|
|
市場調査レポート
商品コード
1769085
in situハイブリダイゼーションの世界市場:オファリング別、技術別、用途別、エンドユーザー別、地域別 - 2030年までの予測In Situ Hybridization Market by Offering, Technology (FISH, CISH), Application, End User - Global Forecast to 2030 |
||||||
カスタマイズ可能
|
|||||||
| in situハイブリダイゼーションの世界市場:オファリング別、技術別、用途別、エンドユーザー別、地域別 - 2030年までの予測 |
|
出版日: 2025年07月08日
発行: MarketsandMarkets
ページ情報: 英文 460 Pages
納期: 即納可能
|
全表示
- 概要
- 図表
- 目次
in situハイブリダイゼーションの市場規模は、予測期間中に7.4%のCAGRで拡大し、2025年の16億4,000万米ドルから2030年には23億5,000万米ドルに達すると予測されています。
in situハイブリダイゼーション(ISH)市場の拡大は、ISHプラットフォームにおける継続的な技術進歩が大きな原動力となっています。強化されたプローブ設計、自動化、多重化機能などの革新は、アッセイの感度、特異性、スループットを著しく向上させています。このような機能強化は、精密医療へのニーズの高まりと臨床検査室開発検査(LDT)の開発に合致しています。このような進歩は、患者固有の遺伝子マーカーの同定を容易にし、個別化治療アプローチにおける重要な要素としてのISHの役割を確固たるものにしています。さらに、がんや神経科学の研究において、空間トランスクリプトミクスの分野が台頭してきており、遺伝子発現を組織の状況に合わせて解析する高感度で多重化されたISH技術の必要性が強調されています。この需要は、ISH分野における継続的な技術革新を後押ししています。特に、ISHは免疫組織化学(IHC)よりも、直接核酸を検出する能力が高いという明確な利点があり、診断の正確性と疾患の特徴付けを高め、市場の成長にさらに貢献しています。
| 調査範囲 | |
|---|---|
| 調査対象年 | 2023年~2030年 |
| 基準年 | 2024年 |
| 予測期間 | 2025年~2030年 |
| 検討単位 | 金額(10億米ドル) |
| セグメント | オファリング別、技術別、用途別、エンドユーザー別、地域別 |
| 対象地域 | 北米、欧州、アジア太平洋、ラテンアメリカ、中東・アフリカ |
技術別のin situハイブリダイゼーション市場は、蛍光in situハイブリダイゼーションと発色in situハイブリダイゼーションに区分されます。蛍光in situハイブリダイゼーション技術はさらに、DNA蛍光in situハイブリダイゼーション、RNA蛍光in situハイブリダイゼーション、PNA蛍光in situハイブリダイゼーションに区分されます。2024年には、蛍光in situハイブリダイゼーション技術分野が技術別で最大の市場シェアを占めました。この技術分野が支配的な地位を占めているのは、DNAとRNAの両レベルで遺伝子異常を検出する際の卓越した感度、特異性、多用途性に起因しています。蛍光in situハイブリダイゼーション(FISH)は、染色体再配列、遺伝子増幅、欠失を同定するための高解像度の洞察を提供し、がん診断、遺伝性疾患の同定、出生前スクリーニングにおいて不可欠なものとなっています。FISHは、さまざまな蛍光プローブを使用することにより、複数のターゲットを同時に検出することができ、臨床と研究の両方のニーズに応えることができます。さらに、個別化医療、特に腫瘍学におけるHER2およびALKの評価においてFISHが広く取り入れられていることが、近年その需要を大幅に強化しています。
in-situハイブリダイゼーション市場は、消耗品、機器、サービス・ソフトウェアに区分されます。消耗品セグメントはさらに、キット・試薬、プローブ・プローブキット、アクセサリー・その他に細分化されます。消耗品セグメントは、予測期間中に最も高い成長率を記録すると予想されています。in situハイブリダイゼーション(ISH)の消耗品市場の拡大は、基本的にがん診断やその他の様々な疾患領域における用途の増加と関連しています。このような利用の増加は、すべてのISHアッセイに不可欠なプローブやプローブキットを含む必須消耗品に対する需要の高まりと直結しています。臨床と研究の両分野で検査量が増加するにつれて、これらの消耗品の需要は着実に伸びています。さらに、ロシュのVENTANA KappaやLambda Dual ISH mRNA Probe Cocktailアッセイなど、ISHに基づく先進的な診断検査の開発と承認が進んでおり、消耗品市場のさらなる拡大が見込まれています。このような技術革新は、B細胞性悪性腫瘍などの診断の正確性と特異性を高めるため、検査機関や医療施設はISH手法をますます検査のレパートリーに取り入れるようになります。このような臨床への取り込みの増加傾向は、対応する消耗品への需要を一貫して促進し、この分野の堅調な成長を下支えします。さらに、業界主要企業にとっては、革新的な製品開発と戦略的サプライチェーンの強化を通じて市場での存在感を高める大きな機会となります。
アジア太平洋は、予測期間を通じてin situハイブリダイゼーション(ISH)市場で最も高い成長率を記録すると予測されています。この成長の原動力は、ヘルスケアインフラの大幅な進歩、政府機関と民間セクターの利害関係者の両方による分子診断と精密医療への投資の高まりです。中国、インド、日本、韓国を含む主要国は、ターゲットを絞った国家プログラムや資金調達メカニズムを通じて、ゲノミクスや個別化医療の研究イニシアチブを積極的に推進しています。同時に、がん、感染症、遺伝性疾患などの慢性疾患の蔓延が進み、ISHのような高度な診断技術に対する需要が高まっています。地域診断企業の急成長は、世界企業との戦略的パートナーシップと相まって、市場アクセスをさらに促進し、技術採用を強化しています。
当レポートでは、世界のin situハイブリダイゼーション市場について調査し、オファリング別、技術別、用途別、エンドユーザー別、地域別動向、および市場に参入する企業のプロファイルなどをまとめています。
目次
第1章 イントロダクション
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 重要考察
第5章 市場概要
- イントロダクション
- 市場力学
- 顧客のビジネスに影響を与える動向/混乱
- バリューチェーン分析
- エコシステム分析
- 技術分析
- 特許分析
- 2025年~2026年の主な会議とイベント
- ポーターのファイブフォース分析
- 主要な利害関係者と購入基準
- 価格分析
- 投資と資金調達のシナリオ
- 規制状況
- AI/生成AIがin situハイブリダイゼーション市場に与える影響
- 2025年の米国関税がin situハイブリダイゼーション市場に与える影響
第6章 in situハイブリダイゼーション市場(オファリング別)
- イントロダクション
- 消耗品
- 機器
- ソフトウェア
- サービス
第7章 in situハイブリダイゼーション市場(技術別)
- イントロダクション
- 蛍光in situハイブリダイゼーション(FISH)
- クロモジェニックin situハイブリダイゼーション(CISH)
第8章 in situハイブリダイゼーション市場(用途別)
- イントロダクション
- 臨床診断
- 研究
- 創薬・開発
第9章 in situハイブリダイゼーション市場(エンドユーザー別)
- イントロダクション
- 病院および診断検査室
- 学術研究機関
- 製薬・バイオテクノロジー企業
- CRO
第10章 in situハイブリダイゼーション市場(地域別)
- イントロダクション
- 北米
- 北米のマクロ経済分析
- 米国
- カナダ
- 欧州
- 欧州のマクロ経済分析
- ドイツ
- 英国
- フランス
- イタリア
- スペイン
- その他
- アジア太平洋
- アジア太平洋のマクロ経済分析
- 中国
- 日本
- インド
- オーストラリア
- 韓国
- その他
- ラテンアメリカ
- ラテンアメリカのマクロ経済分析
- ブラジル
- メキシコ
- その他
- 中東
- 中東のマクロ経済分析
- GCC諸国
- その他のGCC諸国
- その他
- アフリカ
- ゲノミクスインフラと分子診断の需要増加が市場成長を促進
- アフリカのマクロ経済分析
第11章 競合情勢
- イントロダクション
- 主要参入企業の戦略/強み
- 収益分析、2020年~2024年
- 市場シェア分析、2024年
- 企業評価マトリックス:主要参入企業、2024年
- 企業評価マトリックス:スタートアップ/中小企業、2024年
- 企業評価と財務指標
- ブランド/製品比較
- 競合シナリオ
第12章 企業プロファイル
- 主要参入企業
- F. HOFFMANN-LA ROCHE LTD.
- DANAHER
- ABBOTT
- THERMO FISHER SCIENTIFIC INC.
- BIO-TECHNE
- AGILENT TECHNOLOGIES, INC.
- QIAGEN
- BIOCARE MEDICAL, LLC
- BIOVIEW
- CREATIVE BIOARRAY
- ABNOVA CORPORATION
- ZYTOMICS
- ENZO BIOCHEM INC.
- CYTOTEST INC.
- GENEMED BIOTECHNOLOGIES, INC.
- その他の企業
- MOLECULAR INSTRUMENTS, INC.
- OXFORD GENE TECHNOLOGY IP LIMITED
- HISTO-LINE LABORATORIES
- METASYSTEMS
- BIOGENEX
- LGC BIOSEARCH TECHNOLOGIES
- BIODISCOVERY LLC
- KANEKA EUROGENTEC S.A.
- LIFE TECHNOLOGIES(INDIA)PVT. LTD.
- GENECOPOEIA, INC.
第13章 付録
List of Tables
- TABLE 1 IN SITU HYBRIDIZATION MARKET: INCLUSIONS & EXCLUSIONS
- TABLE 2 IN SITU HYBRIDIZATION MARKET: IMPACT ANALYSIS OF SUPPLY- AND DEMAND-SIDE FACTORS
- TABLE 3 IN SITU HYBRIDIZATION MARKET: RISK ANALYSIS
- TABLE 4 IN SITU HYBRIDIZATION MARKET: IMPACT ANALYSIS OF DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- TABLE 5 IN SITU HYBRIDIZATION MARKET: ROLE IN ECOSYSTEM
- TABLE 6 IN SITU HYBRIDIZATION MARKET: DETAILED ANALYSIS OF KEY PATENTS, 2024
- TABLE 7 IN SITU HYBRIDIZATION MARKET: DETAILED LIST OF KEY CONFERENCES & EVENTS, JANUARY 2025-DECEMBER 2026
- TABLE 8 IN SITU HYBRIDIZATION MARKET: PORTER'S FIVE FORCES
- TABLE 9 INFLUENCE OF KEY STAKEHOLDERS ON BUYING PROCESS OF IN SITU HYBRIDIZATION-BASED OFFERINGS
- TABLE 10 KEY BUYING CRITERIA, BY END USER
- TABLE 11 AVERAGE SELLING PRICE TREND OF IN SITU HYBRIDIZATION PRODUCTS, BY KEY PLAYER, 2022-2024 (USD)
- TABLE 12 AVERAGE SELLING PRICE TREND OF IN SITU HYBRIDIZATION CONSUMABLES, BY REGION, 2022-2024 (USD)
- TABLE 13 NORTH AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 14 EUROPE: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 15 ASIA PACIFIC: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 16 REST OF THE WORLD: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 17 US ADJUSTED RECIPROCAL TARIFF RATES
- TABLE 18 IN SITU HYBRIDIZATION PRODUCTS-RELATED TARIFF REVISION
- TABLE 19 IMPACT ON END-USE APPLICATIONS OF IN SITU HYBRIDIZATION PRODUCTS
- TABLE 20 IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 21 IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 22 NORTH AMERICA: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 23 EUROPE: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 24 ASIA PACIFIC: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 25 LATIN AMERICA: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 26 MIDDLE EAST: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 27 GCC COUNTRIES: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 28 IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 29 KITS & REAGENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 30 NORTH AMERICA: KITS & REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 31 EUROPE: KITS & REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 32 ASIA PACIFIC: KITS & REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 33 LATIN AMERICA: KITS & REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 34 MIDDLE EAST: KITS & REAGENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 35 GCC COUNTRIES: KITS & REAGENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 36 PROBE & PROBE KITS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 37 NORTH AMERICA: PROBE & PROBE KITS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 38 EUROPE: PROBE & PROBE KITS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 39 ASIA PACIFIC: PROBE & PROBE KITS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 40 LATIN AMERICA: PROBE & PROBE KITS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 41 MIDDLE EAST: PROBE & PROBE KITS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 42 GCC COUNTRIES: PROBE & PROBE KITS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 43 ACCESSORIES & OTHER CONSUMABLES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 44 NORTH AMERICA: ACCESSORIES & OTHER CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 45 EUROPE: ACCESSORIES & OTHER CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 46 ASIA PACIFIC: ACCESSORIES & OTHER CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 47 LATIN AMERICA: ACCESSORIES & OTHER CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 48 MIDDLE EAST: ACCESSORIES & OTHER CONSUMABLES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 49 GCC COUNTRIES: ACCESSORIES & OTHER CONSUMABLES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 50 IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 51 NORTH AMERICA: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 52 EUROPE: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 53 ASIA PACIFIC: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 54 LATIN AMERICA: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 55 MIDDLE EAST: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 56 GCC COUNTRIES: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 57 IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 58 AUTOMATED IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 59 NORTH AMERICA: AUTOMATED IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 60 EUROPE: AUTOMATED IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 61 ASIA PACIFIC: AUTOMATED IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 62 LATIN AMERICA: AUTOMATED IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 63 MIDDLE EAST: AUTOMATED IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 64 GCC COUNTRIES: AUTOMATED IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 65 MANUAL IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 66 NORTH AMERICA: MANUAL IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 67 EUROPE: MANUAL IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 68 ASIA PACIFIC: MANUAL IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 69 LATIN AMERICA: MANUAL IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 70 MIDDLE EAST: MANUAL IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 71 GCC COUNTRIES: MANUAL IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 72 IN SITU HYBRIDIZATION SOFTWARE MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 73 NORTH AMERICA: IN SITU HYBRIDIZATION SOFTWARE MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 74 EUROPE: IN SITU HYBRIDIZATION SOFTWARE MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 75 ASIA PACIFIC: IN SITU HYBRIDIZATION SOFTWARE MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 76 LATIN AMERICA: IN SITU HYBRIDIZATION SOFTWARE MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 77 MIDDLE EAST: IN SITU HYBRIDIZATION SOFTWARE MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 78 GCC COUNTRIES: IN SITU HYBRIDIZATION SOFTWARE MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 79 IN SITU HYBRIDIZATION SERVICES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 80 NORTH AMERICA: IN SITU HYBRIDIZATION SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 81 EUROPE: IN SITU HYBRIDIZATION SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 82 ASIA PACIFIC: IN SITU HYBRIDIZATION SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 83 LATIN AMERICA: IN SITU HYBRIDIZATION SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 84 MIDDLE EAST: IN SITU HYBRIDIZATION SERVICES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 85 GCC COUNTRIES: IN SITU HYBRIDIZATION SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 86 IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 87 DATA INTERPRETATION SERVICES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 88 NORTH AMERICA: DATA INTERPRETATION SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 89 EUROPE: DATA INTERPRETATION SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 90 ASIA PACIFIC: DATA INTERPRETATION SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 91 LATIN AMERICA: DATA INTERPRETATION SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 92 MIDDLE EAST: DATA INTERPRETATION SERVICES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 93 GCC COUNTRIES: DATA INTERPRETATION SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 94 CUSTOM PROBE DESIGN SERVICES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 95 NORTH AMERICA: CUSTOM PROBE DESIGN SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 96 EUROPE: CUSTOM PROBE DESIGN SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 97 ASIA PACIFIC: CUSTOM PROBE DESIGN SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 98 LATIN AMERICA: CUSTOM PROBE DESIGN SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 99 MIDDLE EAST: CUSTOM PROBE DESIGN SERVICES MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 100 GCC COUNTRES: CUSTOM PROBE DESIGN SERVICES MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 101 IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 102 FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 103 NORTH AMERICA: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 104 EUROPE: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 105 ASIA PACIFIC: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 106 LATIN AMERICA: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 107 MIDDLE EAST: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 108 GCC COUNTRIES: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 109 FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 110 DNA FLUORESCENCE IN SITU HYBRIDIZATION (DNA-FISH) MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 111 NORTH AMERICA: DNA FLUORESCENCE IN SITU HYBRIDIZATION (DNA-FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 112 EUROPE: DNA FLUORESCENCE IN SITU HYBRIDIZATION (DNA-FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 113 ASIA PACIFIC: DNA FLUORESCENCE IN SITU HYBRIDIZATION (DNA-FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 114 LATIN AMERICA: DNA FLUORESCENCE IN SITU HYBRIDIZATION (DNA-FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 115 MIDDLE EAST: DNA FLUORESCENCE IN SITU HYBRIDIZATION (DNA-FISH) MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 116 GCC COUNTRIES: DNA FLUORESCENCE IN SITU HYBRIDIZATION (DNA-FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
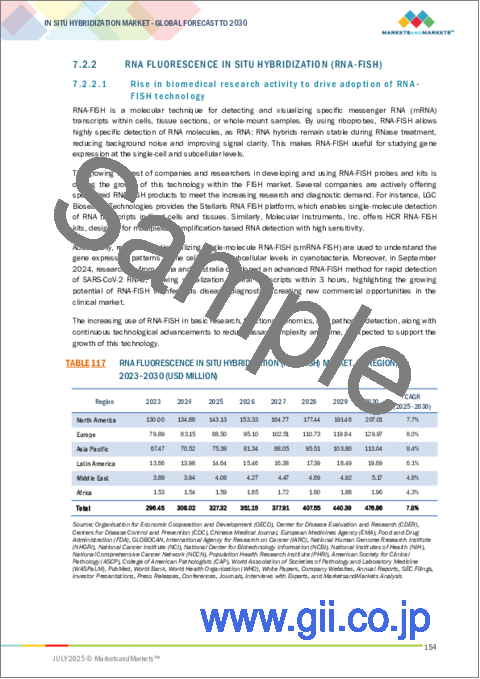
- TABLE 117 RNA FLUORESCENCE IN SITU HYBRIDIZATION (RNA-FISH) MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 118 NORTH AMERICA: RNA FLUORESCENCE IN SITU HYBRIDIZATION (RNA-FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 119 EUROPE: RNA FLUORESCENCE IN SITU HYBRIDIZATION (RNA-FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 120 ASIA PACIFIC: RNA FLUORESCENCE IN SITU HYBRIDIZATION (RNA-FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 121 LATIN AMERICA: RNA FLUORESCENCE IN SITU HYBRIDIZATION (RNA-FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 122 MIDDLE EAST: RNA FLUORESCENCE IN SITU HYBRIDIZATION (RNA-FISH) MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 123 GCC COUNTRIES: RNA FLUORESCENCE IN SITU HYBRIDIZATION (RNA-FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 124 PNA FLUORESCENCE IN SITU HYBRIDIZATION (PNA-FISH) MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 125 NORTH AMERICA: PNA FLUORESCENCE IN SITU HYBRIDIZATION (PNA-FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 126 EUROPE: PNA FLUORESCENCE IN SITU HYBRIDIZATION (PNA-FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 127 ASIA PACIFIC: PNA FLUORESCENCE IN SITU HYBRIDIZATION (PNA-FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 128 LATIN AMERICA: PNA FLUORESCENCE IN SITU HYBRIDIZATION (PNA-FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 129 MIDDLE EAST: PNA FLUORESCENCE IN SITU HYBRIDIZATION (PNA-FISH) MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 130 GCC COUNTRIES: PNA FLUORESCENCE IN SITU HYBRIDIZATION (PNA-FISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 131 CHROMOGENIC IN SITU HYBRIDIZATION (CISH) MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 132 NORTH AMERICA: CHROMOGENIC IN SITU HYBRIDIZATION (CISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 133 EUROPE: CHROMOGENIC IN SITU HYBRIDIZATION (CISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 134 ASIA PACIFIC: CHROMOGENIC IN SITU HYBRIDIZATION (CISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 135 LATIN AMERICA: CHROMOGENIC IN SITU HYBRIDIZATION (CISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 136 MIDDLE EAST: CHROMOGENIC IN SITU HYBRIDIZATION (CISH) MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 137 GCC COUNTRIES: CHROMOGENIC IN SITU HYBRIDIZATION (CISH) MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 138 IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 139 IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 140 NORTH AMERICA: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 141 EUROPE: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 142 ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 143 LATIN AMERICA: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 144 MIDDLE EAST: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 145 GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 146 IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 147 CANCER DIAGNOSTICS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 148 NORTH AMERICA: CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 149 EUROPE: CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 150 ASIA PACIFIC: CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 151 LATIN AMERICA: CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 152 MIDDLE EAST: CANCER DIAGNOSTICS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 153 GCC COUNTRIES: CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 154 INFECTIOUS DISEASE DIAGNOTICS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 155 NORTH AMERICA: INFECTIOUS DISEASE DIAGNOTICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 156 EUROPE: INFECTIOUS DISEASE DIAGNOTICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 157 ASIA PACIFIC: INFECTIOUS DISEASE DIAGNOTICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 158 LATIN AMERICA: INFECTIOUS DISEASE DIAGNOTICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 159 MIDDLE EAST: INFECTIOUS DISEASE DIAGNOTICS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 160 GCC COUNTRIES: INFECTIOUS DISEASE DIAGNOTICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 161 GENETIC DISEASE DIAGNOSTICS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 162 NORTH AMERICA: GENETIC DISEASE DIAGNOSTICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 163 EUROPE: GENETIC DISEASE DIAGNOSTICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 164 ASIA PACIFIC: GENETIC DISEASE DIAGNOSTICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 165 LATIN AMERICA: GENETIC DISEASE DIAGNOSTICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 166 MIDDLE EAST: GENETIC DISEASE DIAGNOSTICS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 167 GCC COUNTRIES: GENETIC DISEASE DIAGNOSTICS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 168 OTHER CLINCAL DIAGNOSTIC APPLICATIONS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 169 NORTH AMERICA: OTHER CLINCAL DIAGNOSTIC APPLICATIONS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 170 EUROPE: OTHER CLINCAL DIAGNOSTIC APPLICATIONS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 171 ASIA PACIFIC: OTHER CLINCAL DIAGNOSTIC APPLICATIONS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 172 LATIN AMERICA: OTHER CLINCAL DIAGNOSTIC APPLICATIONS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 173 MIDDLE EAST: OTHER CLINCAL DIAGNOSTIC APPLICATIONS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 174 GCC COUNTRIES: OTHER CLINCAL DIAGNOSTIC APPLICATIONS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 175 IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 176 NORTH AMERICA: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 177 EUROPE: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 178 ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 179 LATIN AMERICA: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 180 MIDDLE EAST: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 181 GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 182 IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 183 NEUROSCIENCE RESEARCH MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 184 NORTH AMERICA: NEUROSCIENCE RESEARCH MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 185 EUROPE: NEUROSCIENCE RESEARCH MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 186 ASIA PACIFIC: NEUROSCIENCE RESEARCH MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 187 LATIN AMERICA: NEUROSCIENCE RESEARCH MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 188 MIDDLE EAST: NEUROSCIENCE RESEARCH MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 189 GCC COUNTRIES: NEUROSCIENCE RESEARCH MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 190 IMMUNOLOGY RESEARCH MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 191 NORTH AMERICA: IMMUNOLOGY RESEARCH MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 192 EUROPE: IMMUNOLOGY RESEARCH MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 193 ASIA PACIFIC: IMMUNOLOGY RESEARCH MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 194 LATIN AMERICA: IMMUNOLOGY RESEARCH MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 195 MIDDLE EAST: IMMUNOLOGY RESEARCH MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 196 GCC COUNTRIES: IMMUNOLOGY RESEARCH MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 197 OTHER RESEARCH APPLICATIONS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 198 NORTH AMERICA: OTHER RESEARCH APPLICATIONS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 199 EUROPE: OTHER RESEARCH APPLICATIONS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 200 ASIA PACIFIC: OTHER RESEARCH APPLICATIONS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 201 LATIN AMERICA: OTHER RESEARCH APPLICATIONS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 202 MIDDLE EAST: OTHER RESEARCH APPLICATIONS MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 203 GCC COUNTRIES: OTHER RESEARCH APPLICATIONS MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 204 IN SITU HYBRIDIZATION MARKET FOR DRUG DISCOVERY & DEVELOPMENT, BY REGION, 2023-2030 (USD MILLION)
- TABLE 205 NORTH AMERICA: IN SITU HYBRIDIZATION MARKET FOR DRUG DISCOVERY & DEVELOPMENT, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 206 EUROPE: IN SITU HYBRIDIZATION MARKET FOR DRUG DISCOVERY & DEVELOPMENT, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 207 ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET FOR DRUG DISCOVERY & DEVELOPMENT, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 208 LATIN AMERICA: IN SITU HYBRIDIZATION MARKET FOR DRUG DISCOVERY & DEVELOPMENT, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 209 MIDDLE EAST: IN SITU HYBRIDIZATION MARKET FOR DRUG DISCOVERY & DEVELOPMENT, BY REGION, 2023-2030 (USD MILLION)
- TABLE 210 GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET FOR DRUG DISCOVERY & DEVELOPMENT, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 211 IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 212 IN SITU HYBRIDIZATION MARKET FOR HOSPITALS & DIAGNOSTIC LABORATORIES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 213 NORTH AMERICA: IN SITU HYBRIDIZATION MARKET FOR HOSPITALS & DIAGNOSTIC LABORATORIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 214 EUROPE: IN SITU HYBRIDIZATION MARKET FOR HOSPITALS & DIAGNOSTIC LABORATORIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 215 ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET FOR HOSPITALS & DIAGNOSTIC LABORATORIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 216 LATIN AMERICA: IN SITU HYBRIDIZATION MARKET FOR HOSPITALS & DIAGNOSTIC LABORATORIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 217 MIDDLE EAST: IN SITU HYBRIDIZATION MARKET FOR HOSPITALS & DIAGNOSTIC LABORATORIES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 218 GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET FOR HOSPITALS & DIAGNOSTIC LABORATORIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 219 IN SITU HYBRIDIZATION MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 220 NORTH AMERICA: IN SITU HYBRIDIZATION MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 221 EUROPE: IN SITU HYBRIDIZATION MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 222 ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 223 LATIN AMERICA: IN SITU HYBRIDIZATION MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 224 MIDDLE EAST: IN SITU HYBRIDIZATION MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 225 GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET FOR ACADEMIC & RESEARCH INSTITUTES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 226 IN SITU HYBRIDIZATION MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 227 NORTH AMERICA: IN SITU HYBRIDIZATION MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 228 EUROPE: IN SITU HYBRIDIZATION MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 229 ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 230 LATIN AMERICA: IN SITU HYBRIDIZATION MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 231 MIDDLE EAST: IN SITU HYBRIDIZATION MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY REGION, 2023-2030 (USD MILLION)
- TABLE 232 GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET FOR PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 233 IN SITU HYBRIDIZATION MARKET FOR CONTRACT RESEARCH ORGANIZATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 234 NORTH AMERICA: IN SITU HYBRIDIZATION MARKET FOR CONTRACT RESEARCH ORGANIZATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 235 EUROPE: IN SITU HYBRIDIZATION MARKET FOR CONTRACT RESEARCH ORGANIZATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 236 ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET FOR CONTRACT RESEARCH ORGANIZATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 237 LATIN AMERICA: IN SITU HYBRIDIZATION MARKET FOR CONTRACT RESEARCH ORGANIZATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 238 MIDDLE EAST: IN SITU HYBRIDIZATION MARKET FOR CONTRACT RESEARCH ORGANIZATIONS, BY REGION, 2023-2030 (USD MILLION)
- TABLE 239 GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET FOR CONTRACT RESEARCH ORGANIZATIONS, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 240 IN SITU HYBRIDIZATION MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 241 NORTH AMERICA: KEY MACROINDICATORS
- TABLE 242 NORTH AMERICA: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 243 NORTH AMERICA: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 244 NORTH AMERICA: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 245 NORTH AMERICA: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 246 NORTH AMERICA: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 247 NORTH AMERICA: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 248 NORTH AMERICA: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 249 NORTH AMERICA: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 250 NORTH AMERICA: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 251 NORTH AMERICA: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 252 NORTH AMERICA: IN SITU HYBRIDIZATION MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 253 US: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 254 US: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 255 US: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 256 US: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 257 US: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 258 US: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 259 US: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 260 US: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 261 US: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 262 US: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 263 CANADA: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 264 CANADA: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 265 CANADA: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 266 CANADA: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 267 CANADA: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 268 CANADA: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 269 CANADA: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 270 CANADA: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 271 CANADA: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 272 CANADA: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 273 EUROPE: KEY MACROINDICATORS
- TABLE 274 EUROPE: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 275 EUROPE: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 276 EUROPE: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 277 EUROPE: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 278 EUROPE: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 279 EUROPE: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 280 EUROPE: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 281 EUROPE: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 282 EUROPE: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 283 EUROPE: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 284 EUROPE: IN SITU HYBRIDIZATION MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 285 GERMANY: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 286 GERMANY: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 287 GERMANY: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 288 GERMANY: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 289 GERMANY: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 290 GERMANY: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 291 GERMANY: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 292 GERMANY: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 293 GERMANY: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 294 GERMANY: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 295 UK: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 296 UK: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 297 UK: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 298 UK: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 299 UK: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 300 UK: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 301 UK: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 302 UK: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 303 UK: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 304 UK: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 305 FRANCE: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 306 FRANCE: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 307 FRANCE: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 308 FRANCE: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 309 FRANCE: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 310 FRANCE: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 311 FRANCE: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 312 FRANCE: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 313 FRANCE: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 314 FRANCE: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 315 ITALY: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 316 ITALY: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 317 ITALY: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 318 ITALY: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 319 ITALY: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 320 ITALY: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 321 ITALY: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 322 ITALY: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 323 ITALY: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 324 ITALY: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 325 SPAIN: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 326 SPAIN: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 327 SPAIN: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 328 SPAIN: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 329 SPAIN: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 330 SPAIN: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 331 SPAIN: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 332 SPAIN: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 333 SPAIN: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 334 SPAIN: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 335 REST OF EUROPE: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 336 REST OF EUROPE: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 337 REST OF EUROPE: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 338 REST OF EUROPE: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 339 REST OF EUROPE: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 340 REST OF EUROPE: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 341 REST OF EUROPE: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 342 REST OF EUROPE: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 343 REST OF EUROPE: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 344 REST OF EUROPE: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 345 ASIA PACIFIC: KEY MACROINDICATORS
- TABLE 346 ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 347 ASIA PACIFIC: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 348 ASIA PACIFIC: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 349 ASIA PACIFIC: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 350 ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 351 ASIA PACIFIC: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 352 ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 353 ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 354 ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 355 ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 356 ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 357 CHINA: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 358 CHINA: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 359 CHINA: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 360 CHINA: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 361 CHINA: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 362 CHINA: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 363 CHINA: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 364 CHINA: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 365 CHINA: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 366 CHINA: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 367 JAPAN: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 368 JAPAN: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 369 JAPAN: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 370 JAPAN: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 371 JAPAN: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 372 JAPAN: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 373 JAPAN: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 374 JAPAN: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 375 JAPAN: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 376 JAPAN: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 377 INDIA: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 378 INDIA: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 379 INDIA: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 380 INDIA: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 381 INDIA: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 382 INDIA: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 383 INDIA: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 384 INDIA: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 385 INDIA: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 386 INDIA: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 387 AUSTRALIA: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 388 AUSTRALIA: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 389 AUSTRALIA: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 390 AUSTRALIA: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 391 AUSTRALIA: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 392 AUSTRALIA: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 393 AUSTRALIA: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 394 AUSTRALIA: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 395 AUSTRALIA: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 396 AUSTRALIA: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 397 SOUTH KOREA: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 398 SOUTH KOREA: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 399 SOUTH KOREA: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 400 SOUTH KOREA: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 401 SOUTH KOREA: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 402 SOUTH KOREA: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 403 SOUTH KOREA: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 404 SOUTH KOREA: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 405 SOUTH KOREA: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 406 SOUTH KOREA: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 407 REST OF ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 408 REST OF ASIA PACIFIC: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 409 REST OF ASIA PACIFIC: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 410 REST OF ASIA PACIFIC: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 411 REST OF ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 412 REST OF ASIA PACIFIC: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 413 REST OF ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 414 REST OF ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 415 REST OF ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 416 REST OF ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 417 LATIN AMERICA: KEY MACROINDICATORS
- TABLE 418 LATIN AMERICA: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 419 LATIN AMERICA: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 420 LATIN AMERICA: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 421 LATIN AMERICA: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 422 LATIN AMERICA: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 423 LATIN AMERICA: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 424 LATIN AMERICA: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 425 LATIN AMERICA: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 426 LATIN AMERICA: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 427 LATIN AMERICA: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 428 LATIN AMERICA: IN SITU HYBRIDIZATION MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 429 BRAZIL: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 430 BRAZIL: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 431 BRAZIL: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 432 BRAZIL: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 433 BRAZIL: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 434 BRAZIL: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 435 BRAZIL: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 436 BRAZIL: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 437 BRAZIL: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 438 BRAZIL: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 439 MEXICO: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 440 MEXICO: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 441 MEXICO: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 442 MEXICO: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 443 MEXICO: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 444 MEXICO: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 445 MEXICO: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 446 MEXICO: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 447 MEXICO: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 448 MEXICO: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 449 REST OF LATIN AMERICA: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 450 REST OF LATIN AMERICA: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 451 REST OF LATIN AMERICA: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 452 REST OF LATIN AMERICA: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 453 REST OF LATIN AMERICA: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 454 REST OF LATIN AMERICA: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 455 REST OF LATIN AMERICA: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 456 REST OF LATIN AMERICA: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 457 REST OF LATIN AMERICA: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 458 REST OF LATIN AMERICA: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 459 MIDDLE EAST: KEY MACROINDICATORS
- TABLE 460 MIDDLE EAST: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 461 MIDDLE EAST: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 462 MIDDLE EAST: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 463 MIDDLE EAST: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 464 MIDDLE EAST: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 465 MIDDLE EAST: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 466 MIDDLE EAST: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 467 MIDDLE EAST: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 468 MIDDLE EAST: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 469 MIDDLE EAST: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 470 MIDDLE EAST: IN SITU HYBRIDIZATION MARKET, BY REGION, 2023-2030 (USD MILLION)
- TABLE 471 GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 472 GCC COUNTRIES: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 473 GCC COUNTRIES: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 474 GCC COUNTRIES: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 475 GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 476 GCC COUNTRIES: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 477 GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 478 GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 479 GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 480 GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 481 GCC: IN SITU HYBRIDIZATION MARKET, BY COUNTRY, 2023-2030 (USD MILLION)
- TABLE 482 KINGDOM OF SAUDI ARABIA: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 483 KINGDOM OF SAUDI ARABIA: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 484 KINGDOM OF SAUDI ARABIA: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 485 KINGDOM OF SAUDI ARABIA: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 486 KINGDOM OF SAUDI ARABIA: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 487 KINGDOM OF SAUDI ARABIA: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 488 KINGDOM OF SAUDI ARABIA: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 489 KINGDOM OF SAUDI ARABIA: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 490 KINGDOM OF SAUDI ARABIA: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 491 KINGDOM OF SAUDI ARABIA: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 492 UAE: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 493 UAE: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 494 UAE: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 495 UAE: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 496 UAE: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 497 UAE: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 498 UAE: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 499 UAE: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 500 UAE: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 501 UAE: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 502 REST OF GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 503 REST OF GCC COUNTRIES: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 504 REST OF GCC COUNTRIES: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 505 REST OF GCC COUNTRIES: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 506 REST OF GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 507 REST OF GCC COUNTRIES: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 508 REST OF GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 509 REST OF GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 510 REST OF GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 511 REST OF GCC COUNTRIES: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 512 REST OF MIDDLE EAST: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 513 REST OF MIDDLE EAST: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 514 REST OF MIDDLE EAST: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 515 REST OF MIDDLE EAST: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 516 REST OF MIDDLE EAST: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 517 REST OF MIDDLE EAST: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 518 REST OF MIDDLE EAST: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 519 REST OF MIDDLE EAST: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 520 REST OF MIDDLE EAST: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 521 REST OF MIDDLE EAST: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 522 AFRICA: KEY MACROINDICATORS
- TABLE 523 AFRICA: IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2023-2030 (USD MILLION)
- TABLE 524 AFRICA: IN SITU HYBRIDIZATION CONSUMABLES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 525 AFRICA: IN SITU HYBRIDIZATION INSTRUMENTS MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 526 AFRICA: IN SITU HYBRIDIZATION SERVICES MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 527 AFRICA: IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2023-2030 (USD MILLION)
- TABLE 528 AFRICA: FLUORESCENCE IN SITU HYBRIDIZATION (FISH) MARKET, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 529 AFRICA: IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2023-2030 (USD MILLION)
- TABLE 530 AFRICA: IN SITU HYBRIDIZATION MARKET FOR CLINICAL DIAGNOSTIC APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 531 AFRICA: IN SITU HYBRIDIZATION MARKET FOR RESEARCH APPLICATIONS, BY TYPE, 2023-2030 (USD MILLION)
- TABLE 532 AFRICA: IN SITU HYBRIDIZATION MARKET, BY END USER, 2023-2030 (USD MILLION)
- TABLE 533 OVERVIEW OF STRATEGIES DEPLOYED BY KEY PLAYERS IN IN SITU HYBRIDIZATION MARKET
- TABLE 534 IN SITU HYBRIDIZATION MARKET: DEGREE OF COMPETITION
- TABLE 535 IN SITU HYBRIDIZATION MARKET: REGION FOOTPRINT
- TABLE 536 IN SITU HYBRIDIZATION MARKET: OFFERING FOOTPRINT
- TABLE 537 IN SITU HYBRIDIZATION MARKET: TECHNOLOGY FOOTPRINT
- TABLE 538 IN SITU HYBRIDIZATION MARKET: DETAILED LIST OF KEY STARTUPS/SME PLAYERS
- TABLE 539 IN SITU HYBRIDIZATIONN MARKET: COMPETITIVE BENCHMARKING OF KEY STARTUPS/SME PLAYERS, BY OFFERING AND REGION
- TABLE 540 IN SITU HYBRIDIZATION MARKET: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022-JUNE 2025
- TABLE 541 IN SITU HYBRIDIZATION MARKET: DEALS, JANUARY 2022-JUNE 2025
- TABLE 542 F. HOFFMANN-LA ROCHE LTD.: COMPANY OVERVIEW
- TABLE 543 F. HOFFMANN-LA ROCHE LTD.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 544 F. HOFFMANN-LA ROCHE LTD.: PRODUCT LAUNCHES & APPROVALS, JANUARY 2022-JUNE 2025
- TABLE 545 DANAHER: COMPANY OVERVIEW
- TABLE 546 DANAHER: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 547 DANAHER: PRODUCT LAUNCHES, JANUARY 2022-JUNE 2025
- TABLE 548 DANAHER: DEALS, JANUARY 2022-JUNE 2025
- TABLE 549 ABBOTT: COMPANY OVERVIEW
- TABLE 550 ABBOTT: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 551 THERMO FISHER SCIENTIFIC INC.: COMPANY OVERVIEW
- TABLE 552 THERMO FISHER SCIENTIFIC INC.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 553 BIO-TECHNE: COMPANY OVERVIEW
- TABLE 554 BIO-TECHNE: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 555 BIO-TECHNE: PRODUCT LAUNCHES, JANUARY 2022-JUNE 2025
- TABLE 556 BIO-TECHNE: DEALS, JANUARY 2022-JUNE 2025
- TABLE 557 AGILENT TECHNOLOGIES, INC.: COMPANY OVERVIEW
- TABLE 558 AGILENT TECHNOLOGIES, INC.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 559 QIAGEN: COMPANY OVERVIEW
- TABLE 560 QIAGEN: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 561 BIOCARE MEDICAL, LLC: COMPANY OVERVIEW
- TABLE 562 BIOCARE MEDICAL, LLC: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 563 BIOCARE MEDICAL, LLC: DEALS, JANUARY 2022-JUNE 2025
- TABLE 564 BIOVIEW: COMPANY OVERVIEW
- TABLE 565 BIOVIEW: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 566 CREATIVE BIOARRAY: COMPANY OVERVIEW
- TABLE 567 CREATIVE BIOARRAY: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 568 ABNOVA CORPORATION: COMPANY OVERVIEW
- TABLE 569 ABNOVA CORPORATION: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 570 ZYTOMICS: COMPANY OVERVIEW
- TABLE 571 ZYTOMICS: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 572 ENZO BIOCHEM INC.: COMPANY OVERVIEW
- TABLE 573 ENZO BIOCHEM INC.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 574 ENZO BIOCHEM INC.: PRODUCT LAUNCHES, JANUARY 2022-JUNE 2025
- TABLE 575 CYTOTEST INC.: COMPANY OVERVIEW
- TABLE 576 CYTOTEST INC.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 577 GENEMED BIOTECHNOLOGIES, INC.: COMPANY OVERVIEW
- TABLE 578 GENEMED BIOTECHNOLOGIES, INC.: PRODUCTS/SERVICES/SOLUTIONS OFFERED
- TABLE 579 MOLECULAR INSTRUMENTS, INC.: COMPANY OVERVIEW
- TABLE 580 OXFORD GENE TECHNOLOGY IP LIMITED: COMPANY OVERVIEW
- TABLE 581 HISTO-LINE LABORATORIES: COMPANY OVERVIEW
- TABLE 582 METASYSTEMS: COMPANY OVERVIEW
- TABLE 583 BIOGENEX: COMPANY OVERVIEW
- TABLE 584 LGC BIORESEARCH TECHNOLOGIES: COMPANY OVERVIEW
- TABLE 585 BIODISCOVERY LLC: COMPANY OVERVIEW
- TABLE 586 KANEKA EUROGENTEC S.A.: COMPANY OVERVIEW
- TABLE 587 LIFE TECHNOLOGIES (INDIA) PVT. LTD.: COMPANY OVERVIEW
- TABLE 588 GENECOPOEIA, INC.: COMPANY OVERVIEW
List of Figures
- FIGURE 1 IN SITU HYBRIDIZATION MARKET SEGMENTATION & REGIONAL SCOPE
- FIGURE 2 IN SITU HYBRIDIZATION MARKET: YEARS CONSIDERED
- FIGURE 3 IN SITU HYBRIDIZATION MARKET: RESEARCH DESIGN
- FIGURE 4 IN SITU HYBRIDIZATION MARKET: BREAKDOWN OF PRIMARIES (SUPPLY- AND DEMAND-SIDE PARTICIPANTS)
- FIGURE 5 IN SITU HYBRIDIZATION MARKET SIZE ESTIMATION (SUPPLY-SIDE ANALYSIS), 2024
- FIGURE 6 GLOBAL MARKET SIZE ESTIMATION: COMPANY REVENUE ANALYSIS (BOTTOM-UP APPROACH), 2024
- FIGURE 7 IN SITU HYBRIDIZATION MARKET: REVENUE ANALYSIS OF HOFFMANN-LA ROCHE LTD. (2024)
- FIGURE 8 IN SITU HYBRIDIZATION MARKET SIZE VALIDATION FROM PRIMARY SOURCES
- FIGURE 9 SEGMENTAL MARKET SIZE ESTIMATION: TOP-DOWN APPROACH
- FIGURE 10 IN SITU HYBRIDIZATION MARKET: CAGR PROJECTIONS (2025-2030)
- FIGURE 11 IN SITU HYBRIDIZATION MARKET: GROWTH ANALYSIS OF MARKET DYNAMICS
- FIGURE 12 IN SITU HYBRIDIZATION MARKET: DATA TRIANGULATION METHODOLOGY
- FIGURE 13 IN SITU HYBRIDIZATION MARKET, BY OFFERING, 2025 VS. 2030 (USD MILLION)
- FIGURE 14 IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY, 2025 VS. 2030 (USD MILLION)
- FIGURE 15 IN SITU HYBRIDIZATION MARKET, BY APPLICATION, 2025 VS. 2030 (USD MILLION)
- FIGURE 16 IN SITU HYBRIDIZATION MARKET, BY END USER, 2025 VS. 2030 (USD MILLION)
- FIGURE 17 REGIONAL ANALYSIS OF IN SITU HYBRIDIZATION MARKET
- FIGURE 18 INCREASING ADOPTION OF IN SITU HYBRIDIZATION TECHNOLOGIES IN LABORATOY-DEVELOPED TESTS TO DRIVE MARKET
- FIGURE 19 US AND CONSUMABLES ACCOUNTED FOR LARGEST NORTH AMERICAN MARKET SHARE IN 2024
- FIGURE 20 HOSPITALS & DIAGNOSTIC LABORATORIES TO COMMAND LARGEST END USER MARKET SHARE IN 2024
- FIGURE 21 CHINA TO REGISTER HIGHEST GROWTH RATE DURING STUDY PERIOD
- FIGURE 22 IN SITU HYBRIDIZATION MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- FIGURE 23 IN SITU HYBRIDIZATION MARKET: TRENDS/DISRUPTIONS IMPACTING CUSTOMER'S BUSINESS
- FIGURE 24 IN SITU HYBRIDIZATION PRODUCTS MARKET: VALUE CHAIN ANALYSIS
- FIGURE 25 IN SITU HYBRIDIZATION MARKET: ECOSYSTEM ANALYSIS
- FIGURE 26 TOP APPLICANTS/OWNERS (COMPANIES) FOR IN SITU HYBRIDIZATION PATENTS
- FIGURE 27 IN SITU HYBRIDIZATION MARKET: PORTER'S FIVE FORCES ANALYSIS
- FIGURE 28 INFLUENCE OF KEY STAKEHOLDERS ON BUYING PROCESS OF IN SITU HYBRIDIZATION-BASED OFFERINGS
- FIGURE 29 KEY BUYING CRITERIA, BY END USER
- FIGURE 30 IMPACT OF AI ON IN SITU HYBRIDIZATION ECOSYSTEM
- FIGURE 31 NORTH AMERICA: IN SITU HYBRIDIZATION MARKET SNAPSHOT
- FIGURE 32 ASIA PACIFIC: IN SITU HYBRIDIZATION MARKET SNAPSHOT
- FIGURE 33 REVENUE ANALYSIS OF KEY PLAYERS IN IN SITU HYBRIDIZATION MARKET, 2020-2024 (USD MILLION)
- FIGURE 34 MARKET SHARE ANALYSIS OF KEY PLAYERS IN IN SITU HYBRIDIZATION MARKET (2024)
- FIGURE 35 IN SITU HYBRIDIZATION MARKET: COMPANY EVALUATION MATRIX (KEY PLAYERS), 2024
- FIGURE 36 IN SITU HYBRIDIZATION MARKET: COMPANY FOOTPRINT
- FIGURE 37 IN SITU HYBRIDIZATION MARKET: COMPANY EVALUATION MATRIX (STARTUPS/SMES), 2024
- FIGURE 38 EV/EBITDA OF KEY VENDORS
- FIGURE 39 YEAR-TO-DATE (YTD) PRICE TOTAL RETURN AND 5-YEAR STOCK BETA OF KEY VENDORS
- FIGURE 40 BRAND/PRODUCT COMPARATIVE ANALYSIS OF IN SITU HYBRIDIZATION-BASED ASSAYS
- FIGURE 41 F. HOFFMANN-LA ROCHE LTD.: COMPANY SNAPSHOT
- FIGURE 42 DANAHER: COMPANY SNAPSHOT
- FIGURE 43 ABBOTT: COMPANY SNAPSHOT
- FIGURE 44 THERMO FISHER SCIENTIFIC INC.: COMPANY SNAPSHOT
- FIGURE 45 BIO-TECHNE: COMPANY SNAPSHOT
- FIGURE 46 AGILENT TECHNOLOGIES, INC.: COMPANY SNAPSHOT
- FIGURE 47 QIAGEN: COMPANY SNAPSHOT
- FIGURE 48 ENZO BIOCHEM INC.: COMPANY SNAPSHOT
The in situ hybridization market is expected to reach USD 2.35 billion in 2030 from USD 1.64 billion in 2025, at a CAGR of 7.4% during the forecast period. The expansion of the in situ hybridization (ISH) market is largely driven by continuous technological advancements within ISH platforms. Innovations such as enhanced probe designs, automation, and multiplexing capabilities have markedly improved assay sensitivity, specificity, and throughput. These enhancements align with the increasing need for precision medicine and the development of laboratory-developed tests (LDTs). Such advancements facilitate the identification of patient-specific genetic markers, solidifying ISH's role as a critical component in personalized treatment approaches. Furthermore, the emerging field of spatial transcriptomics within cancer and neuroscience research underscores the necessity for highly sensitive and multiplexed ISH technologies to analyze gene expression in its tissue context. This demand is propelling ongoing innovation in the ISH sector. Notably, ISH presents distinct advantages over immunohistochemistry (IHC), particularly with its ability to detect direct nucleic acid, which enhances diagnostic accuracy and disease characterization, further contributing to the market's growth.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2023-2030 |
| Base Year | 2024 |
| Forecast Period | 2025-2030 |
| Units Considered | Value (USD Billion) |
| Segments | Offering, Technology, Application, End User, Region |
| Regions covered | North America, Europe, Asia Pacific, Latin America, Middle East, and Africa |
"The fluorescent in situ hybridization technology segment accounted for the largest technology market share in 2024."
The in situ hybridization market by technology is segmented into fluorescent in situ hybridization and chromogenic in situ hybridization. The fluorescent in situ hybridization technology is further segmented into DNA fluorescent in situ hybridization, RNA fluorescent in situ hybridization, and PNA fluorescent in situ hybridization. In 2024, the fluorescent in situ hybridization technology segment accounted for the largest market share, by technology. The dominant position of this technology segment is attributable to its exceptional sensitivity, specificity, and versatility in detecting genetic anomalies at both DNA and RNA levels. Fluorescence In Situ Hybridization (FISH) provides high-resolution insights for identifying chromosomal rearrangements, gene amplifications, and deletions, making it indispensable in cancer diagnostics, the identification of genetic disorders, and prenatal screenings. FISH enables concurrent detection of multiple targets through the use of various fluorescent probes, catering to both clinical and research needs. Furthermore, the extensive incorporation of FISH in personalized medicine, particularly for HER2 and ALK assessments in oncology, has significantly bolstered its demand in recent years.
"Consumables segment is projected to register the highest CAGR in the global in situ hybridization market during the forecast period."
By offering, the in situ hybridization market is segmented into consumables, instruments, services & software. The consumables segment is further sub-segmented into kits & reagents, probes & probe kits, and accessories & other consumables. The consumables segment is expected to register the highest growth rate during the forecast period. The expansion of the market for consumables in in situ hybridization (ISH) procedures is fundamentally linked to their increasing application in cancer diagnostics and various other disease areas. This uptick in utilization directly correlates with a heightened demand for essential consumables, including probes and probe kits, integral to every ISH assay. As testing volumes escalate in both clinical and research environments, the demand for these materials is poised for steady growth. Furthermore, the ongoing development and approval of advanced ISH-based diagnostic tests, such as Roche's VENTANA Kappa and Lambda Dual ISH mRNA Probe Cocktail assay, are anticipated to further broaden the consumables market. Such innovations enhance the accuracy and specificity of diagnostics for conditions such as B-cell malignancies, thereby incentivizing laboratories and healthcare facilities to increasingly incorporate ISH methodologies into their testing repertoire. This upward trajectory in clinical uptake will consistently drive the demand for corresponding consumables, underpinning robust growth in this segment. Additionally, it presents significant opportunities for key industry players to fortify their market presence through innovative product development and strategic supply chain enhancements.
"The Asia Pacific region is growing at the highest CAGR in the in situ hybridization market during the study period."
The Asia Pacific region is anticipated to experience the highest growth rate in the in situ hybridization (ISH) market throughout the forecast period. This growth is driven by significant advancements in healthcare infrastructure and heightened investments in molecular diagnostics and precision medicine from both government entities and private sector stakeholders. Key nations, including China, India, Japan, and South Korea, are actively advancing research initiatives in genomics and personalized medicine through targeted national programs and funding mechanisms. Concurrently, the increasing prevalence of chronic diseases-such as cancer, infectious diseases, and genetic disorders-is propelling the demand for sophisticated diagnostic technologies like ISH. The burgeoning presence of regional diagnostic firms, coupled with strategic partnerships with global entities, is further facilitating market access and enhancing technology adoption.
The primary interviews conducted for this report can be categorized as follows:
- By Company Type: Tier 1- 44%, Tier 2- 32%, and Tier 3- 24%
- By Designation: (Managers)- 45%, (CXOs, Directors)- 30%, and (Executives)- 25%
- By Region: North America- 40%, Europe- 25%, Asia Pacific- 20%, Rest of the World- 15%
F. Hoffmann-La Roche Ltd (Switzerland), Merck KGaA (Germany), Thermo Fisher Scientific Inc. (US), Abbott (US), QIAGEN (Netherlands), Bio-Techne. (US), and Biocare Medical (US) are some of the key players in the in situ hybridization market.
The study includes an in-depth competitive analysis of these key players in the in situ hybridization market, with their company profiles, recent developments, and key market strategies.
Research Coverage:
This research report categorizes the in situ hybridization market by offering (consumables (kits & reagents, probes & probe kits, accessories & other consumables), instruments, services & software), technology (fluorescent in situ hybridization (DNA fluorescent in situ hybridization, RNA fluorescent in situ hybridization, PNA fluorescent in situ hybridization), chromogenic in situ hybridization), application (clinical diagnostics (cancer diagnostics, genetic disorder detection, infectious disease diagnostics, and others), research applications (neuroscience, immunology, others), drug discovery & development), end user (hospitals & diagnostic laboratories, academic & research institutes, pharmaceutical & biotechnology companies, contract research organizations) and by region (North America, Europe, Asia Pacific, Latin America, Middle East, and Africa). The report provides in-depth information on significant factors influencing the growth of the in situ hybridization market, including drivers, trends, challenges, and opportunities. A thorough analysis of major industry players has been undertaken to provide insights into their business profiles, products/services, key strategies, collaborations, partnerships, and agreements. Additionally, the report encompasses recent developments, such as new product launches and acquisitions within the in situ hybridization market.
Key Benefits of Buying the Report:
The report will help market leaders/new entrants by providing the closest approximations of the revenue numbers for the overall in situ hybridization market and its subsegments. It will also help stakeholders better understand the competitive landscape and gain more insights to position their business better and make suitable go-to-market strategies. This report will enable stakeholders to understand the market's pulse and provide them with information on the key market drivers, restraints, opportunities, and challenges.
The report provides insights on the following pointers:
- Analysis of key drivers [technological advancements enhancing in situ hybridization capabilities, increasing adoption of in situ hybridization in precision medicine and laboratory-developed tests (LDTs), rising demand for spatial transcriptomics in cancer and neuroscience research, expansion of multiplex in situ hybridization techniques for multi-gene detection], restraints [high costs of in situ hybridization techniques, competition from alternative molecular diagnostic techniques (qPCR, NGS)], opportunities [advantages over other techniques such as immunohistochemistry (IHC), expansion in emerging markets, growing adoption of companion diagnostics (CDx) solutions], and challenges [technical complexities associated with in situ hybridization].
- Product Development/Innovation: Detailed insights on newly launched and approved products/services of the in situ hybridization market
- Market Development: Comprehensive information about lucrative markets - the report analyses the market across varied regions.
- Market Diversification: Exhaustive information about new products/services, untapped geographies, recent developments, and investments in the in situ hybridization market
- Competitive Assessment: In-depth assessment of market share, growth strategies and service offerings of leading players like F. Hoffmann-La Roche Ltd (Switzerland), Danaher (US), Agilent Technologies, Inc. (US), Abbott (US), Thermo Fisher Scientific Inc. (US), Bioview (Israel), NEOGENOMICS LABORATORIES (US), Qiagen (Netherlands), Bio-Techne. (US) and Biocare Medical (US), among others, in the in situ hybridization market.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.3 STUDY SCOPE
- 1.3.1 MARKET SEGMENTATION & REGIONAL SCOPE
- 1.3.2 INCLUSIONS & EXCLUSIONS
- 1.3.3 YEARS CONSIDERED
- 1.3.4 CURRENCY CONSIDERED
- 1.4 STAKEHOLDERS
- 1.5 SUMMARY OF CHANGES
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- 2.1.1 SECONDARY DATA
- 2.1.1.1 Key sources of secondary data
- 2.1.1.2 Key objectives of secondary research
- 2.1.2 PRIMARY DATA
- 2.1.2.1 Breakdown of primaries
- 2.1.2.2 Key objectives of primary research
- 2.1.1 SECONDARY DATA
- 2.2 MARKET ESTIMATION METHODOLOGY
- 2.2.1 GLOBAL MARKET SIZE ESTIMATION
- 2.2.2 SEGMENTAL MARKET ASSESSMENT (TOP-DOWN APPROACH)
- 2.3 MARKET GROWTH RATE FORECAST
- 2.4 DATA TRIANGULATION
- 2.5 STUDY ASSUMPTIONS
- 2.6 RESEARCH LIMITATIONS
- 2.7 RISK ANALYSIS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
- 4.1 IN SITU HYBRIDIZATION MARKET OVERVIEW
- 4.2 NORTH AMERICA: IN SITU HYBRIDIZATION MARKET, BY OFFERING AND COUNTRY
- 4.3 IN SITU HYBRIDIZATION MARKET SHARE, BY END USER, 2024
- 4.4 IN SITU HYBRIDIZATION MARKET: GEOGRAPHIC GROWTH OPPORTUNITIES
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- 5.2.1 DRIVERS
- 5.2.1.1 Technological innovations for advanced in situ hybridization capabilities
- 5.2.1.2 Increasing adoption of in situ hybridization in precision medicines and laboratory-developed tests
- 5.2.1.3 Rising demand for spatial transcriptomics in cancer and neuroscience research
- 5.2.2 RESTRAINTS
- 5.2.2.1 Competition from alternative molecular diagnostic techniques
- 5.2.2.2 High cost of in situ hybridization techniques in research and clinical applications
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Advantages of in situ hybridization over competing technologies
- 5.2.3.2 Rising adoption of companion diagnostic solutions
- 5.2.3.3 High growth opportunities in emerging economies
- 5.2.4 CHALLENGES
- 5.2.4.1 Technical complexities associated with in situ hybridization
- 5.2.1 DRIVERS
- 5.3 TRENDS/DISRUPTIONS IMPACTING CUSTOMER'S BUSINESS
- 5.4 VALUE CHAIN ANALYSIS
- 5.5 ECOSYSTEM ANALYSIS
- 5.5.1 ROLE IN ECOSYSTEM
- 5.6 TECHNOLOGY ANALYSIS
- 5.6.1 KEY TECHNOLOGIES
- 5.6.1.1 Fluorescence In Situ Hybridization (FISH)
- 5.6.1.2 Chromogenic In Situ Hybridization (CISH)
- 5.6.1.3 RNAscope
- 5.6.2 COMPLEMENTARY TECHNOLOGIES
- 5.6.2.1 Immunofluorescence (IF)
- 5.6.2.2 Next-generation Sequencing (NGS)
- 5.6.3 ADJACENT TECHNOLOGIES
- 5.6.3.1 Immunohistochemistry (IHC)
- 5.6.1 KEY TECHNOLOGIES
- 5.7 PATENT ANALYSIS
- 5.7.1 TOP APPLICANTS/OWNERS (COMPANIES) FOR IN SITU HYBRIDIZATION PATENTS
- 5.7.2 LIST OF KEY PATENTS
- 5.8 KEY CONFERENCES & EVENTS, 2025-2026
- 5.9 PORTER'S FIVE FORCES ANALYSIS
- 5.9.1 INTENSITY OF COMPETITIVE RIVALRY
- 5.9.2 THREAT OF SUBSTITUTES
- 5.9.3 BARGAINING POWER OF BUYERS
- 5.9.4 BARGAINING POWER OF SUPPLIERS
- 5.9.5 THREAT OF NEW ENTRANTS
- 5.10 KEY STAKEHOLDERS & BUYING CRITERIA
- 5.10.1 KEY STAKEHOLDERS IN BUYING PROCESS
- 5.10.2 KEY BUYING CRITERIA
- 5.11 PRICING ANALYSIS
- 5.11.1 AVERAGE SELLING PRICE TREND OF IN SITU HYBRIDIZATION PRODUCTS, BY KEY PLAYER, 2022-2024
- 5.11.2 AVERAGE SELLING PRICE TREND OF IN SITU HYBRIDIZATION CONSUMABLES, BY REGION, 2022-2024
- 5.12 INVESTMENT & FUNDING SCENARIO
- 5.13 REGULATORY LANDSCAPE
- 5.13.1 REGULATORY FRAMEWORK
- 5.13.1.1 North America
- 5.13.1.2 Europe
- 5.13.1.3 Asia Pacific
- 5.13.2 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.13.1 REGULATORY FRAMEWORK
- 5.14 IMPACT OF AI/GEN AI ON IN SITU HYBRIDIZATION MARKET
- 5.14.1 KEY USE CASES
- 5.14.2 IMPACT OF AI ON IN SITU HYBRIDIZATION ECOSYSTEM
- 5.14.3 FUTURE OF AI IN IN SITU HYBRIDIZATION ECOSYSTEM
- 5.15 IMPACT OF 2025 US TARIFF ON IN SITU HYBRIDIZATION MARKET
- 5.15.1 INTRODUCTION
- 5.15.2 KEY TARIFF RATES
- 5.15.3 PRICE IMPACT ANALYSIS
- 5.15.4 IMPACT ON COUNTRY/REGION
- 5.15.4.1 North America
- 5.15.4.1.1 US
- 5.15.4.2 Europe
- 5.15.4.3 Asia Pacific
- 5.15.4.1 North America
- 5.15.5 IMPACT ON END-USE INDUSTRIES
- 5.15.5.1 Hospitals & diagnostic laboratories
- 5.15.5.2 Academic & research institutes
- 5.15.5.3 Pharmaceutical & biotechnology companies
- 5.15.5.4 Contract research organizations
6 IN SITU HYBRIDIZATION MARKET, BY OFFERING
- 6.1 INTRODUCTION
- 6.2 CONSUMABLES
- 6.2.1 KITS & REAGENTS
- 6.2.1.1 Increasing demand for companion diagnostics and personalized medicines to support market growth
- 6.2.2 PROBE & PROBE KITS
- 6.2.2.1 Technological advancements in probe design and chemistry to propel market growth
- 6.2.3 ACCESSORIES & OTHER CONSUMABLES
- 6.2.3.1 Growing adoption of in situ hybridization in research and clinical diagnostics to aid market growth
- 6.2.1 KITS & REAGENTS
- 6.3 INSTRUMENTS
- 6.3.1 AUTOMATED IN SITU HYBRIDIZATION INSTRUMENTS
- 6.3.1.1 Increased adoption of faster and more reliable automated systems to boost market growth
- 6.3.2 MANUAL IN SITU HYBRIDIZATION INSTRUMENTS
- 6.3.2.1 Persistent demand in emerging economies to maintain market growth
- 6.3.1 AUTOMATED IN SITU HYBRIDIZATION INSTRUMENTS
- 6.4 SOFTWARE
- 6.4.1 INTEGRATION OF ARTIFICIAL INTELLIGENCE AND MACHINE LEARNING IN SOFTWARE TO SUPPORT GROWTH
- 6.5 SERVICES
- 6.5.1 DATA INTERPRETATION SERVICES
- 6.5.1.1 Increasing use of multiplex assays and spatial biology techniques to boost segment growth
- 6.5.2 CUSTOM PROBE DESIGN SERVICES
- 6.5.2.1 Rising demand for customized probes in research and drug development to favor market growth
- 6.5.1 DATA INTERPRETATION SERVICES
7 IN SITU HYBRIDIZATION MARKET, BY TECHNOLOGY
- 7.1 INTRODUCTION
- 7.2 FLUORESCENCE IN SITU HYBRIDIZATION (FISH)
- 7.2.1 DNA FLUORESCENCE IN SITU HYBRIDIZATION (DNA-FISH)
- 7.2.1.1 Extensive use in research and companion diagnostics to fuel segment growth
- 7.2.2 RNA FLUORESCENCE IN SITU HYBRIDIZATION (RNA-FISH)
- 7.2.2.1 Rise in biomedical research activity to drive adoption of RNA-FISH technology
- 7.2.3 PNA FLUORESCENCE IN SITU HYBRIDIZATION (PNA-FISH)
- 7.2.3.1 High sensitivity offered by PNA FISH to fuel precise and reliable detection at low probe concentrations
- 7.2.1 DNA FLUORESCENCE IN SITU HYBRIDIZATION (DNA-FISH)
- 7.3 CHROMOGENIC IN SITU HYBRIDIZATION (CISH)
- 7.3.1 COST-EFFECTIVENESS, PRESERVATION OF TISSUE MORPHOLOGY, AND EASE OF INTERPRETATION TO BOOST SEGMENT GROWTH
8 IN SITU HYBRIDIZATION MARKET, BY APPLICATION
- 8.1 INTRODUCTION
- 8.2 CLINICAL DIAGNOSTIC APPLICATIONS
- 8.2.1 CANCER DIAGNOSTICS
- 8.2.1.1 Rising number of cancer cases and increasing focus on precision medicines to drive segment
- 8.2.2 INFECTIOUS DISEASE DIAGNOSTICS
- 8.2.2.1 Growing demand for high sensitivity and specificity diagnostic methods in resource-limited settings to spur market growth
- 8.2.3 GENETIC DISEASE DIAGNOSTICS
- 8.2.3.1 Rising adoption of prenatal and neonatal screening to propel segment growth
- 8.2.4 OTHER CLINICAL DIAGNOSTIC APPLICATIONS
- 8.2.1 CANCER DIAGNOSTICS
- 8.3 RESEARCH APPLICATIONS
- 8.3.1 NEUROSCIENCE RESEARCH
- 8.3.1.1 Growing focus on neurological research to increase R&D in neuroscience-based ISH technologies
- 8.3.2 IMMUNOLOGY RESEARCH
- 8.3.2.1 Rising prevalence of autoimmune diseases to support market growth
- 8.3.3 OTHER RESEARCH APPLICATIONS
- 8.3.1 NEUROSCIENCE RESEARCH
- 8.4 DRUG DISCOVERY & DEVELOPMENT
- 8.4.1 RISING DEMAND FOR NUCLEIC ACID-BASED THERAPIES TO FAVOR MARKET GROWTH
9 IN SITU HYBRIDIZATION MARKET, BY END USER
- 9.1 INTRODUCTION
- 9.2 HOSPITALS & DIAGNOSTIC LABORATORIES
- 9.2.1 RISING DEMAND FOR MOLECULAR TESTS AND INCREASING NEED FOR ACCURATE DISEASE DIAGNOSIS TO DRIVE MARKET
- 9.3 ACADEMIC & RESEARCH INSTITUTES
- 9.3.1 INCREASING GOVERNMENT AND PRIVATE FUNDING FOR LIFE SCIENCE AND BIOMEDICAL RESEARCH TO FUEL MARKET GROWTH
- 9.4 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
- 9.4.1 INCREASING FOCUS ON DEVELOPING PRECISION TREATMENTS TO PROPEL MARKET GROWTH
- 9.5 CONTRACT RESEARCH ORGANIZATIONS
- 9.5.1 INCREASING OUTSOURCING OF RESEARCH ACTIVITIES IN TISSUE DIAGNOSTICS TO SUPPORT MARKET GROWTH
10 IN SITU HYBRIDIZATION MARKET, BY REGION
- 10.1 INTRODUCTION
- 10.2 NORTH AMERICA
- 10.2.1 MACROECONOMIC ANALYSIS FOR NORTH AMERICA
- 10.2.2 US
- 10.2.2.1 US to dominate North American in situ hybridization market during study period
- 10.2.3 CANADA
- 10.2.3.1 Increasing cancer burden and expanding in situ hybridization applications in cancer research to propel market growth
- 10.3 EUROPE
- 10.3.1 MACROECONOMIC ANALYSIS FOR EUROPE
- 10.3.2 GERMANY
- 10.3.2.1 Research advancements and corporate developments to fuel market growth
- 10.3.3 UK
- 10.3.3.1 High government healthcare investments in genomic research to support market growth
- 10.3.4 FRANCE
- 10.3.4.1 Rising government investment in pharmaceutical industry to propel market growth
- 10.3.5 ITALY
- 10.3.5.1 High R&D investments and advanced pharmaceutical industry to favor market growth
- 10.3.6 SPAIN
- 10.3.6.1 Growing focus on cancer biomarker development to aid market growth
- 10.3.7 REST OF EUROPE
- 10.4 ASIA PACIFIC
- 10.4.1 MACROECONOMIC ANALYSIS FOR ASIA PACIFIC
- 10.4.2 CHINA
- 10.4.2.1 Increased focus on precision medicine and local manufacturing of in situ hybridization technologies to spur market growth
- 10.4.3 JAPAN
- 10.4.3.1 Stringent regulatory guidelines and large geriatric population to augment market growth
- 10.4.4 INDIA
- 10.4.4.1 Rising awareness of cancer and genetic disorders to support market growth
- 10.4.5 AUSTRALIA
- 10.4.5.1 Robust government support and active genetic research to propel market growth
- 10.4.6 SOUTH KOREA
- 10.4.6.1 Increased focus on genomic and diagnostic research to aid market adoption of in situ hybridization products
- 10.4.7 REST OF ASIA PACIFIC
- 10.5 LATIN AMERICA
- 10.5.1 MACROECONOMIC ANALYSIS FOR LATIN AMERICA
- 10.5.2 BRAZIL
- 10.5.2.1 Growing focus on precision diagnostics to drive market
- 10.5.3 MEXICO
- 10.5.3.1 Increasing collaboration among key players to spur market growth
- 10.5.4 REST OF LATIN AMERICA
- 10.6 MIDDLE EAST
- 10.6.1 MACROECONOMIC ANALYSIS FOR MIDDLE EAST
- 10.6.2 GCC COUNTRIES
- 10.6.2.1 Kingdom of Saudi Arabia
- 10.6.2.1.1 Expanding role of in situ hybridization products in research and clinical practice to boost market growth
- 10.6.2.2 UAE
- 10.6.2.2.1 Rising government support and incorporation of in situ hybridization technologies by hospitals to aid market growth
- 10.6.2.1 Kingdom of Saudi Arabia
- 10.6.3 REST OF GCC COUNTRIES
- 10.6.4 REST OF MIDDLE EAST
- 10.7 AFRICA
- 10.7.1 GROWING DEMAND FOR GENOMICS INFRASTRUCTURE AND MOLECULAR DIAGNOSTICS TO PROPEL MARKET GROWTH
- 10.7.2 MACROECONOMIC ANALYSIS FOR AFRICA
11 COMPETITIVE LANDSCAPE
- 11.1 INTRODUCTION
- 11.2 KEY PLAYERS STRATEGIES/RIGHT TO WIN
- 11.2.1 OVERVIEW OF STRATEGIES ADOPTED BY KEY PLAYERS IN IN SITU HYBRIDIZATION MARKET
- 11.3 REVENUE ANALYSIS, 2020-2024
- 11.4 MARKET SHARE ANALYSIS, 2024
- 11.5 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2024
- 11.5.1 STARS
- 11.5.2 EMERGING LEADERS
- 11.5.3 PERVASIVE PLAYERS
- 11.5.4 PARTICIPANTS
- 11.5.5 COMPANY FOOTPRINT: KEY PLAYERS, 2024
- 11.5.5.1 Company footprint
- 11.5.5.2 Region Footprint
- 11.5.5.3 Offering footprint
- 11.5.5.4 Technology footprint
- 11.6 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2024
- 11.6.1 PROGRESSIVE COMPANIES
- 11.6.2 RESPONSIVE COMPANIES
- 11.6.3 DYNAMIC COMPANIES
- 11.6.4 STARTING BLOCKS
- 11.6.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2024
- 11.6.5.1 Detailed list of key startups/SMEs
- 11.6.5.2 Competitive benchmarking of key startups/SMEs
- 11.7 COMPANY VALUATION & FINANCIAL METRICS
- 11.7.1 FINANCIAL METRICS
- 11.7.2 COMPANY VALUATION
- 11.8 BRAND/PRODUCT COMPARISON
- 11.9 COMPETITIVE SCENARIO
- 11.9.1 PRODUCT LAUNCHES & APPROVALS
- 11.9.2 DEALS
12 COMPANY PROFILES
- 12.1 KEY PLAYERS
- 12.1.1 F. HOFFMANN-LA ROCHE LTD.
- 12.1.1.1 Business overview
- 12.1.1.2 Products/Services/Solutions offered
- 12.1.1.3 Recent developments
- 12.1.1.3.1 Product launches & approvals
- 12.1.1.4 MnM view
- 12.1.1.4.1 Right to win
- 12.1.1.4.2 Strategic choices
- 12.1.1.4.3 Weaknesses & competitive threats
- 12.1.2 DANAHER
- 12.1.2.1 Business overview
- 12.1.2.2 Products/Services/Solutions offered
- 12.1.2.3 Recent developments
- 12.1.2.3.1 Product launches
- 12.1.2.3.2 Deals
- 12.1.2.4 MnM view
- 12.1.2.4.1 Right to win
- 12.1.2.4.2 Strategic choices
- 12.1.2.4.3 Weaknesses & competitive threats
- 12.1.3 ABBOTT
- 12.1.3.1 Business overview
- 12.1.3.2 Products/Services/Solutions offered
- 12.1.3.3 MnM view
- 12.1.3.3.1 Right to win
- 12.1.3.3.2 Strategic choices
- 12.1.3.3.3 Weaknesses & competitive threats
- 12.1.4 THERMO FISHER SCIENTIFIC INC.
- 12.1.4.1 Business overview
- 12.1.4.2 Products/Services/Solutions offered
- 12.1.4.3 MnM view
- 12.1.4.3.1 Right to win
- 12.1.4.3.2 Strategic choices
- 12.1.4.3.3 Weaknesses & competitive threats
- 12.1.5 BIO-TECHNE
- 12.1.5.1 Business overview
- 12.1.5.2 Products/Services/Solutions offered
- 12.1.5.3 Recent developments
- 12.1.5.3.1 Product launches
- 12.1.5.3.2 Deals
- 12.1.5.4 MnM view
- 12.1.5.4.1 Right to win
- 12.1.5.4.2 Strategic choices
- 12.1.5.4.3 Weaknesses & competitive threats
- 12.1.6 AGILENT TECHNOLOGIES, INC.
- 12.1.6.1 Business overview
- 12.1.6.2 Products/Services/Solutions offered
- 12.1.7 QIAGEN
- 12.1.7.1 Business overview
- 12.1.7.2 Products/Services/Solutions offered
- 12.1.8 BIOCARE MEDICAL, LLC
- 12.1.8.1 Business overview
- 12.1.8.2 Products/Services/Solutions offered
- 12.1.8.3 Recent developments
- 12.1.8.3.1 Deals
- 12.1.9 BIOVIEW
- 12.1.9.1 Business overview
- 12.1.9.2 Products/Services/Solutions offered
- 12.1.10 CREATIVE BIOARRAY
- 12.1.10.1 Business overview
- 12.1.10.2 Products/Services/Solutions offered
- 12.1.11 ABNOVA CORPORATION
- 12.1.11.1 Business overview
- 12.1.11.2 Products/Services/Solutions offered
- 12.1.12 ZYTOMICS
- 12.1.12.1 Business overview
- 12.1.12.2 Products/Services/Solutions offered
- 12.1.13 ENZO BIOCHEM INC.
- 12.1.13.1 Business overview
- 12.1.13.2 Products/Services/Solutions offered
- 12.1.13.3 Recent developments
- 12.1.13.3.1 Product launches
- 12.1.14 CYTOTEST INC.
- 12.1.14.1 Business overview
- 12.1.14.2 Products/Services/Solutions offered
- 12.1.15 GENEMED BIOTECHNOLOGIES, INC.
- 12.1.15.1 Business overview
- 12.1.15.2 Products/Services/Solutions offered
- 12.1.1 F. HOFFMANN-LA ROCHE LTD.
- 12.2 OTHER PLAYERS
- 12.2.1 MOLECULAR INSTRUMENTS, INC.
- 12.2.2 OXFORD GENE TECHNOLOGY IP LIMITED
- 12.2.3 HISTO-LINE LABORATORIES
- 12.2.4 METASYSTEMS
- 12.2.5 BIOGENEX
- 12.2.6 LGC BIOSEARCH TECHNOLOGIES
- 12.2.7 BIODISCOVERY LLC
- 12.2.8 KANEKA EUROGENTEC S.A.
- 12.2.9 LIFE TECHNOLOGIES (INDIA) PVT. LTD.
- 12.2.10 GENECOPOEIA, INC.
13 APPENDIX
- 13.1 DISCUSSION GUIDE
- 13.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 13.3 CUSTOMIZATION OPTIONS
- 13.4 RELATED REPORTS
- 13.5 AUTHOR DETAILS