
|
市場調査レポート
商品コード
1349828
HER2低発現胃または胃食道接合部(GEJ)腺がん市場:1次調査(KOLの洞察) - 市場インテリジェンス - 疫学と2034年までの市場予測HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma | Primary Research (KOL's Insight) | Market Intelligence | Epidemiology & Market Forecast-2034 |
||||||
|
|||||||
| HER2低発現胃または胃食道接合部(GEJ)腺がん市場:1次調査(KOLの洞察) - 市場インテリジェンス - 疫学と2034年までの市場予測 |
|
出版日: 2023年09月22日
発行: Mellalta Meets LLP
ページ情報: 英文 200 Pages
納期: 5~7営業日
|
- 全表示
- 概要
- 目次
HER2低発現胃または胃食道接合部(GEJ)腺がん市場は、化学療法、免疫療法、標的療法が大きく貢献しています。2034年までに、新規の新興治療薬の取り込みが、HER2低発現胃または胃食道接合部(GEJ)腺がん治療薬市場に劇的な変化をもたらす主要なブレークポイントとして機能するとみられています。
専門家は、胃がんや他のいくつかのがんはHER2を発現し、HER2標的療法に反応すると考えています。このため、これらのがん、特にHER2の発現が低い患者におけるT-DXdの使用に関する研究への関心が高まっています。
胃および胃食道接合部(GEJ)腺がんは、治療選択肢が限られている侵攻性の悪性腫瘍です。HER2低発現腫瘍は、免疫組織化学的(IHC)分析でスコア1+、またはIHCスコア2+でin situハイブリダイゼーション(ISH)陰性と定義されます。この腫瘍のサブセットは、胃またはGEJ腺がんにおいて十分に特徴づけられておらず、その臨床的意義は不明なままです。しかしながら、最近の証拠は、HER2低発現が予後に影響を及ぼし、治療決定の指針となる可能性があることを示唆しています。
HER2低発現の胃または胃食道接合部腺がん患者は、治療選択の点で課題を提起しています。トラスツズマブなどの従来のHER2標的療法は、このサブグループにおいて限られた有効性しか示していません。従って、これらの患者の転帰を改善するために、代替治療戦略を検討する必要があります。
当レポートでは、世界のHER2低発現胃または胃食道接合部(GEJ)腺がん市場について調査し、市場の現状とともに、症例数の動向、患者動向、競合製品の市場における位置づけ、市場の機会などを提供しています。
目次
第1章 エグゼクティブサマリー
第2章 HER2低発現胃または胃食道接合部(GEJ)腺がんの疾患の背景
- HER2低発現胃または胃食道接合部(GEJ)腺がんの定義
- 原因と症状
- 病態生理学
- HER2の低発現に寄与する因子
第3章 HER2低発現胃または胃食道接合部(GEJ)腺がんの診断
第4章 現在の治療法と医療行為
- 主な調査結果
- 治療アルゴリズム
第5章 アンメットニーズ
第6章 新しい治療法
- 主な調査結果
- パイプラインの概要
- HER2低発現胃または胃食道接合部(GEJ)腺がん領域における注目すべき進展
第7章 HER2低発現胃または胃食道接合部(GEJ)腺がんの上市スケジュールと主要な市場イベント
第8章 HER2低発現胃または胃食道接合部(GEJ)腺がん- 価格と償還
第9章 KOLの洞察(米国、EU、日本)
第10章 将来の治療パラダイム
- HER2低発現胃または胃食道接合部(GEJ)腺がんの競合情勢と承認
- 将来の治療アルゴリズムと競合の位置付け
- 新しい治療法に関する重要なデータの概要
- 現在および今後の治療の年間コスト
- HER2低発現胃または胃食道接合部(GEJ)腺がんにおける後期治療の戦略的考慮事項
第11章 市場の見通し
- 主な調査結果
- 概要
- 2034年までの国別市場予測
第12章 市場促進要因と抑制要因
第13章 付録
第14章 調査手法
The HER2-Low Gastric / Gastroesophageal Junction (GEJ) Adenocarcinoma market is hugely contributed by chemotherapy, immunotherapy, and targeted therapies. By 2034, the uptake of novel emerging therapies will serve as a major breakpoint to get a drastic change in the HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma therapeutics market.
"Experts believe that gastric cancer and some other cancers express HER2 and respond to HER2-targeted therapies. This has led to a growing interest in studying the use of T-DXd in these cancers, particularly in patients with low expression of HER2."
Gastric and gastroesophageal junction (GEJ) adenocarcinomas are aggressive malignancies with limited treatment options. HER2-low tumors are defined as having a score of 1+ on immunohistochemical (IHC) analysis or an IHC score of 2+ with negative results on in situ hybridization (ISH). This subset of tumors has not been well characterized in gastric or GEJ adenocarcinomas, and their clinical significance remains unclear. However, recent evidence suggests that HER2-low expression may have prognostic implications and could potentially guide treatment decisions.
Patients with HER2 low gastric or gastroesophageal junction adenocarcinoma pose a challenge in terms of treatment selection. Traditional HER2-targeted therapies, such as trastuzumab, have shown limited efficacy in this subgroup. Therefore, alternative treatment strategies need to be explored to improve outcomes for these patients.
Mellalta's HER2-Low Gastric / Gastroesophageal Junction (GEJ) Adenocarcinoma Report- Market Summary
| Report Attributes | Details |
| Market Size 2034: | $ billion |
| Key Market Players: | Duality Biologics; AstraZeneca/Daiichi Sankyo |
| Forecast Period: | 2020-2034. |
| Countries Covered: | US, France, Germany, Italy, Spain, UK, China and Japan. |
| Current SOC Chemotherapy: | Immunotherapy; Targeted Therapies. |
| Future SOC: | Targeted Therapies; Combination Approach. |
| Key Unmet Need: | Improved HER2 Assessment; Limited Treatment Option. |
| Key Clinical Insights: | The poor prognosis observed in patients with HER2-low gastric or GEJ adenocarcinoma highlights the need for novel treatment approaches and pharmaceutical companies are now exploring the development of novel agents specifically designed to address the needs of this patient population. |
| Provider-Patient (PPP) Perspective: | Cost-effectiveness of treatment; Better Diagnostic Identification; Access to appropriate treatments and improved options. |
Mellalta's HER2-Low Gastric / Gastroesophageal Junction (GEJ) Adenocarcinoma Report - Epidemiology
The total incident cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma in the G7 countries are anticipated to increase by a significant number of cases by 2034 for the study period (2020-2034). As per estimates, the United States will present with the highest incidence of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma cases in 2034. Among the EU5, Germany had the highest HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma cases, followed by the UK, France, Italy, and Spain. Japan is reported to have the highest number of treated cases after the United States, Germany, and the UK.
A retrospective study evaluated HER-2 expression in early-stage gastric cancer using immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) and found that 8.3% of patients had HER2-positive tumors, 31.8% had HER2-low tumors, and 50.3% had HER2-negative tumors.
Mellalta's HER2-Low Gastric / Gastroesophageal Junction (GEJ) Adenocarcinoma Report - Current Market Size & Forecast Trends
The current standard of care is limited to chemotherapy, immunotherapy, and targeted therapy. Recent developments have shown that ADCs, such as DS-8201 and Disitamab vedotin, release intracellular toxins that can exert a killing effect on neighboring cells without target expression. This bystander effect allows patients with HER2 Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma to benefit from HER2-targeted therapy. Therefore, ADCs may expand the population that can benefit from HER2-targeted therapy and are expected to be a novel option for patients whose tumors have low HER2 expression.
The advent of anti-HER2 therapies, such as trastuzumab and trastuzumab deruxtecan (T-DXd), has revolutionized the treatment landscape for HER2-positive gastric or gastroesophageal junction adenocarcinoma. However, the efficacy of these therapies in HER2-low tumors remains uncertain. Recent studies have shown promising results with T-DXd in patients with HER2-low gastric cancer, suggesting that HER2-low tumors may still benefit from HER2-targeted treatments. Based on the results of the DESTINY-Gastric01 trial, T-DXd was approved in Japan for the treatment of patients with HER2-positive unresectable advanced or recurrent gastric cancer that has progressed after chemotherapy. T-DXd is now recommended in the Japanese Gastric Cancer Association treatment guidelines as the third-line treatment for HER2-positive gastric cancer. Additional studies on T-DXd for the treatment of AGC are also ongoing. The DESTINY-Gastric02 trial (NCT04014075) is an open-label, single-arm phase II trial to assess the efficacy and safety of T-DXd in HER2-positive AGC patients who have been treated with previous trastuzumab containing chemotherapy in non-Asian populations. The primary endpoint is the ORR by independent central review, similar to the DESTINY-Gastric01 trial. Seventy-two patients will be enrolled.
Mellalta Meets Current Treatment Strategies in Gastric Cancer
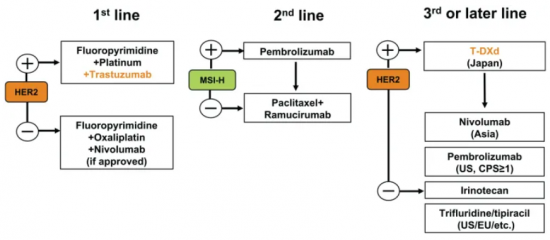
Current Treatment Strategies in Gastric Cancer Source: Daisuke Kotani et al 2021
In the 2024-2034 forecast period, there will be tremendous growth and shift in therapeutic market with the launch of novel emerging therapies like DB-1303 (Duality Biologics), Trastuzumab deruxtecan (AstraZeneca/Daiichi Sankyo), and more. We expect a greater uptake of the new therapies which will result in better treatment outcomes for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma market space. The launch of these upcoming therapies will drive the highly competitive therapeutic market in the coming time.
Questions Answered:
- What is the size of clinically and commercially relevant drug-treatable HER2 low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma populations, and how will drug-treatment rates of HER2 change over time?
- Potential challenges and opportunities in implementing targeted therapies for HER2-low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma.
- What are the most promising agents in the pipeline, and how will they shape the future of this therapy market?
- What key drivers and constraints will affect the HER2 low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma therapy market over the forecast period?
Report Highlights:
- HER2-Low Gastroesophageal Junction (GEJ) Adenocarcinoma - Current Market Trends
- HER2-Low Gastroesophageal Junction (GEJ) Adenocarcinoma - Current & Forecasted Cases across the G7 Countries
- HER2-Low Gastroesophageal Junction (GEJ) Adenocarcinoma - Market Opportunities and Sales Potential for Agents
- HER2-Low Gastroesophageal Junction (GEJ) Adenocarcinoma - Patient-based Market Forecast to 2034
- HER2-Low Gastroesophageal Junction (GEJ) Adenocarcinoma - Untapped Business Opportunities
- HER2-Low Gastroesophageal Junction (GEJ) Adenocarcinoma - Product Positioning Vis-a-vis Competitors' Products
- HER2-Low Gastroesophageal Junction (GEJ) Adenocarcinoma - KOLs Insight
Table of Content
1. Executive Summary
- 1.1. Key Findings
- 1.2. Key Market Challenges and Opportunities
- 1.3. What Do the Experts Say?
2. HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma Disease Background
- 2.1. HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma Definition
- 2.2. Cause & Symptoms
- 2.3. Pathophysiology
- 2.4. Factors contributing to the HER2-Low Expression in Breast Cancer
3. HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma-Diagnosis
- 3.1. HER2 Assessment with Immunohistochemistry (IHC) and In Situ Hybridization (ISH) (ASCO/CAP Guidelines)
- 3.2. Epidemiology and Patient Populations
- 3.3. Key Findings
- 3.4. Methods and data Sources
- 3.4.1. Country Specific Incident cases of Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (US, Germany, France, Italy, Spain, UK, and Japan)
- 3.4.2. Country Specific Incident cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.3. Country Specific Treated cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.4. Key Sources for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma Epidemiology and Model Parameters
- 3.4.4.1. United States
- 3.4.4.1.1. United States Incident cases of Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (US, Germany, France, Italy, Spain, UK, and Japan)
- 3.4.4.1.2. United States Incident cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.4.1.3. United States Treated cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.4.2. Germany
- 3.4.4.2.1. Germany Incident cases of Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (US, Germany, France, Italy, Spain, UK, and Japan)
- 3.4.4.2.2. Germany Incident cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.4.2.3. Germany Treated cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.4.3. France
- 3.4.4.4. France Incident cases of Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (US, Germany, France, Italy, Spain, UK, and Japan)
- 3.4.4.5. France Incident cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.4.6. France Treated cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.4.1. United States
- 3.4.5. Italy
- 3.4.5.1. Italy Incident cases of Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (US, Germany, France, Italy, Spain, UK, and Japan)
- 3.4.5.2. Italy Incident cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.5.3. Italy Treated cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.5.4. Spain
- 3.4.5.4.1. Spain Incident cases of Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (US, Germany, France, Italy, Spain, UK, and Japan)
- 3.4.5.4.2. Spain Incident cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.5.4.3. Spain Treated cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.5.5. United Kingdom
- 3.4.5.5.1. United Kingdom Incident cases of Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (US, Germany, France, Italy, Spain, UK, and Japan)
- 3.4.5.5.2. United Kingdom Incident cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.5.5.3. United Kingdom Treated cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.5.6. Japan
- 3.4.5.6.1. Japan Incident cases of Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (US, Germany, France, Italy, Spain, UK, and Japan)
- 3.4.5.6.2. Japan Incident cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.5.6.3. Japan Treated cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
4. Current Therapy and Medical Practice
- 4.1. Key Findings
- 4.2. Treatment Algorithm
5. Unmet Needs
6. Emerging Therapy
- 6.1. Key Findings
- 6.2. Pipeline Overview
- 6.3. Notable Developments in the HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma space
- 6.3.1. Product Analysis
- 6.3.1.1. DB-1303 (Duality Biologics)
- 6.3.1.1.1. Product Profile
- 6.3.1.1.2. Clinical Development
- 6.3.1.1.3. Market & Sales Opportunity Forecasted to 2034
- 6.3.1.2. Trastuzumab deruxtecan (AstraZeneca/Daiichi Sankyo)
- 6.3.1.2.1. Product Profile
- 6.3.1.2.2. Clinical Development
- 6.3.1.2.3. Market & Sales Opportunity Forecasted to 2034
- 6.3.1.3. Product A (Company A)
- 6.3.1.3.1. Product Profile
- 6.3.1.3.2. Clinical Development
- 6.3.1.3.3. Market & Sales Opportunity Forecasted to 2034
- 6.3.1.4. Product B (Company B)
- 6.3.1.4.1. Product Profile
- 6.3.1.4.2. Clinical Development
- 6.3.1.4.3. Market & Sales Opportunity Forecasted to 2034
- 6.3.1.4.4. Others
- 6.3.1.1. DB-1303 (Duality Biologics)
- 6.3.1. Product Analysis
7. Launch Timeline & Key Market Events for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
8. HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma - Pricing & Reimbursement
9. KOLs Insight (US, EU, JP)
- 9.1. Unmet Needs
- 9.2. Analysis of the progresses in terms of approvals & current pipeline;
- 9.3. Impact on the treatment algorithm and product positioning
- 9.4. Relevance of new targets/platforms/ therapies Uptake Share %
- 9.5. Physicians Preferences for the new therapies
10. Future Treatment Paradigm
- 10.1. HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma Competitor Landscape and Approvals Anticipated
- 10.2. Future Treatment Algorithms and Competitor Positioning
- 10.3. Key Data Summary for Emerging Treatment
- 10.4. Annual Cost of Current & Emerging Treatment
- 10.5. Late Phase Therapy Strategic Considerations in HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
11. Market Outlook
- 11.1. Key Findings
- 11.2. Overview
- 11.3. Country Specific Market Forecast to 2034
- 11.3.1. Sales of Drugs to Treat HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma in the Major Pharmaceutical Markets, 2020-2034
- 11.3.2. Patient Share of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma by Therapies
- 11.3.3. Market Forecast by Country
- 11.3.3.1. United States
- 11.3.3.1.1. United States Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma 2020-2034 (USD Million)
- 11.3.3.1.2. United States Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma by Therapy 2020-2034 (USD Million)
- 11.3.3.2. Germany
- 11.3.3.2.1. Germany Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma 2020-2034 (USD Million)
- 11.3.3.2.2. Germany Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma by Therapy 2020-2034 (USD Million)
- 11.3.3.3. France
- 11.3.3.3.1. France Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma 2020-2034 (USD Million)
- 11.3.3.3.2. France Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma by Therapy 2020-2034 (USD Million)
- 11.3.3.4. Italy
- 11.3.3.4.1. Italy Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma 2020-2034 (USD Million)
- 11.3.3.4.2. Italy Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma by Therapy 2020-2034 (USD Million)
- 11.3.3.5. Spain
- 11.3.3.5.1. Spain Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma 2020-2034 (USD Million)
- 11.3.3.5.2. Spain Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma by Therapy 2020-2034 (USD Million)
- 11.3.3.6. United Kingdom
- 11.3.3.6.1. United Kingdom Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma 2020-2034 (USD Million)
- 11.3.3.6.2. United Kingdom Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma by Therapy 2020-2034 (USD Million)
- 11.3.3.7. Japan
- 11.3.3.7.1. Japan Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma 2020-2034 (USD Million)
- 11.3.3.7.2. Japan Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma by Therapy 2020-2034 (USD Million)
- 11.3.3.1. United States
12. Market Drivers and Constraints
- 12.1. What Factors Are Driving the Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma?
- 12.2. What Factors Are Constraining the Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma?


