|
|
市場調査レポート
商品コード
1419626
再生医療の世界市場 (~2028年):製品 (幹細胞・免疫療法・遺伝子治療・組織工学)・用途 (筋骨格系・癌・皮膚科・創傷治療・心血管疾患・眼疾患) 別Regenerative Medicine Market by Product (Stem Cell, Immunotherapy, Gene Therapy, Tissue Engineering), Application (Musculoskeletal, Cancer, Dermatology, Wound care, Cardiovascular Diseases, Eye Disorders) - Global Forecast to 2028 |
||||||
カスタマイズ可能
|
|||||||
| 再生医療の世界市場 (~2028年):製品 (幹細胞・免疫療法・遺伝子治療・組織工学)・用途 (筋骨格系・癌・皮膚科・創傷治療・心血管疾患・眼疾患) 別 |
|
出版日: 2024年01月26日
発行: MarketsandMarkets
ページ情報: 英文 293 Pages
納期: 即納可能
|
全表示
- 概要
- 目次
レポート概要
| 調査範囲 | |
|---|---|
| 調査対象年 | 2021-2028年 |
| 基準年 | 2022年 |
| 予測期間 | 2023-2028年 |
| 検討単位 | 金額 (米ドル) |
| セグメント | 製品・治療領域別 |
| 対象地域 | 北米・欧州・アジア太平洋・ラテンアメリカ・中東&アフリカ |
世界の再生医療の市場規模は、2023年の160億米ドルから、予測期間中はCAGRは25.1%で推移し、2028年には490億米ドルに達すると予測されています。
新たな治療領域における広い展望、主要企業による提携・協力などの要因が、今後数年間で市場を押し上げると予測されています。さらに、個別化医療への注目の高まりと再生医療規制認可の増加も、有利な市場成長をもたらすと思われます。しかし一方で、長期的なデータの欠如、倫理的・法的な懸念、治療費の高騰が市場成長を抑制すると予測されています。
製品別では、細胞治療の部門が圧倒的シェアを占める見通しです。幹細胞治療の人気の高まりが、細胞治療部門の拡大を牽引すると予想されています。
治療領域別では、筋骨格系疾患の部門が2022年に最大のシェアを示しています。この背景には、整形外科疾患の罹患率の増加や筋骨格系の再生研究に対する関心の高まりがあります。
地域別では、アジア太平洋地域が予測期間中に大きなCAGRで成長すると予測されています。慢性疾患の高負担、1人当たり所得の増加、新技術に対する需要の高まりが、同地域の再生医療市場の成長を支えるものと期待されています。
当レポートでは、世界の再生医療の市場を調査し、市場概要、市場影響因子および市場機会の分析、技術・特許の動向、法規制環境、パイプラインの動向、市場規模の推移・予測、各種区分・地域別の詳細分析、競合情勢、主要企業のプロファイルなどをまとめています。
目次
第1章 イントロダクション
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 重要考察
第5章 市場概要
- 市場力学
- 促進要因
- 抑制要因
- 機会
- 課題
- 顧客のビジネスに影響を与える動向/ディスラプション
- 価格分析
- サプライチェーン分析
- バリューチェーン分析
- エコシステム分析
- 技術分析
- 特許分析
- 主な会議とイベント
- 関税と規制状況
- ポーターのファイブフォース分析
- 主なステークホルダーと購入基準
- パイプライン分析
第6章 再生医療市場:製品別
- 細胞治療
- 幹細胞治療
- 細胞ベースの免疫療法
- 遺伝子治療
- ヒト組織工学
第7章 再生医療市場:治療領域別
- 腫瘍
- 筋骨格系疾患
- 皮膚科・創傷治療
- 心血管疾患
- 眼科
- 神経科
- その他
第8章 再生医療市場:地域別
- 北米
- 欧州
- アジア太平洋
- ラテンアメリカ
- 中東
- アフリカ
第9章 競合情勢
- 主要企業の採用戦略
- 収益シェア分析
- 市場シェア分析
- 企業評価マトリックス
- 新興企業/中小企業の評価マトリックス
- 競合シナリオと動向
第10章 企業プロファイル
- 主要企業
- NOVARTIS AG
- BIOGEN INC.
- GILEAD SCIENCES, INC.
- SAREPTA THERAPEUTICS, INC.
- AMGEN, INC.
- SMITH+NEPHEW
- MEDIPOST CO., LTD.
- JCR PHARMACEUTICALS CO., LTD.
- TAKEDA PHARMACEUTICAL COMPANY LIMITED
- CORESTEM, INC.
- VERICEL CORPORATION
- MIMEDX GROUP, INC.
- ORGANOGENESIS INC.
- MEDTRONIC
- BRISTOL-MYERS SQUIBB COMPANY
- その他の企業
- ORTHOCELL LTD.
- MESOBLAST LTD.
- BIORESTORATIVE THERAPIES, INC.
- PLURISTEM THERAPEUTICS INC.
- TEGOSCIENCE
- ANTEROGEN.CO.,LTD.
- BLUEBIRD BIO, INC.
- STEMPEUTICS RESEARCH PVT. LTD.
- SIBIONO GENETECH CO. LTD.
- ASPECT BIOSYSTEMS
第11章 付録
Report Description
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2021-2028 |
| Base Year | 2022 |
| Forecast Period | 2023-2028 |
| Units Considered | Value (USD) Billion |
| Segments | By Product, By Therapeutic area |
| Regions covered | North America, Europe, the Asia Pacific, Latin America, the Middle East and Africa |
The global regenerative medicines market is projected to reach USD 49.0 Billion by 2028 from USD 16.0 Billion in 2023, at a CAGR of 25.1% during the forecast period of 2023 to 2028. Factors such as the wide scope in new therapeutic areas and collaborations and partnerships by major market players are predicted to uplift the market in the coming years. Additionally, rise in the focus of personalized medicine and rising regenerative medicine regulatory approvals will provide lucrative market growth. However, lack of long-term data and ethical and legal concerns and high cost of treatments are predicted to restrict the market.
"The cell therapy segment held the dominant share in the regenerative medicine market."
Based on product, the global regenerative medicine market is segmented into gene therapy, cell therapy (stem cell therapy [cell transplantations, stem cell therapy products {autologous therapy, allogenic therapy] cell-based immunotherapy products), and tissue engineering products. The increasing popularity of stem therapy is expected to drive the expansion of the cell therapy market.
"Musculoskeletal disorders segment accounted for the largest share of the therapeutic area segment in 2022."
Based on therapeutic area, the regenerative medicine market is segmented into oncology, musculoskeletal disorders, neurology, ophthalmology, dermatology & wound care, cardiovascular diseases, and other applications. This can be attributed to the increasing preavalence of orthopedic disorders and growing interest on musculoskeletal regeneration research.
"APAC region is likely to grow at a faster pace."
The regenerative medicine market is segmented into North America, Europe, Asia Pacific, Latin America, Middle East and Africa. APAC region is anticipated to grow at a significant CAGR during the forecast period. High burden of chronic diseases, growing per capita income, and the rising demand for new technologies are expected to support regenerative medicine market growth in the region.
The primary interviews conducted for this report can be categorized as follows:
- By Respondent: Supply Side- 80%, and Demand Side - 20%
- By Designation (Supply Side): Managers - 45%, CXOs & Directors - 30%, Executives- 25%
- By Region: North America -55%, Europe -10%, Asia-Pacific -20%, Latin America -10%, MEA- 5%
List of Companies Profiled in the Report:
- Novartis AG (Switzerland)
- Biogen, Inc. (US)
- Sarepta Therapeutics, Inc. (US)
- Gilead Sciences, Inc. (US)
- Amgen Inc. (US)
- Smith+Nephew (UK)
- MEDIPOST Co., Ltd. (Korea)
- JCR Pharmaceuticals Co., Ltd. (Japan)
- Takeda Pharmaceutical Company Limited (Japan)
- CORESTEM, Inc (South Korea)
- Vericel Corporation (US)
- MIMEDX Group, Inc. (US)
- Organogenesis Inc. (US)
- Medtronic (Ireland)
- Bristol-Myers Squibb Company (US)
- Orthocell Ltd. (Australia)
- Mesoblast Ltd. (Australia)
- BioRestorative Therapies, Inc. (US)
- Pluristem Therapeutics Inc. (US)
- TEGOSCIENCE (South Korea)
- ANTEROGEN.CO.,LTD. (South Korea)
- Bluebird bio, Inc. (US)
- Stempeutics Research Pvt Ltd. (India)
- Sibiono GeneTech Co. Ltd. (China)
- Aspect Biosystems Ltd. (Canada)
- Athersys, Inc. (US)
Research Coverage:
This report provides a detailed picture of the regenerative medicine market. It aims to estimate the size and future growth potential of the market across different segments, such as product, therapeutic area and region. The report also includes an in-depth competitive analysis of the key market players, along with their company profiles, recent developments, and key market strategies.
Key Benefits of Buying the Report:
The report will help market leaders/new entrants by providing them with the closest approximations of the revenue numbers for the overall regenerative medicine market and its subsegments. It will also help stakeholders better understand the competitive landscape and gain more insights to better position their business and make suitable go-to-market strategies. This report will enable stakeholders to understand the market's pulse and provide them with information on the key market drivers, restraints, opportunities, and challenges.
The report provides insights on the following pointers:
- Analysis of key drivers (Growing focus on personalized medicine, steep rise of regulatory approvals in regenerative medicine, rising collaborations and partnerships by major market players, increasing scope in new therapeutic areas), restraints (Lack of long-term data, Ethical and legal concerns and high cost of treatments), opportunities (Integration with artificial intelligence and big data, Harnessing the potential of 3D printing, Growing number of organ transplants) and challenges (Lack of favorable reimbursement policies across various regions) are influencing the growth of regenerative medicine market.
- Product Development/Innovation: Detailed insights on newly launched products of the regenerative medicine market.
- Market Development: Comprehensive information about lucrative markets - the report analyses the regenerative medicine market across varied regions.
- Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the regenerative medicine market.
- Competitive Assessment: In-depth assessment of market shares, growth strategies, and product offerings of leading players include Novartis AG (Switzerland), Biogen, Inc. (US), Sarepta Therapeutics, Inc. (US), Gilead Sciences, Inc. (US), Amgen Inc. (US), Smith+Nephew (UK), MEDIPOST Co., Ltd. (Korea), JCR Pharmaceuticals Co., Ltd. (Japan), Takeda Pharmaceutical Company Limited (Japan) and CORESTEM, Inc (South Korea) and among others in the regenerative medicine market.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.2.1 INCLUSIONS & EXCLUSIONS
- 1.3 MARKET SCOPE
- 1.3.1 MARKETS COVERED
- 1.3.2 YEARS CONSIDERED
- 1.3.3 CURRENCY CONSIDERED
- 1.4 RESEARCH LIMITATIONS
- 1.5 STAKEHOLDERS
- 1.6 SUMMARY OF CHANGES
- 1.7 IMPACT OF RECESSION ON REGENERATIVE MEDICINE MARKET
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- FIGURE 1 RESEARCH DESIGN
- 2.1.1 SECONDARY DATA
- 2.1.2 PRIMARY DATA
- FIGURE 2 BREAKDOWN OF PRIMARIES: REGENERATIVE MEDICINE MARKET
- 2.2 MARKET SIZE ESTIMATION
- FIGURE 3 MARKET SIZE ESTIMATION FOR SUPPLY-SIDE ANALYSIS: REGENERATIVE MEDICINE MARKET, 2022
- FIGURE 4 MARKET SIZE ESTIMATION: REVENUE SHARE ANALYSIS, 2022
- FIGURE 5 NOVARTIS AG: REVENUE SHARE ANALYSIS, 2022
- FIGURE 6 PRODUCT-BASED REVENUE ANALYSIS, 2022
- 2.2.1 PRIMARY INSIGHTS
- FIGURE 7 VALIDATION FROM PRIMARY EXPERTS
- 2.2.2 SEGMENT ASSESSMENT METHODOLOGY: REGENERATIVE MEDICINE MARKET
- FIGURE 8 MARKET SIZE ESTIMATION METHODOLOGY: TOP-DOWN APPROACH
- FIGURE 9 CAGR PROJECTIONS: REGENERATIVE MEDICINE MARKET
- FIGURE 10 GROWTH ANALYSIS OF DRIVERS, RESTRAINTS, CHALLENGES, AND OPPORTUNITIES IN REGENERATIVE MEDICINE MARKET
- 2.3 MARKET BREAKDOWN & DATA TRIANGULATION
- FIGURE 11 DATA TRIANGULATION METHODOLOGY
- 2.4 STUDY ASSUMPTIONS
- 2.5 RISK ANALYSIS
- 2.6 IMPACT OF RECESSION ON REGENERATIVE MEDICINE MARKET
- TABLE 1 GLOBAL INFLATION RATE PROJECTION, 2024-2028 (% GROWTH)
- TABLE 2 US HEALTH EXPENDITURE, 2019-2022 (USD MILLION)
- TABLE 3 US HEALTH EXPENDITURE, 2023-2027 (USD MILLION)
3 EXECUTIVE SUMMARY
- FIGURE 12 REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2023 VS. 2028 (USD MILLION)
- FIGURE 13 REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2023 VS. 2028 (USD MILLION)
- FIGURE 14 GEOGRAPHICAL SNAPSHOT OF REGENERATIVE MEDICINE MARKET
4 PREMIUM INSIGHTS
- 4.1 REGENERATIVE MEDICINE MARKET OVERVIEW
- FIGURE 15 FOCUS ON PERSONALISED MEDICINE TO DRIVE MARKET GROWTH
- 4.2 NORTH AMERICA: REGENERATIVE MEDICINE MARKET
- FIGURE 16 CELL THERAPY TO COMMAND LARGEST SHARE OF NORTH AMERICAN REGENERATIVE MEDICINE MARKET
- 4.3 REGENERATIVE MEDICINE MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
- FIGURE 17 CHINA TO REGISTER HIGHEST GROWTH DURING FORECAST PERIOD
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- FIGURE 18 REGENERATIVE MEDICINE MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- 5.2.1 DRIVERS
- 5.2.1.1 Growing focus on personalized medicine
- FIGURE 19 PERCENTAGE OF FDA APPROVALS FOR PERSONALIZED MEDICINE PRODUCTS (2018-2022)
- 5.2.1.2 Emerging applications in new therapeutic areas
- TABLE 4 REGENERATIVE MEDICINE MARKET: EMERGING APPLICATIONS
- 5.2.1.3 Increasing collaborations and partnerships by major market players
- 5.2.1.4 Increasing regulatory approvals in regenerative medicine
- TABLE 5 LIST OF KEY PRODUCT APPROVALS IN REGENERATIVE MEDICINE MARKET, 2021-2023
- 5.2.2 RESTRAINTS
- 5.2.2.1 Lack of long-term data
- 5.2.2.2 Ethical and legal concerns and high cost of treatments
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Harnessing the potential of 3D printing
- 5.2.3.2 Integration with artificial intelligence and big data
- 5.2.3.3 Growing number of organ transplants
- 5.2.4 CHALLENGES
- 5.2.4.1 Lack of favorable reimbursement policies across various regions
- 5.3 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS' BUSINESSES
- FIGURE 20 REVENUE SHIFT TOWARD REGENERATIVE MEDICINE COMPANIES
- 5.4 PRICING ANALYSIS
- 5.4.1 AVERAGE SELLING PRICE TREND OF KEY PLAYERS, BY PRODUCT
- TABLE 6 REGENERATIVE MEDICINE PRICE TREND
- 5.5 SUPPLY CHAIN ANALYSIS
- FIGURE 21 SUPPLY CHAIN ANALYSIS: REGENERATIVE MEDICINE MARKET
- 5.6 VALUE CHAIN ANALYSIS
- FIGURE 22 VALUE CHAIN ANALYSIS: REGENERATIVE MEDICINE MARKET
- 5.7 ECOSYSTEM ANALYSIS
- FIGURE 23 REGENERATIVE MEDICINE MARKET: ECOSYSTEM ANALYSIS
- TABLE 7 ROLE IN ECOSYSTEM
- 5.8 TECHNOLOGY ANALYSIS
- FIGURE 24 KEY STEPS IN GENE THERAPY
- TABLE 8 STEM CELL THERAPIES AND GENE THERAPIES
- 5.9 PATENT ANALYSIS
- TABLE 9 REGENERATIVE MEDICINE MARKET: INDICATIVE LIST OF PATENTS
- 5.10 KEY CONFERENCES & EVENTS IN 2024-2025
- 5.10.1 REGENERATIVE MEDICINE CONFERENCES IN 2024-2025
- TABLE 10 REGENERATIVE MEDICINE CONFERENCES IN 2024-2025
- 5.11 TARIFF & REGULATORY LANDSCAPE
- 5.11.1 FDA APPROVALS
- 5.11.2 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 11 NORTH AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 12 EUROPE: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 13 ASIA PACIFIC: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 14 REST OF THE WORLD: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.12 PORTER'S FIVE FORCES ANALYSIS
- TABLE 15 REGENERATIVE MEDICINE MARKET: PORTER'S FIVE FORCES ANALYSIS
- 5.12.1 THREAT OF NEW ENTRANTS
- 5.12.2 THREAT OF SUBSTITUTES
- 5.12.3 BARGAINING POWER OF BUYERS
- 5.12.4 BARGAINING POWER OF SUPPLIERS
- 5.12.5 DEGREE OF COMPETITION
- 5.13 KEY STAKEHOLDERS & BUYING CRITERIA
- 5.13.1 KEY STAKEHOLDERS IN BUYING PROCESS
- FIGURE 25 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS OF REGENERATIVE MEDICINE PRODUCTS
- 5.13.2 BUYING CRITERIA FOR REGENERATIVE MEDICINE PRODUCTS
- FIGURE 26 KEY BUYING CRITERIA FOR END USERS
- 5.14 PIPELINE ANALYSIS
- TABLE 16 GENE THERAPY PRODUCTS UNDER CLINICAL TRIAL
- TABLE 17 CELL THERAPY PRODUCTS UNDER CLINICAL TRIAL
6 REGENERATIVE MEDICINE MARKET, BY PRODUCT
- 6.1 INTRODUCTION
- TABLE 18 REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- 6.2 CELL THERAPY
- TABLE 19 REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 20 REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY REGION, 2021-2028 (USD MILLION)
- TABLE 21 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 22 EUROPE: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 23 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 24 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.2.1 STEM CELL THERAPY
- TABLE 25 REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 26 REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY REGION, 2021-2028 (USD MILLION)
- TABLE 27 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 28 EUROPE: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 29 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 30 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.2.1.1 Cell transplantations
- 6.2.1.1.1 Potential of Allo-SCT in treating AML to drive segmental growth
- 6.2.1.1 Cell transplantations
- TABLE 31 REGENERATIVE MEDICINE MARKET FOR CELL TRANSPLANTATIONS, BY REGION, 2021-2028 (USD MILLION)
- TABLE 32 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR CELL TRANSPLANTATIONS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 33 EUROPE: REGENERATIVE MEDICINE MARKET FOR CELL TRANSPLANTATIONS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 34 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR CELL TRANSPLANTATIONS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 35 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR CELL TRANSPLANTATIONS, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.2.1.2 Stem cell therapy products
- TABLE 36 REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 37 REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY REGION, 2021-2028 (USD MILLION)
- TABLE 38 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 39 EUROPE: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 40 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 41 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.2.1.2.1 Autologous therapy
- 6.2.1.2.1.1 Low risk of post-treatment complications to support segment growth
- 6.2.1.2.1 Autologous therapy
- TABLE 42 REGENERATIVE MEDICINE MARKET FOR AUTOLOGOUS THERAPY, BY REGION, 2021-2028 (USD MILLION)
- TABLE 43 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR AUTOLOGOUS THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
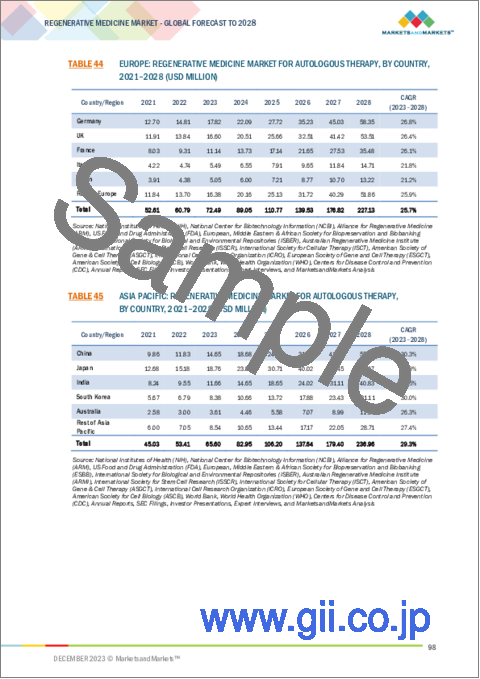
- TABLE 44 EUROPE: REGENERATIVE MEDICINE MARKET FOR AUTOLOGOUS THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 45 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR AUTOLOGOUS THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 46 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR AUTOLOGOUS THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.2.1.2.2 Allogeneic therapy
- 6.2.1.2.2.1 High potential in disease treatment to ensure demand and development
- 6.2.1.2.2 Allogeneic therapy
- TABLE 47 REGENERATIVE MEDICINE MARKET FOR ALLOGENEIC THERAPY, BY REGION, 2021-2028 (USD MILLION)
- TABLE 48 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR ALLOGENEIC THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 49 EUROPE: REGENERATIVE MEDICINE MARKET FOR ALLOGENEIC THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 50 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR ALLOGENEIC THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 51 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR ALLOGENEIC THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.2.2 CELL-BASED IMMUNOTHERAPY
- 6.2.2.1 Growing adoption of T-cell therapy in cancer treatment to support market growth
- TABLE 52 REGENERATIVE MEDICINE MARKET FOR CELL-BASED IMMUNOTHERAPY, BY REGION, 2021-2028 (USD MILLION)
- TABLE 53 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR CELL-BASED IMMUNOTHERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 54 EUROPE: REGENERATIVE MEDICINE MARKET FOR CELL-BASED IMMUNOTHERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 55 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR CELL-BASED IMMUNOTHERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 56 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR CELL-BASED IMMUNOTHERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.3 GENE THERAPY
- 6.3.1 STRONG PRODUCT PIPELINE DUE TO BROADENING APPLICATIONS IN DISEASE TREATMENT TO DRIVE MARKET
- TABLE 57 APPROVED GENE THERAPIES, 2022
- TABLE 58 REGENERATIVE MEDICINE MARKET FOR GENE THERAPY, BY REGION, 2021-2028 (USD MILLION)
- TABLE 59 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR GENE THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 60 EUROPE: REGENERATIVE MEDICINE MARKET FOR GENE THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 61 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR GENE THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 62 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR GENE THERAPY, BY COUNTRY, 2021-2028 (USD MILLION)
- 6.4 TISSUE ENGINEERING
- 6.4.1 HIGH DEMAND FOR TISSUE ENGINEERING PRODUCTS IN DERMATOLOGY & WOUND CARE APPLICATIONS TO DRIVE MARKET
- TABLE 63 REGENERATIVE MEDICINE MARKET FOR TISSUE ENGINEERING, BY REGION, 2021-2028 (USD MILLION)
- TABLE 64 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR TISSUE ENGINEERING, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 65 EUROPE: REGENERATIVE MEDICINE MARKET FOR TISSUE ENGINEERING, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 66 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR TISSUE ENGINEERING, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 67 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR TISSUE ENGINEERING, BY COUNTRY, 2021-2028 (USD MILLION)
7 REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA
- 7.1 INTRODUCTION
- TABLE 68 NUMBER OF CLINICAL TRIALS FOR REGENERATIVE MEDICINE PRODUCTS, BY THERAPEUTIC AREA, 2021 VS. 2022
- TABLE 69 REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 7.2 ONCOLOGY
- 7.2.1 INCREASING STEM CELL TRANSPLANTS TO DRIVE MARKET
- TABLE 70 INDICATIVE LIST OF COMMERCIALIZED REGENERATIVE CELL THERAPIES FOR ONCOLOGY APPLICATIONS
- TABLE 71 REGENERATIVE MEDICINE MARKET FOR ONCOLOGY, BY REGION, 2021-2028 (USD MILLION)
- TABLE 72 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR ONCOLOGY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 73 EUROPE: REGENERATIVE MEDICINE MARKET FOR ONCOLOGY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 74 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR ONCOLOGY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 75 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR ONCOLOGY, BY COUNTRY, 2021-2028 (USD MILLION)
- 7.3 MUSCULOSKELETAL DISORDERS
- 7.3.1 RISING RESEARCH ON MUSCULOSKELETAL REGENERATION AND PREVALENCE OF ORTHOPEDIC DISORDERS TO DRIVE MARKET
- TABLE 76 REGENERATIVE MEDICINE MARKET FOR MUSCULOSKELETAL DISORDERS, BY REGION, 2021-2028 (USD MILLION)
- TABLE 77 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR MUSCULOSKELETAL DISORDERS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 78 EUROPE: REGENERATIVE MEDICINE MARKET FOR MUSCULOSKELETAL DISORDERS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 79 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR MUSCULOSKELETAL DISORDERS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 80 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR MUSCULOSKELETAL DISORDERS, BY COUNTRY, 2021-2028 (USD MILLION)
- 7.4 DERMATOLOGY & WOUND CARE
- 7.4.1 RISING USE OF WOUND CARE BIOLOGICS TO DRIVE MARKET
- TABLE 81 INDICATIVE LIST OF REGENERATIVE MEDICINE PRODUCTS COMMERCIALIZED FOR TREATMENT OF ACUTE & CHRONIC WOUNDS
- TABLE 82 REGENERATIVE MEDICINE MARKET FOR DERMATOLOGY & WOUND CARE, BY REGION, 2021-2028 (USD MILLION)
- TABLE 83 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR DERMATOLOGY & WOUND CARE, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 84 EUROPE: REGENERATIVE MEDICINE MARKET FOR DERMATOLOGY & WOUND CARE, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 85 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR DERMATOLOGY & WOUND CARE, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 86 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR DERMATOLOGY & WOUND CARE, BY COUNTRY, 2021-2028 (USD MILLION)
- 7.5 CARDIOVASCULAR DISEASES
- 7.5.1 INCREASING USE OF MSCS FOR CLINICAL RESEARCH ON IHD TO SUPPORT MARKET GROWTH
- TABLE 87 REGENERATIVE MEDICINE MARKET FOR CARDIOVASCULAR DISEASES, BY REGION, 2021-2028 (USD MILLION)
- TABLE 88 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR CARDIOVASCULAR DISEASES, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 89 EUROPE: REGENERATIVE MEDICINE MARKET FOR CARDIOVASCULAR DISEASES, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 90 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR CARDIOVASCULAR DISEASES, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 91 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR CARDIOVASCULAR DISEASES, BY COUNTRY, 2021-2028 (USD MILLION)
- 7.6 OPHTHALMOLOGY
- 7.6.1 REGENERATION OF EYE TISSUES FOR AMD TO SUPPORT MARKET GROWTH
- TABLE 92 LIST OF PIPELINE PRODUCTS FOR REGENERATIVE MEDICINE IN OPHTHALMOLOGY
- TABLE 93 REGENERATIVE MEDICINE MARKET FOR OPHTHALMOLOGY, BY REGION, 2021-2028 (USD MILLION)
- TABLE 94 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR OPHTHALMOLOGY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 95 EUROPE: REGENERATIVE MEDICINE MARKET FOR OPHTHALMOLOGY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 96 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR OPHTHALMOLOGY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 97 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR OPHTHALMOLOGY, BY COUNTRY, 2021-2028 (USD MILLION)
- 7.7 NEUROLOGY
- 7.7.1 GROWING NUMBER OF GENE THERAPY PRODUCTS APPROVED FOR NEUROLOGICAL DISEASE TREATMENT TO DRIVE MARKET GROWTH
- TABLE 98 REGENERATIVE MEDICINE MARKET FOR NEUROLOGY, BY REGION, 2021-2028 (USD MILLION)
- TABLE 99 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR NEUROLOGY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 100 EUROPE: REGENERATIVE MEDICINE MARKET FOR NEUROLOGY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 101 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR NEUROLOGY, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 102 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR NEUROLOGY, BY COUNTRY, 2021-2028 (USD MILLION)
- 7.8 OTHER THERAPEUTIC AREAS
- TABLE 103 REGENERATIVE MEDICINE MARKET FOR OTHER THERAPEUTIC AREAS, BY REGION, 2021-2028 (USD MILLION)
- TABLE 104 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR OTHER THERAPEUTIC AREAS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 105 EUROPE: REGENERATIVE MEDICINE MARKET FOR OTHER THERAPEUTIC AREAS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 106 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR OTHER THERAPEUTIC AREAS, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 107 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR OTHER THERAPEUTIC AREAS, BY COUNTRY, 2021-2028 (USD MILLION)
8 REGENERATIVE MEDICINE MARKET, BY REGION
- 8.1 INTRODUCTION
- FIGURE 27 NUMBER OF PLAYERS IN REGENERATIVE MEDICINE MARKET, BY REGION, 2020-2022
- TABLE 108 NUMBER OF CLINICAL TRIALS FOR REGENERATIVE MEDICINE (2022)
- TABLE 109 REGENERATIVE MEDICINE MARKET, BY REGION, 2021-2028 (USD MILLION)
- 8.2 NORTH AMERICA
- FIGURE 28 NORTH AMERICA: REGENERATIVE MEDICINE MARKET SNAPSHOT
- TABLE 110 NORTH AMERICA: REGENERATIVE MEDICINE MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 111 NORTH AMERICA: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 112 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 113 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 114 NORTH AMERICA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 115 NORTH AMERICA: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.2.1 US
- 8.2.1.1 Rising number of pipeline products for regenerative medicine to drive market
- TABLE 116 US: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 117 US: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 118 US: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 119 US: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 120 US: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.2.2 CANADA
- 8.2.2.1 Rising government initiatives for regenerative medicine research to drive market
- TABLE 121 CANADA: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 122 CANADA: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 123 CANADA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 124 CANADA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 125 CANADA: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.2.3 NORTH AMERICA: RECESSION IMPACT
- 8.3 EUROPE
- TABLE 126 EUROPE: REGENERATIVE MEDICINE MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 127 EUROPE: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 128 EUROPE: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 129 EUROPE: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 130 EUROPE: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 131 EUROPE: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.3.1 GERMANY
- 8.3.1.1 Rising focus on clinical research and patent approvals to drive market
- TABLE 132 GERMANY: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 133 GERMANY: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 134 GERMANY: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 135 GERMANY: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 136 GERMANY: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.3.2 UK
- 8.3.2.1 Incorporating regenerative medicine into commercial healthcare services under NHS to drive market
- TABLE 137 UK: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 138 UK: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 139 UK: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 140 UK: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 141 UK: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.3.3 FRANCE
- 8.3.3.1 Structured regulatory framework to drive regenerative medicine product pipeline
- TABLE 142 FRANCE: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 143 FRANCE: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 144 FRANCE: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 145 FRANCE: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 146 FRANCE: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.3.4 ITALY
- 8.3.4.1 Rising prevalence of neurological and cardiovascular disorders to drive market
- TABLE 147 ITALY: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 148 ITALY: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 149 ITALY: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 150 ITALY: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 151 ITALY: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.3.5 SPAIN
- 8.3.5.1 Favorable regulatory environment to drive product pipeline
- TABLE 152 SPAIN: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 153 SPAIN: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 154 SPAIN: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 155 SPAIN: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 156 SPAIN: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.3.6 REST OF EUROPE
- TABLE 157 REST OF EUROPE: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 158 REST OF EUROPE: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 159 REST OF EUROPE: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 160 REST OF EUROPE: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 161 REST OF EUROPE: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.3.7 EUROPE: RECESSION IMPACT
- 8.4 ASIA PACIFIC
- FIGURE 29 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET SNAPSHOT
- TABLE 162 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 163 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 164 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 165 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 166 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 167 ASIA PACIFIC: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.4.1 CHINA
- 8.4.1.1 Favorable government support for development of regenerative medicine to drive market
- TABLE 168 CHINA: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 169 CHINA: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 170 CHINA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 171 CHINA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 172 CHINA: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.4.2 JAPAN
- 8.4.2.1 Regulatory amendments to pharmaceutical legislation to drive commercialization of therapies
- TABLE 173 JAPAN: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 174 JAPAN: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 175 JAPAN: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 176 JAPAN: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 177 JAPAN: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.4.3 INDIA
- 8.4.3.1 Introduction of novel therapies for CVD and cancer to support market growth
- TABLE 178 INDIA: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 179 INDIA: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 180 INDIA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 181 INDIA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 182 INDIA: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.4.4 SOUTH KOREA
- 8.4.4.1 Strong research base and availability of funds to support market growth
- TABLE 183 SOUTH KOREA: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 184 SOUTH KOREA: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 185 SOUTH KOREA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 186 SOUTH KOREA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 187 SOUTH KOREA: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.4.5 AUSTRALIA
- 8.4.5.1 Increasing incidence of sports injuries to support market growth
- TABLE 188 AUSTRALIA: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 189 AUSTRALIA: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 190 AUSTRALIA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 191 AUSTRALIA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 192 AUSTRALIA: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.4.6 REST OF ASIA PACIFIC
- TABLE 193 REST OF ASIA PACIFIC: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 194 REST OF ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 195 REST OF ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 196 REST OF ASIA PACIFIC: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 197 REST OF ASIA PACIFIC: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.4.7 ASIA PACIFIC: RECESSION IMPACT
- 8.5 LATIN AMERICA
- TABLE 198 LATIN AMERICA: REGENERATIVE MEDICINE MARKET, BY COUNTRY, 2021-2028 (USD MILLION)
- TABLE 199 LATIN AMERICA: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 200 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 201 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 202 LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 203 LATIN AMERICA: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.5.1 BRAZIL
- 8.5.1.1 Gradual increase in pharmaceutical R&D to support market growth
- TABLE 204 BRAZIL: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 205 BRAZIL: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 206 BRAZIL: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 207 BRAZIL: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 208 BRAZIL: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.5.2 REST OF LATIN AMERICA
- TABLE 209 REST OF LATIN AMERICA: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 210 REST OF LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 211 REST OF LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 212 REST OF LATIN AMERICA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 213 REST OF LATIN AMERICA: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.5.3 LATIN AMERICA: RECESSION IMPACT
- 8.6 MIDDLE EAST
- 8.6.1 GROWING FOCUS ON GENETIC MEDICINE DEVELOPMENT PROJECTS TO DRIVE MARKET GROWTH IN COMING YEARS
- TABLE 214 MIDDLE EAST: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 215 MIDDLE EAST: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 216 MIDDLE EAST: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 217 MIDDLE EAST: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 218 MIDDLE EAST: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.6.2 MIDDLE EAST: RECESSION IMPACT
- 8.7 AFRICA
- 8.7.1 GROWING COLLABORATIONS TO SUPPORT GROWTH IN COMING YEARS
- TABLE 219 AFRICA: REGENERATIVE MEDICINE MARKET, BY PRODUCT, 2021-2028 (USD MILLION)
- TABLE 220 AFRICA: REGENERATIVE MEDICINE MARKET FOR CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 221 AFRICA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 222 AFRICA: REGENERATIVE MEDICINE MARKET FOR STEM CELL THERAPY PRODUCTS, BY TYPE, 2021-2028 (USD MILLION)
- TABLE 223 AFRICA: REGENERATIVE MEDICINE MARKET, BY THERAPEUTIC AREA, 2021-2028 (USD MILLION)
- 8.7.2 AFRICA: RECESSION IMPACT
9 COMPETITIVE LANDSCAPE
- 9.1 INTRODUCTION
- 9.2 RIGHT-TO-WIN APPROACHES ADOPTED BY KEY PLAYERS
- TABLE 224 REGENERATIVE MEDICINE MARKET: STRATEGIES ADOPTED BY KEY PLAYERS
- 9.3 REVENUE SHARE ANALYSIS
- FIGURE 30 REVENUE ANALYSIS OF KEY PLAYERS (2020-2022)
- 9.4 MARKET SHARE ANALYSIS
- FIGURE 31 MARKET SHARE ANALYSIS OF KEY PLAYERS (2022)
- TABLE 225 REGENERATIVE MEDICINE MARKET: DEGREE OF COMPETITION
- 9.5 COMPANY EVALUATION MATRIX
- 9.5.1 STARS
- 9.5.2 EMERGING LEADERS
- 9.5.3 PERVASIVE PLAYERS
- 9.5.4 PARTICIPANTS
- FIGURE 32 REGENERATIVE MEDICINE MARKET: COMPANY EVALUATION MATRIX, 2022
- 9.5.5 COMPANY FOOTPRINT ANALYSIS
- TABLE 226 REGENERATIVE MEDICINE MARKET: PRODUCT FOOTPRINT ANALYSIS OF KEY PLAYERS
- TABLE 227 REGENERATIVE MEDICINE MARKET: REGIONAL FOOTPRINT ANALYSIS OF KEY PLAYERS
- 9.6 START-UP/SME EVALUATION MATRIX
- 9.6.1 PROGRESSIVE COMPANIES
- 9.6.2 RESPONSIVE COMPANIES
- 9.6.3 DYNAMIC COMPANIES
- 9.6.4 STARTING BLOCKS
- FIGURE 33 REGENERATIVE MEDICINE MARKET: START-UP/SME EVALUATION MATRIX, 2022
- 9.6.5 COMPETITIVE BENCHMARKING
- TABLE 228 REGENERATIVE MEDICINE MARKET: DETAILED LIST OF KEY START-UP/SME PLAYERS
- TABLE 229 REGENERATIVE MEDICINE MARKET: COMPETITIVE BENCHMARKING OF START-UP/SME PLAYERS
- 9.7 COMPETITIVE SCENARIO & TRENDS
- 9.7.1 PRODUCT LAUNCHES
- TABLE 230 REGENERATIVE MEDICINE MARKET: PRODUCT APPROVALS/LAUNCHES, JANUARY 2020 TO DECEMBER 2023
- 9.7.2 DEALS
- TABLE 231 REGENERATIVE MEDICINE MARKET: DEALS, JANUARY 2020 TO DECEMBER 2023
- 9.7.3 OTHER DEVELOPMENTS
- TABLE 232 REGENERATIVE MEDICINE MARKET: OTHER DEVELOPMENTS, JANUARY 2020 TO DECEMBER 2023
10 COMPANY PROFILES
- 10.1 KEY MARKET PLAYERS
- (Business Overview, Products Offered, Recent Developments, and MnM View (Key strengths/Right to Win, Strategic Choices Made, and Weaknesses and Competitive Threats))**
- 10.1.1 NOVARTIS AG
- TABLE 233 NOVARTIS AG: COMPANY OVERVIEW
- FIGURE 34 NOVARTIS AG: COMPANY SNAPSHOT (2022)
- 10.1.2 BIOGEN INC.
- TABLE 234 BIOGEN INC.: COMPANY OVERVIEW
- FIGURE 35 BIOGEN INC.: COMPANY SNAPSHOT (2022)
- 10.1.3 GILEAD SCIENCES, INC.
- TABLE 235 GILEAD SCIENCES, INC.: COMPANY OVERVIEW
- FIGURE 36 GILEAD SCIENCES, INC.: COMPANY SNAPSHOT (2022)
- 10.1.4 SAREPTA THERAPEUTICS, INC.
- TABLE 236 SAREPTA THERAPEUTICS, INC.: COMPANY OVERVIEW
- FIGURE 37 SAREPTA THERAPEUTICS: COMPANY SNAPSHOT (2022)
- 10.1.5 AMGEN, INC.
- TABLE 237 AMGEN, INC.: COMPANY OVERVIEW
- FIGURE 38 AMGEN, INC.: COMPANY SNAPSHOT (2022)
- 10.1.6 SMITH+NEPHEW
- TABLE 238 SMITH+NEPHEW: COMPANY OVERVIEW
- FIGURE 39 SMITH+NEPHEW: COMPANY SNAPSHOT (2022)
- 10.1.7 MEDIPOST CO., LTD.
- TABLE 239 MEDIPOST CO., LTD.: COMPANY OVERVIEW
- FIGURE 40 MEDIPOST CO., LTD.: COMPANY SNAPSHOT
- 10.1.8 JCR PHARMACEUTICALS CO., LTD.
- TABLE 240 JCR PHARMACEUTICALS CO., LTD.: COMPANY OVERVIEW
- FIGURE 41 JCR PHARMACEUTICALS CO., LTD.: COMPANY SNAPSHOT (2022)
- 10.1.9 TAKEDA PHARMACEUTICAL COMPANY LIMITED
- TABLE 241 TAKEDA PHARMACEUTICAL COMPANY LIMITED: COMPANY OVERVIEW
- FIGURE 42 TAKEDA PHARMACEUTICAL COMPANY LIMITED: COMPANY SNAPSHOT (2022)
- 10.1.10 CORESTEM, INC.
- TABLE 242 CORESTEM, INC.: COMPANY OVERVIEW
- FIGURE 43 CORESTEM, INC.: COMPANY SNAPSHOT (2021)
- 10.1.11 VERICEL CORPORATION
- TABLE 243 VERICEL CORPORATION: COMPANY OVERVIEW
- FIGURE 44 VERICEL CORPORATION: COMPANY SNAPSHOT (2022)
- 10.1.12 MIMEDX GROUP, INC.
- TABLE 244 MIMEDX GROUP, INC.: COMPANY OVERVIEW
- FIGURE 45 MIMEDX GROUP, INC.: COMPANY SNAPSHOT (2022)
- 10.1.13 ORGANOGENESIS INC.
- TABLE 245 ORGANOGENESIS INC.: COMPANY OVERVIEW
- FIGURE 46 ORGANOGENESIS INC.: COMPANY SNAPSHOT (2022)
- 10.1.14 MEDTRONIC
- TABLE 246 MEDTRONIC: COMPANY OVERVIEW
- FIGURE 47 MEDTRONIC: COMPANY SNAPSHOT (2022)
- 10.1.15 BRISTOL-MYERS SQUIBB COMPANY
- TABLE 247 BRISTOL-MYERS SQUIBB COMPANY: COMPANY OVERVIEW
- FIGURE 48 BRISTOL-MYERS SQUIBB COMPANY: COMPANY SNAPSHOT (2022)
- 10.2 OTHER PLAYERS
- 10.2.1 ORTHOCELL LTD.
- TABLE 248 ORTHOCELL LTD.: COMPANY OVERVIEW
- 10.2.2 MESOBLAST LTD.
- TABLE 249 MESOBLAST LTD.: COMPANY OVERVIEW
- 10.2.3 BIORESTORATIVE THERAPIES, INC.
- TABLE 250 BIORESTORATIVE THERAPIES, INC.: COMPANY OVERVIEW
- 10.2.4 PLURISTEM THERAPEUTICS INC.
- TABLE 251 PLURISTEM THERAPEUTICS INC.: COMPANY OVERVIEW
- 10.2.5 TEGOSCIENCE
- TABLE 252 TEGOSCIENCE: COMPANY OVERVIEW
- 10.2.6 ANTEROGEN.CO.,LTD.
- TABLE 253 ANTEROGEN.CO.,LTD.: COMPANY OVERVIEW
- 10.2.7 BLUEBIRD BIO, INC.
- TABLE 254 BLUEBIRD BIO, INC.: COMPANY OVERVIEW
- 10.2.8 STEMPEUTICS RESEARCH PVT. LTD.
- TABLE 255 STEMPEUTICS RESEARCH PVT. LTD.: COMPANY OVERVIEW
- 10.2.9 SIBIONO GENETECH CO. LTD.
- TABLE 256 SIBIONO GENETECH CO. LTD.: COMPANY OVERVIEW
- 10.2.10 ASPECT BIOSYSTEMS
- TABLE 257 ASPECT BIOSYSTEMS: COMPANY OVERVIEW
- TABLE 258 ATHERSYS, INC.: COMPANY OVERVIEW
- *Details on Business Overview, Products Offered, Recent Developments, and MnM View (Key strengths/Right to Win, Strategic Choices Made, and Weaknesses and Competitive Threats) might not be captured in case of unlisted companies.
11 APPENDIX
- 11.1 DISCUSSION GUIDE
- 11.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 11.3 CUSTOMIZATION OPTIONS
- 11.4 RELATED REPORTS
- 11.5 AUTHOR DETAILS