 5 Authorities/Organizations Funding and Investing In Regenerative Medicine-related Research & Development
- Table 6 Regenerative Medicine Market: Pipeline Analysis
- Table 7 Global Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 8 Estimated Number of New Leukemia Cases, by Region, 2022-2030 (Thousands)
- Table 9 Global Regenerative Medicine Market for Cell Therapy, by Type, 2022-2031 (USD Million)
- Table 10 Global Regenerative Medicine Market for Cell Therapy, by Country/Region, 2022-2031 (USD Million)
- Table 11 Companies Offering Cell-Based Immunotherapy Solutions
- Table 12 Global Regenerative Medicine Market for Cell-Based Immunotherapy, by Country/Region, 2022-2031 (USD Million)
- Table 13 Companies Offering Stem Cell Therapy Solutions
- Table 14 Characteristics Features of Stem Cell Therapy for Regenerative Medicine
- Table 15 Global Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 16 Global Regenerative Medicine Market for Stem Cell Therapy, by Country/Region, 2022-2031 (USD Million)
- Table 17 Companies Offering Allogenic Therapy Solutions
- Table 18 Global Regenerative Medicine Market for Allogenic Therapy, by Country/Region, 2022-2031 (USD Million)
- Table 19 Companies Offering Autologous Cell Therapy
- Table 20 Global Regenerative Medicine Market for Autologous Cell Therapy, by Country/Region, 2022-2031 (USD Million)
- Table 21 Companies Offering Platelet-Rich Plasma (PRP) Therapy
- Table 22 Global Regenerative Medicine for Platelet-Rich Plasma (PRP) Therapy, by Country/Region, 2022-2031 (USD Million)
- Table 23 Companies Offering Gene Therapy Products
- Table 24 Global Regenerative Medicine Market for Gene Therapy, by Country/Region, 2022-2031 (USD Million)
- Table 25 Companies Offering Tissue Engineering Products
- Table 26 Global Regenerative Medicine Market for Tissue Engineering Products, by Country/Region, 2022-2031 (USD Million)
- Table 27 Global Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 28 Global Regenerative Medicine Market for Musculoskeletal Disorders, by Country/Region, 2022-2031 (USD Million)
- Table 29 Global Regenerative Medicine Market for Oncology, by Country/Region, 2022-2031 (USD Million)
- Table 30 Global Regenerative Medicine Market for Ophthalmology, by Country/Region, 2022-2031 (USD Million)
- Table 31 Global Regenerative Medicine Market for Immunology & Inflammation, by Country/Region, 2022-2031 (USD Million)
- Table 32 Global Regenerative Medicine Market for Neurology, by Country/Region, 2022-2031 (USD Million)
- Table 33 Global Regenerative Medicine Market for Dermatology, by Country/Region, 2022-2031 (USD Million)
- Table 34 Global Regenerative Medicine Market for Cardiology, by Country/Region, 2022-2031 (USD Million)
- Table 35 Global Regenerative Medicine Market for Other Applications, by Country/Region, 2022-2031 (USD Million)
- Table 36 Global Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 37 Global Regenerative Medicine Market for Hospitals & Clinics, by Country/Region, 2022-2031 (USD Million)
- Table 38 Global Regenerative Medicine Market for Ambulatory Surgical Centers, by Country/Region, 2022-2031 (USD Million)
- Table 39 Global Regenerative Medicine Market, by Country/Region, 2022-2031 (USD Million)
- Table 40 North America: Regenerative Medicine Market, by Country, 2022-2031 (USD Million)
- Table 41 North America: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 42 North America: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 43 North America: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 44 North America: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 45 North America: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 46 U.S.: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 47 U.S.: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 48 U.S.: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 49 U.S.: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 50 U.S.: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 51 Canada: Approved Regenerative Medicine, 2021-2024
- Table 52 Canada: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 53 Canada: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 54 Canada: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 55 Canada: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 56 Canada: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 57 Europe: Regenerative Medicine Sector Data, Q2 2024 Vs. Q1 2023
- Table 58 Europe: Regenerative Medicine Market, by Country/Region, 2022-2031 (USD Million)
- Table 59 Europe: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 60 Europe: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 61 Europe: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 62 Europe: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 63 Europe: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 64 Germany: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 65 Germany: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 66 Germany: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 67 Germany: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 68 Germany: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 69 U.K.: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 70 U.K.: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 71 U.K.: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 72 U.K.: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 73 U.K.: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 74 France: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 75 France: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 76 France: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 77 France: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 78 France: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 79 Switzerland: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 80 Switzerland: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 81 Switzerland: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 82 Switzerland: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 83 Switzerland: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 84 Italy: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 85 Italy: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 86 Italy: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 87 Italy: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 88 Italy: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 89 Spain: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 90 Spain: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 91 Spain: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 92 Spain: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 93 Spain: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 94 Netherlands: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 95 Netherlands: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 96 Netherlands: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 97 Netherlands: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 98 Netherlands: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 99 Sweden: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 100 Sweden: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 101 Sweden: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 102 Sweden: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 103 Sweden: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 104 Rest of Europe: Estimated Number of New Cancer Cases, by Country, 2022 Vs. 2030
- Table 105 Rest of Europe: Recent Developments In The Regenerative Medicine Market
- Table 106 Rest of Europe: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 107 Rest of Europe: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 108 Rest of Europe: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 109 Rest of Europe: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 110 Rest of Europe: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 111 Asia-Pacific: Regenerative Medicine Sector Data, Q2 2024 Vs. Q1 2023
- Table 112 Asia-Pacific: Regenerative Medicine Market, by Country/Region, 2022-2031 (USD Million)
- Table 113 Asia-Pacific: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 114 Asia-Pacific: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 115 Asia-Pacific: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 116 Asia-Pacific: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 117 Asia-Pacific: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 118 Japan: Approved Regenerative Medicine
- Table 119 Japan: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 120 Japan: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 121 Japan: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 122 Japan: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 123 Japan: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 124 China: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 125 China: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 126 China: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 127 China: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 128 China: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 129 Australia: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 130 Australia: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 131 Australia: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 132 Australia: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 133 Australia: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 134 India: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 135 India: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 136 India: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 137 India: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 138 India: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 139 South Korea: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 140 South Korea: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 141 South Korea: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 142 South Korea: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 143 South Korea: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 144 Rest of Asia-Pacific: Recent Developments in Regenerative Medicine Market, by Country
- Table 145 Rest of Asia-Pacific: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 146 Rest of Asia-Pacific: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 147 Rest of Asia-Pacific: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 148 Rest of Asia-Pacific: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 149 Rest of Asia-Pacific: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 150 Latin America: Regenerative Medicine Market, by Country/Region, 2022-2031 (USD Million)
- Table 151 Latin America: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 152 Latin America: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 153 Latin America: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 154 Latin America: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 155 Latin America: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 156 Brazil: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 157 Brazil: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 158 Brazil: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 159 Brazil: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 160 Brazil: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 161 Mexico: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 162 Mexico: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 163 Mexico: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 164 Mexico: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 165 Mexico: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 166 Rest of Latin America: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 167 Rest of Latin America: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 168 Rest of Latin America: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 169 Rest of Latin America: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 170 Rest of Latin America: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 171 Middle East & Africa: Regenerative Medicine Market, by Product, 2022-2031 (USD Million)
- Table 172 Middle East & Africa: Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 173 Middle East & Africa: Stem Cell Therapy Market, by Type, 2022-2031 (USD Million)
- Table 174 Middle East & Africa: Regenerative Medicine Market, by Application, 2022-2031 (USD Million)
- Table 175 Middle East & Africa: Regenerative Medicine Market, by End User, 2022-2031 (USD Million)
- Table 176 Recent Developments, by Company, 2021-2024
LIST OF FIGURES
- Figure 1 Research Process
- Figure 2 Secondary Sources Referenced for this Study.
- Figure 3 Primary Research Techniques
- Figure 4 Key Executives Interviewed
- Figure 5 Breakdown of Primary Interviews (Supply-side & Demand-side)
- Figure 6 Market Sizing And Growth Forecast Approach
- Figure 7 Global Regenerative Medicine Market, by Product, 2024 VS. 2031 (USD Million)
- Figure 8 Global Regenerative Medicine Market, by Application, 2024 VS. 2031 (USD Million)
- Figure 9 Global Regenerative Medicine Market, by End User, 2024 VS. 2031 (USD Million)
- Figure 10 Global Regenerative Medicine Market, by Geography
- Figure 11 Impact Analysis of Market Dynamics
- Figure 12 NIH Funding for Regenerative Medicine, 2019-2025 (USD Million)
- Figure 13 Japan: Regenerative Medicines Regulatory Pathway
- Figure 14 India: Approval Pathway for Regenerative Medicines
- Figure 15 Saudi Arabia: Drug Licensing - Marketing Authorization Process
- Figure 16 Porter's Five Forces Analysis
- Figure 17 Investments In Regenerative Medicine, 2017-2023 (USD Billion)
- Figure 18 Global Regenerative Medicine Market, by Product, 2024 Vs. 2031 (USD Million)
- Figure 19 Cell-based Immunotherapy Workflow
- Figure 20 Global Regenerative Medicine Market, by Application, 2024 VS. 2031 (USD Million)
- Figure 21 Global Regenerative Medicine Market, by End User, 2024 VS. 2031 (USD Million)
- Figure 22 Regenerative Medicine Market, by Geography, 2024 VS. 2031 (USD Million)
- Figure 23 North America: Regenerative Medicine Market Snapshot
- Figure 24 Europe: Regenerative Medicine Market Snapshot
- Figure 25 Asia-Pacific: Regenerative Medicine Market Snapshot
- Figure 26 Latin America: Regenerative Medicine Market Snapshot
- Figure 27 Key Growth Strategies Adopted by Leading Players, 2021-2024
- Figure 28 Regenerative Medicine Market: Competitive Benchmarking, by Product
- Figure 29 Regenerative Medicine Market: Competitive Benchmarking, by Region
- Figure 30 Competitive Dashboard: Regenerative Medicine Market
- Figure 31 Global Regenerative Medicine Market Share Analysis, by Key Players (2023)
- Figure 32 Novartis AG: Financial Snapshot (2023)
- Figure 33 Gilead Sciences, Inc.: Financial Overview (2023)
- Figure 34 Johnson & Johnson.: Financial Overview (2023)
- Figure 35 Bristol-Myers Squibb Company: Financial Overview (2023)
- Figure 36 Biogen Inc.: Financial Snapshot (2023)
- Figure 37 Smith & Nephew Plc: Financial Overview (2023)
- Figure 38 Amgen Inc.: Financial Overview (2023)
- Figure 39 Vericel CORPORATION.: Financial Overview (2023)
- Figure 40 Integra Lifesciences Holdings Corporation.: Financial Snapshot (2023)
- Figure 41 Pfizer Inc.: Financial Snapshot (2023)
- Figure 42 AbbVie Inc: Financial Overview (2023)
- Figure 43 Sarepta Therapeutics, Inc.: Financial Snapshot (2023)
- Figure 44 F. Hoffmann-La Roche AG: Financial Snapshot (2023)
- Figure 45 CORESTEMCHEMON, Inc.: Financial Overview (2022)
- Figure 46 CSL LIMITED: Financial Overview (2024)
- Figure 47 Takeda Pharmaceutical Company Limited: Financial Overview (2023)
- Figure 48 Ferring Pharmaceuticals: Financial Overview (2023)
- Figure 49 Medtronic plc: Financial Overview (2024)
- Figure 50 bluebird bio, Inc.: Financial Overview (2023)
- Figure 51 Vertex Pharmaceuticals Incorporated: Financial Overview (2023)
Regenerative Medicine Market Size, Share, Forecast, & Trends Analysis by Product (Gene Therapy, Stem Cell, Tissue Engineering) Application (Musculoskeletal, Cancer, Cardiovascular, Immunology, Dermatology, Ophthalmology) End User-Global Forecast to 2031
The global regenerative medicine market is projected to reach $37.27 billion by 2031 at a CAGR of 21.5% from 2024 to 2031.
Succeeding extensive secondary and primary research and in-depth analysis of the market scenario, the report comprises the analysis of key industry drivers, restraints, challenges, and opportunities.
The growth of the global regenerative medicine market is driven by increasing approvals for new regenerative medicines, advancements in regenerative medicine, the rising incidence of chronic diseases, increasing funding for regenerative medicine development, and the growing applications of regenerative medicine in newer therapeutic areas. However, high treatment costs and ethical issues related to stem cells restrain the growth of this market.
Furthermore, the rising demand for personalized medicines, the increasing number of organ transplant procedures, and the strong product pipeline for regenerative medicines are expected to generate market growth opportunities. However, unfavorable reimbursement policies, complexities in the manufacture of regenerative medicines, and the lack of standardized frameworks for the safety & efficacy of regenerative medicines are major challenges in the global regenerative medicine market.
The report offers a competitive landscape based on an extensive assessment of the product portfolio offerings, geographic presences, and key strategic developments adopted by leading market players in the industry in the last three to four years (2020-2024). The key players operating in the regenerative medicine market are Novartis AG (Switzerland), Biogen Inc. (U.S.), Kite Pharma, Inc. (U.S.), Spark Therapeutics, Inc. (U.S.), Integra LifeSciences Corporation (U.S.), Sarepta Therapeutics, Inc. (U.S.), Takeda Pharmaceutical Company Limited (Japan), Amgen Inc. (U.S.), CORESTEMCHEMON Inc. (South Korea), Smith & Nephew plc (England), Vertex Pharmaceuticals Incorporated (U.S.), CSL Behring, LLC (U.S.), Janssen Global Services, LLC (U.S.), Medtronic plc (Ireland), AbbVie Inc. (U.S.), Bristol-Myers Squibb Company (U.S.), Ferring Pharmaceuticals A/S (Sweden), Pfizer Inc. (U.S.), bluebird bio Inc. (U.S.), and Vericel Corporation (U.S.).
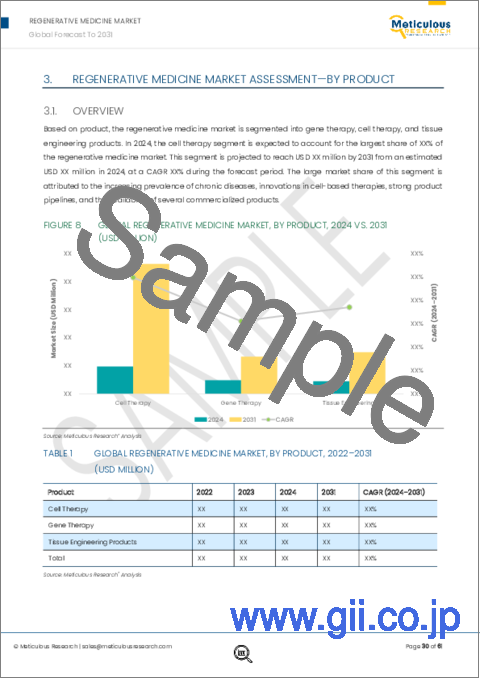
Among all the products studied in this report, the cell therapy segment is projected to register the highest CAGR of 25.0% during the forecast period. The cell therapy segment is further segmented into stem cell therapy, cell-based immunotherapy, and platelet-rich plasma therapy. Cell-based immunotherapy is projected to register the highest CAGR during the forecast period. The rapid growth of this segment is attributed to the increasing cancer prevalence, rising awareness regarding the use of CAR T-cell therapy, and rising number of approvals & clinical trials for cell-based immunotherapy. The funding for the development of cell-based immunotherapy is also increasing, further driving the market growth. For instance, in May 2024, German Cancer Aid provided a $2.8 million (€2.6 million) grant to the Leibniz Institute for Immunotherapy (LIT) and University Hospital Regensburg (UKR) (Germany). The funding was earmarked for a clinical study focused on the use of stem-like CAR T-cells to treat patients with advanced lymphomas.
Among all the applications studied in this report, the oncology segment is projected to register the highest CAGR of 26.5% during the forecast period. Regenerative medicine offers various approaches, such as stem cell therapy, gene therapy, and tissue engineering, for treating cancer. These approaches provide personalized treatment options and help minimize the risk of side effects associated with traditional cancer treatments. Additionally, the rising funding for cancer treatment research contributes to the growth of this segment. For instance, in February 2024, BioNTech SE (Germany) invested USD 200 million in Autolus Therapeutics (U.K.) and established a strategic collaboration to support the cancer therapy pipeline.
Among all the end users studied in this report, the hospitals and clinics segment is projected to register the highest CAGR during the forecast period. Hospitals & clinics have developed infrastructure and highly skilled healthcare professionals, are easily accessible, and offer advanced treatments, including various therapies. Hence, patients are more inclined toward visiting hospitals & clinics. Many hospitals are also launching dedicated units for regenerative medicine, driving market growth. For instance, in April 2023, Jaslok Hospital (India) opened a Restorative and Regenerative Medicine Department aimed at treating complex diseases. This department offers orthobiologics for various cell-based treatments for bone and joint disorders, as well as platelet-rich plasma therapies for orthopedic, vascular, and cosmetic applications.
An in-depth analysis of the geographical scenario of the global regenerative medicine market provides detailed qualitative and quantitative insights into the five major geographies (North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa) along with the coverage of major countries in each region. Asia-Pacific is projected to register the highest CAGR of 25.0% during the forecast period. In 2024, Japan is expected to account for the largest share of the regenerative medicine market in Asia-Pacific. The large market share of Japan is attributed to the large population base aged 65 and older, government initiatives for the adoption of regenerative medicine, and supportive regulatory framework for the approval of regenerative medicine. For instance, Japan has implemented a conditional approval system for regenerative medicine, allowing companies to bring new therapies to market more quickly. The national healthcare system also covers these new therapies, providing further incentives for foreign companies to introduce their products in Japan.
Scope of the Report:
Regenerative Medicine Market Assessment-by Product
- Gene Therapy
- Cell Therapy
- Stem Cell Therapy
- Autologous Therapy
- Allogenic Therapy
- Platelet Rich Plasma Therapy
- Cell-based Immunotherapy
- Tissue Engineering
Regenerative Medicine Market Assessment-by Application
- Cardiology
- Ophthalmology
- Oncology
- Neurology
- Immunology & Inflammation
- Musculoskeletal
- Dermatology
- Other Applications
Note: Other Applications include wound care, orthopedics, and blood disorders
Regenerative Medicine Market Assessment-by End User
- Hospitals & Clinics
- Ambulatory Surgical Centers
Regenerative Medicine Market Assessment-by Geography
- North America
- U.S.
- Canada
- Europe
- Germany
- France
- U.K
- Italy
- Spain
- Switzerland
- Netherlands
- Sweden
- Rest of Europe
- Asia-Pacific
- China
- Japan
- India
- South Korea
- Australia
- Rest of Asia-Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
TABLE OF CONTENTS
1. Introduction
- 1.1. Market Definition & Scope
- 1.2. Market Ecosystem
- 1.3. Currency and Limitations
- 1.4. Key Stakeholders
2. Research Methodology
- 2.1. Research Process
- 2.2. Data Collection and Validation Process
- 2.2.1. Secondary Research
- 2.2.2. Primary Research/Interviews With Key Opinion Leaders from the Industry
- 2.3. Market Sizing & Forecasting
- 2.3.1. Market Size Estimation Approach
- 2.3.2. Growth Forecast Approach
- 2.3.3. Market Share Analysis
- 2.4. Assumptions for the Study
3. Executive Summary
4. Market Insights
- 4.1. Overview
- 4.2. Factors Affecting Market Growth
- 4.2.1. Drivers
- 4.2.1.1. Approvals for New Regenerative Medicines
- 4.2.1.2. Increasing Funding for Regenerative Medicine Development
- 4.2.1.3. Increasing Applications of Regenerative Medicine in Newer Therapy Areas
- 4.2.2. Restraints
- 4.2.2.1. Ethical Issues Related to Stem Cells
- 4.2.3. Opportunities
- 4.2.3.1. Rising Demand for Personalized Medicines
- 4.2.4. Challenges
- 4.2.4.1. Complexities in the Manufacture of Regenerative Medicines
- 4.2.5. Factor Analysis
- 4.2.1. Drivers
- 4.3. Trends
- 4.3.1. Utilization of 3D Bioprinting in Regenerative Medicine
- 4.3.2. Integration of AI Tools in Regenerative Medicine Development
- 4.3.3. Induced Pluripotent Stem Cells
- 4.4. Regulatory Analysis
- 4.4.1. North America
- 4.4.1.1. U.S.
- 4.4.1.2. Canada
- 4.4.2. Asia-Pacific
- 4.4.2.1. Japan
- 4.4.2.2. China
- 4.4.2.3. India
- 4.4.3. Europe
- 4.4.4. Middle East & Africa
- 4.4.5. PRP Qualification Criteria
- 4.4.1. North America
- 4.5. Porter's Five Forces Analysis
- 4.5.1. Bargaining Power of Suppliers
- 4.5.2. Bargaining Power of Buyers
- 4.5.3. Threat of Substitutes
- 4.5.4. Threat of New Entrants
- 4.5.5. Degree of Competition
- 4.6. Investment and Funding Scenario
- 4.7. Pipeline Analysis
5. Regenerative Medicine Market Assessment-by Product
- 5.1. Overview
- 5.2. Cell Therapy
- 5.2.1. Cell-based Immunotherapy
- 5.2.2. Stem Cell Therapy
- 5.2.2.1. Allogenic Therapy
- 5.2.2.2. Autologous Therapy
- 5.2.3. Platelet-rich Plasma Therapy
- 5.3. Gene Therapy
- 5.4. Tissue Engineering Products
6. Regenerative Medicine Market Assessment-by Application
- 6.1. Overview
- 6.2. Musculoskeletal
- 6.3. Oncology
- 6.4. Ophthalmology
- 6.5. Immunology & Inflammation
- 6.6. Neurology
- 6.7. Dermatology
- 6.8. Cardiology
- 6.9. Other Applications
7. Regenerative Medicine Market Assessment-by End User
- 7.1. Introduction
- 7.2. Hospitals & Clinics
- 7.3. Ambulatory Surgical Centers
8. Regenerative Medicine Market Assessment-by Geography
- 8.1. Overview
- 8.2. North America
- 8.2.1. U.S.
- 8.2.2. Canada
- 8.3. Europe
- 8.3.1. Germany
- 8.3.2. U.K.
- 8.3.3. France
- 8.3.4. Switzerland
- 8.3.5. Italy
- 8.3.6. Spain
- 8.3.7. Netherlands
- 8.3.8. Sweden
- 8.3.9. Rest of Europe
- 8.4. Asia-Pacific
- 8.4.1. Japan
- 8.4.2. China
- 8.4.3. Australia
- 8.4.4. India
- 8.4.5. South Korea
- 8.4.6. Rest of Asia-Pacific
- 8.5. Latin America
- 8.5.1. Brazil
- 8.5.2. Mexico
- 8.5.3. Rest of Latin America
- 8.6. Middle East & Africa
9. Competition Analysis
- 9.1. Overview
- 9.2. Key Growth Strategies
- 9.3. Competitive Benchmarking
- 9.4. Competitive Dashboard
- 9.4.1. Industry Leaders
- 9.4.2. Market Differentiators
- 9.4.3. Vanguards
- 9.4.4. Emerging Companies
- 9.5. Market Share Analysis (2023)
- 9.5.1. Novartis AG (Switzerland)
- 9.5.2. Kite Pharma, Inc. (U.S.)
- 9.5.3. Biogen, Inc. (U.S.)
- 9.5.4. Bristol-Myers Squibb Company (U.S.)
- 9.5.5. Janssen Global Services, LLC (U.S.)
10. Company Profiles (Business Overview, Financial Overview, Product Portfolio, Strategic Developments, SWOT Analysis*)
- 10.1. Novartis AG
- 10.2. Kite Pharma, Inc. (A Subsidiary of Gilead Sciences, Inc.)
- 10.3. Janssen Global Services, LLC (A Subsidiary of Johnson & Johnson)
- 10.4. Bristol-Myers Squibb Company
- 10.5. Biogen Inc.
- 10.6. Smith & Nephew Plc
- 10.7. Amgen Inc.
- 10.8. Vericel Corporation
- 10.9. Integra Lifesciences Holdings Corporation
- 10.10. Pfizer Inc.
- 10.11. AbbVie Inc.
- 10.12. Sarepta Therapeutics, Inc.
- 10.13. Spark Therapeutics, Inc. (A Subsidiary of F. Hoffmann-La Roche Ag)
- 10.14. CORESTEMCHEMON, Inc.
- 10.15. CSL Behring, LLC (A Subsidiary of CSL Limited)
- 10.16. Takeda Pharmaceutical Company Limited
- 10.17. Ferring Pharmaceuticals
- 10.18. Medtronic plc
- 10.19. Bluebird Bio, Inc.
- 10.20. Vertex Pharmaceuticals Incorporated
(Note: SWOT Analysis of the Top 5 Companies Will Be Provided)
11. Appendix
- 11.1. Available Customization
- 11.2. Related Reports