|
|
市場調査レポート
商品コード
1519291
CAR-T細胞療法の世界市場:市場規模、シェア、動向:製品別、標的抗原別、適応症別、人口統計別、エンドユーザー別、地域別 - 2029年までの予測CAR T-cell Therapy Market Size, Share & Trends by Product, Target Antigen, Indication, Demographic, End User & Region - Global Forecast to 2029 |
||||||
カスタマイズ可能
|
|||||||
| CAR-T細胞療法の世界市場:市場規模、シェア、動向:製品別、標的抗原別、適応症別、人口統計別、エンドユーザー別、地域別 - 2029年までの予測 |
|
出版日: 2024年07月22日
発行: MarketsandMarkets
ページ情報: 英文 266 Pages
納期: 即納可能
|
全表示
- 概要
- 図表
- 目次
世界のCAR-T細胞療法の市場規模は、2024年の55億米ドルから2029年には290億米ドルに達すると推定され、2024年から2029年の予測期間中のCAGRは39.6%になるとみられています。
市場成長の原動力は、世界のがん罹患率の上昇です。CAR-T細胞療法の技術的進歩、治療開発への投資と資金調達の増加は、市場成長を促進すると思われます。しかし、CAR-T細胞療法に伴う副作用や高額な治療費は、予測期間中の市場成長を抑制すると予想される主な要因の一部です。
| 調査範囲 | |
|---|---|
| 調査対象年 | 2022年~2029年 |
| 基準年 | 2023年 |
| 予測期間 | 2024年~2029年 |
| 検討単位 | 金額(10億米ドル) |
| セグメント別 | 製品別、標的抗原別、適応症別、人口統計別、エンドユーザー別、地域別 |
| 対象地域 | 北米、欧州、アジア太平洋、ラテンアメリカ、中東・アフリカ |
製品別では、2023年にはイエスカルタセグメントが最大の市場シェアを占めました。市場成長の要因は、再発/難治性がんの治療におけるYescartaの高い奏効率と持続的寛解が治療採用の増加につながったことです。
エンドユーザー別では、世界のCAR-T細胞療法市場は病院、長期療養施設、専門センターに区分されます。2023年には、病院セグメントが世界のCAR-T細胞療法市場の主要促進要因として浮上し、予測期間中に最も高いCAGRを示しました。病院はCAR-T細胞療法を標準的な腫瘍学治療プロトコールに組み込んでいます。この傾向は、CAR-T細胞治療を受け入れる病院が増えるにつれて、CAR-T細胞治療の需要を押し上げています。さらに、病院はCAR-T細胞療法の臨床試験を促進する上で重要な役割を果たしています。研究イニシアティブや製薬企業との提携に積極的に参加することで、病院はエビデンスベースの拡大や規制当局の承認促進に貢献し、市場の成長を促しています。
当レポートでは、世界のCAR-T細胞療法市場について調査し、製品別、標的抗原別、適応症別、人口統計別、エンドユーザー別、地域別動向、および市場に参入する企業のプロファイルなどをまとめています。
目次
第1章 イントロダクション
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 重要考察
第5章 市場概要
- イントロダクション
- 市場力学
- 顧客のビジネスに影響を与える動向/混乱
- バリューチェーン分析
- エコシステム分析
- 技術分析
- 特許分析
- 価格分析
- 2024年~2025年の主な会議とイベント
- 規制状況
- 投資と資金調達のシナリオ
- ポーターのファイブフォース分析
- 主な利害関係者と購入基準
第6章 CAR-T細胞療法市場(製品別)
- イントロダクション
- イエスカルタ
- キムリア
- カルヴィクティ
- アベックマ
- テカルトゥス
- ブレヤンジ
- その他
第7章 CAR-T細胞療法市場(標的抗原別)
- イントロダクション
- CD19
- BCMA
- その他
第8章 CAR-T細胞療法市場(適応症別)
- イントロダクション
- 多発性骨髄腫
- B細胞リンパ腫
- 急性リンパ性白血病
- その他
第9章 CAR-T細胞療法市場(人口統計別)
- イントロダクション
- 成人
- 小児
第10章 CAR-T細胞療法市場(エンドユーザー別)
- イントロダクション
- 病院
- 専門センター
- 長期療養施設
第11章 CAR-T細胞療法市場(地域別)
- イントロダクション
- 北米
- 欧州
- アジア太平洋
- ラテンアメリカ
- 中東・アフリカ
第12章 競合情勢
- 概要
- 主要参入企業の戦略/強み
- 収益分析
- 市場シェア分析
- 企業評価マトリックス:主要参入企業、2023年
- 企業評価マトリックス:スタートアップ/中小企業、2023年
- 評価と財務指標
- ブランド/製品比較
- 競合シナリオ
第13章 企業プロファイル
- 主要参入企業
- BRISTOL-MYERS SQUIBB COMPANY
- GILEAD SCIENCES, INC.
- NOVARTIS AG
- JOHNSON & JOHNSON
- JW(CAYMAN)THERAPEUTICS CO. LTD.
- IMMUNOADOPTIVE CELL THERAPY PRIVATE LIMITED(IMMUNOACT)
- CARSGEN THERAPEUTICS HOLDINGS LIMITED
- IASO BIOTHERAPEUTICS
- その他の企業
- GUANGZHOU BIO-GENE TECHNOLOGY CO., LTD(HEDY GROUP CO., LTD.)
- CARTESIAN THERAPEUTICS, INC.
- AUTOLUS THERAPEUTICS
- ALLOGENE THERAPEUTICS
- CRISPR THERAPEUTICS
- WUGEN
第14章 付録
List of Tables
- TABLE 1 IMPACT ANALYSIS OF SUPPLY-SIDE AND DEMAND-SIDE FACTORS
- TABLE 2 GLOBAL INFLATION RATE PROJECTIONS, 2023-2029 (% GROWTH)
- TABLE 3 ADVERSE EFFECTS ASSOCIATED WITH CAR T-CELL THERAPY
- TABLE 4 KEY PRODUCT PROVIDERS IN CAR T-CELL THERAPY MARKET
- TABLE 5 KEY END USERS IN CAR T-CELL THERAPY MARKET
- TABLE 6 KEY REGULATORY BODIES IN CAR T-CELL THERAPY MARKET
- TABLE 7 NUMBER OF PATENTS FILED IN CAR T-CELL THERAPY MARKET, BY DOCUMENT TYPE, 2014-2024
- TABLE 8 TOP 12 PATENT OWNERS IN CAR T-CELL THERAPY MARKET, 2014-2024
- TABLE 9 DETAILED ANALYSIS OF KEY PATENTS IN CAR T-CELL THERAPY MARKET
- TABLE 10 AVERAGE SELLING PRICE FOR LEADING CAR T-CELL THERAPEUTIC DRUGS
- TABLE 11 AVERAGE SELLING PRICE FOR MAJOR CAR T-CELL THERAPEUTIC DRUGS, BY REGION
- TABLE 12 INDICATIVE PRICING ANALYSIS FOR CAR T-CELL THERAPY, BY PRODUCT, 2021-2023
- TABLE 13 KEY CONFERENCES & EVENTS, JUNE 2024-DECEMBER 2025
- TABLE 14 NORTH AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 15 EUROPE: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 16 ASIA PACIFIC: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 17 LATIN AMERICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 18 MIDDLE EAST & AFRICA: REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- TABLE 19 CAR T-CELL THERAPY MARKET: PORTER'S FIVE FORCES ANALYSIS
- TABLE 20 INFLUENCE OF STAKEHOLDERS ON BUYING PROCESS
- TABLE 21 KEY BUYING CRITERIA FOR PRODUCTS
- TABLE 22 CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 23 CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (UNITS)
- TABLE 24 YESCARTA MARKET, BY REGION, 2022-2029 (USD MILLION)
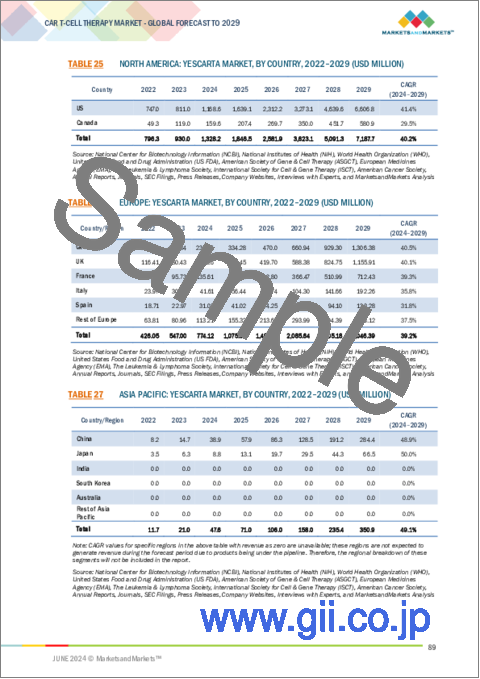
- TABLE 25 NORTH AMERICA: YESCARTA MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 26 EUROPE: YESCARTA MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 27 ASIA PACIFIC: YESCARTA MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 28 LATIN AMERICA: YESCARTA MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 29 KYMRIAH MARKET, BY REGION, 2022-2029 (USD MILLION)
- TABLE 30 NORTH AMERICA: KYMRIAH MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 31 EUROPE: KYMRIAH MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 32 ASIA PACIFIC: KYMRIAH MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 33 LATIN AMERICA: KYMRIAH MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 34 CARVYKTI MARKET, BY REGION, 2022-2029 (USD MILLION)
- TABLE 35 NORTH AMERICA: CARVYKTI MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 36 EUROPE: CARVYKTI MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 37 ASIA PACIFIC: CARVYKTI MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 38 LATIN AMERICA: CARVYKTI MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 39 ABECMA MARKET, BY REGION, 2022-2029 (USD MILLION)
- TABLE 40 NORTH AMERICA: ABECMA MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 41 EUROPE: ABECMA MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 42 ASIA PACIFIC: ABECMA MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 43 LATIN AMERICA: ABECMA MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 44 TECARTUS MARKET, BY REGION, 2022-2029 (USD MILLION)
- TABLE 45 NORTH AMERICA: TECARTUS MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 46 EUROPE: TECARTUS MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 47 ASIA PACIFIC: TECARTUS MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 48 LATIN AMERICA: TECARTUS MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 49 BREYANZI MARKET, BY REGION, 2022-2029 (USD MILLION)
- TABLE 50 NORTH AMERICA: BREYANZI MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 51 EUROPE: BREYANZI MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 52 ASIA PACIFIC: BREYANZI MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 53 LATIN AMERICA: BREYANZI MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 54 OTHER CAR T-CELL THERAPY PRODUCTS MARKET, BY REGION, 2022-2029 (USD MILLION)
- TABLE 55 NORTH AMERICA: OTHER CAR T-CELL THERAPY PRODUCTS MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 56 EUROPE: OTHER CAR T-CELL THERAPY PRODUCTS MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 57 ASIA PACIFIC: OTHER CAR T-CELL THERAPY PRODUCTS MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 58 LATIN AMERICA: OTHER CAR T-CELL THERAPY PRODUCTS MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 59 CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 60 COMMERCIALLY AVAILABLE CD19 ANTIGEN-TARGETING CAR T-CELL THERAPIES
- TABLE 61 CD19-TARGETING CAR T-CELL THERAPY MARKET, BY REGION, 2022-2029 (USD MILLION)
- TABLE 62 NORTH AMERICA: CD19-TARGETING CAR T-CELL THERAPY MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 63 EUROPE: CD19-TARGETING CAR T-CELL THERAPY MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 64 ASIA PACIFIC: CD19-TARGETING CAR T-CELL THERAPY MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 65 LATIN AMERICA: CD19-TARGETING CAR T-CELL THERAPY MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 66 COMMERCIALLY AVAILABLE BCMA ANTIGEN-TARGETING CAR T-CELL THERAPIES
- TABLE 67 BCMA-TARGETING CAR T-CELL THERAPY MARKET, BY REGION, 2022-2029 (USD MILLION)
- TABLE 68 NORTH AMERICA: BCMA-TARGETING CAR T-CELL THERAPY MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 69 EUROPE: BCMA-TARGETING CAR T-CELL THERAPY MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 70 ASIA PACIFIC: BCMA-TARGETING CAR T-CELL THERAPY MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 71 LATIN AMERICA: BCMA-TARGETING CAR T-CELL THERAPY MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 72 OTHER CAR T-CELL THERAPY TARGETS MARKET, BY REGION, 2022-2029 (USD MILLION)
- TABLE 73 NORTH AMERICA: OTHER CAR T-CELL THERAPY TARGETS MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 74 EUROPE: OTHER CAR T-CELL THERAPY TARGETS MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 75 ASIA PACIFIC: OTHER CAR T-CELL THERAPY TARGETS MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 76 LATIN AMERICA: OTHER CAR T-CELL THERAPY TARGETS MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 77 CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 78 MULTIPLE MYELOMA, BY REGION, 2022-2029 (USD MILLION)
- TABLE 79 NORTH AMERICA: MULTIPLE MYELOMA MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 80 EUROPE: MULTIPLE MYELOMA MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 81 ASIA PACIFIC: MULTIPLE MYELOMA MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 82 LATIN AMERICA: MULTIPLE MYELOMA MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 83 CAR T-CELL THERAPY MARKET FOR B-CELL LYMPHOMA, BY REGION, 2022-2029 (USD MILLION)
- TABLE 84 NORTH AMERICA: CAR T-CELL THERAPY MARKET FOR B-CELL LYMPHOMA, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 85 EUROPE: CAR T-CELL THERAPY MARKET FOR B-CELL LYMPHOMA, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 86 ASIA PACIFIC: CAR T-CELL THERAPY MARKET FOR B-CELL LYMPHOMA, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 87 LATIN AMERICA: CAR T-CELL THERAPY MARKET FOR B-CELL LYMPHOMA, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 88 CAR T-CELL THERAPY MARKET FOR ACUTE LYMPHOBLASTIC LEUKEMIA, BY REGION, 2022-2029 (USD MILLION)
- TABLE 89 NORTH AMERICA: CAR T-CELL THERAPY MARKET FOR ACUTE LYMPHOBLASTIC LEUKEMIA, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 90 EUROPE: CAR T-CELL THERAPY MARKET FOR ACUTE LYMPHOBLASTIC LEUKEMIA, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 91 ASIA PACIFIC: CAR T-CELL THERAPY MARKET FOR ACUTE LYMPHOBLASTIC LEUKEMIA, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 92 LATIN AMERICA: CAR T-CELL THERAPY MARKET FOR ACUTE LYMPHOBLASTIC LEUKEMIA, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 93 CAR T-CELL THERAPY MARKET FOR OTHER INDICATIONS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 94 NORTH AMERICA: CAR T-CELL THERAPY MARKET FOR OTHER INDICATIONS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 95 EUROPE: CAR T-CELL THERAPY MARKET FOR OTHER INDICATIONS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 96 ASIA PACIFIC: CAR T-CELL THERAPY MARKET FOR OTHER INDICATIONS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 97 LATIN AMERICA: CAR T-CELL THERAPY MARKET FOR OTHER INDICATIONS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 98 CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 99 CAR T-CELL THERAPY MARKET FOR ADULTS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 100 NORTH AMERICA: CAR T-CELL THERAPY MARKET FOR ADULTS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 101 EUROPE: CAR T-CELL THERAPY MARKET FOR ADULTS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 102 ASIA PACIFIC: CAR T-CELL THERAPY MARKET FOR ADULTS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 103 LATIN AMERICA: CAR T-CELL THERAPY MARKET FOR ADULTS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 104 CAR T-CELL THERAPY MARKET FOR PEDIATRICS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 105 NORTH AMERICA: CAR T-CELL THERAPY MARKET FOR PEDIATRICS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 106 EUROPE: CAR T-CELL THERAPY MARKET FOR PEDIATRICS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 107 ASIA PACIFIC: CAR T-CELL THERAPY MARKET FOR PEDIATRICS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 108 LATIN AMERICA: CAR T-CELL THERAPY MARKET FOR PEDIATRICS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 109 CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 110 CAR T-CELL THERAPY MARKET FOR HOSPITALS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 111 NORTH AMERICA: CAR T-CELL THERAPY MARKET FOR HOSPITALS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 112 EUROPE: CAR T-CELL THERAPY MARKET FOR HOSPITALS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 113 ASIA PACIFIC: CAR T-CELL THERAPY MARKET FOR HOSPITALS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 114 LATIN AMERICA: CAR T-CELL THERAPY MARKET FOR HOSPITALS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 115 CAR T-CELL THERAPY MARKET FOR SPECIALTY CENTERS, BY REGION, 2022-2029 (USD MILLION)
- TABLE 116 NORTH AMERICA: CAR T-CELL THERAPY MARKET FOR SPECIALTY CENTERS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 117 EUROPE: CAR T-CELL THERAPY MARKET FOR SPECIALTY CENTERS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 118 ASIA PACIFIC: CAR T-CELL THERAPY MARKET FOR SPECIALTY CENTERS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 119 LATIN AMERICA: CAR T-CELL THERAPY MARKET FOR SPECIALTY CENTERS, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 120 CAR T-CELL THERAPY MARKET FOR LONG-TERM CARE FACILITIES, BY REGION, 2022-2029 (USD MILLION)
- TABLE 121 NORTH AMERICA: CAR T-CELL THERAPY MARKET FOR LONG-TERM CARE FACILITIES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 122 EUROPE: CAR T-CELL THERAPY MARKET FOR LONG-TERM CARE FACILITIES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 123 ASIA PACIFIC: CAR T-CELL THERAPY MARKET FOR LONG-TERM CARE FACILITIES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 124 LATIN AMERICA: CAR T-CELL THERAPY MARKET FOR LONG-TERM CARE FACILITIES, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 125 CAR T-CELL THERAPY MARKET, BY REGION, 2022-2029 (USD MILLION)
- TABLE 126 NORTH AMERICA: CAR T-CELL THERAPY MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 127 NORTH AMERICA: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 128 NORTH AMERICA: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 129 NORTH AMERICA: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 130 NORTH AMERICA: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 131 NORTH AMERICA: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 132 US: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 133 US: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 134 US: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 135 US: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 136 US: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 137 CANADA: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 138 CANADA: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 139 CANADA: CAR T-CELL THERAPY MARKET, BY INDICATION,
022-2029 (USD MILLION)
- TABLE 140 CANADA: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 141 CANADA: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 142 EUROPE: CAR T-CELL THERAPY MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 143 EUROPE: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 144 EUROPE: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 145 EUROPE: CAR T-CELL THERAPY MARKET, BY INDICATION,
022-2029 (USD MILLION)
- TABLE 146 EUROPE: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 147 EUROPE: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 148 GERMANY: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 149 GERMANY: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 150 GERMANY: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 151 GERMANY: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 152 GERMANY: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 153 UK: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 154 UK: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 155 UK: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 156 UK: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 157 UK: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 158 FRANCE: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 159 FRANCE: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 160 FRANCE: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 161 FRANCE: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 162 FRANCE: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 163 ITALY: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 164 ITALY: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 165 ITALY: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 166 ITALY: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 167 ITALY: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 168 SPAIN: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 169 SPAIN: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 170 SPAIN: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 171 SPAIN: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 172 SPAIN: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 173 REST OF EUROPE: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 174 REST OF EUROPE: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 175 REST OF EUROPE: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 176 REST OF EUROPE: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 177 REST OF EUROPE: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 178 ASIA PACIFIC: CAR T-CELL THERAPY MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 179 ASIA PACIFIC: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 180 ASIA PACIFIC: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 181 ASIA PACIFIC: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 182 ASIA PACIFIC: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 183 ASIA PACIFIC: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 184 CHINA: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 185 CHINA: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 186 CHINA: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 187 CHINA: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 188 CHINA: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 189 JAPAN: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 190 JAPAN: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 191 JAPAN: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 192 JAPAN: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 193 JAPAN: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 194 INDIA: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 195 INDIA: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 196 INDIA: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 197 INDIA: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 198 INDIA: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 199 AUSTRALIA: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 200 AUSTRALIA: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 201 AUSTRALIA: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 202 AUSTRALIA: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 203 AUSTRALIA: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 204 SOUTH KOREA: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 205 SOUTH KOREA: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 206 SOUTH KOREA: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 207 SOUTH KOREA: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 208 SOUTH KOREA: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 209 REST OF ASIA PACIFIC: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 210 REST OF ASIA PACIFIC: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 211 REST OF ASIA PACIFIC: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 212 REST OF ASIA PACIFIC: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 213 REST OF ASIA PACIFIC: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 214 LATIN AMERICA: CAR T-CELL THERAPY MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
- TABLE 215 LATIN AMERICA: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 216 LATIN AMERICA: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 217 LATIN AMERICA: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 218 LATIN AMERICA: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 219 LATIN AMERICA: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 220 BRAZIL: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 221 BRAZIL: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 222 BRAZIL: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 223 BRAZIL: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 224 BRAZIL: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 225 REST OF LATIN AMERICA: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 226 REST OF LATIN AMERICA: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 227 REST OF LATIN AMERICA: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 228 REST OF LATIN AMERICA: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 229 REST OF LATIN AMERICA: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 230 MIDDLE EAST & AFRICA: CAR T-CELL THERAPY MARKET, BY PRODUCT, 2022-2029 (USD MILLION)
- TABLE 231 MIDDLE EAST & AFRICA: CAR T-CELL THERAPY MARKET, BY TARGET, 2022-2029 (USD MILLION)
- TABLE 232 MIDDLE EAST & AFRICA: CAR T-CELL THERAPY MARKET, BY INDICATION, 2022-2029 (USD MILLION)
- TABLE 233 MIDDLE EAST & AFRICA: CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2022-2029 (USD MILLION)
- TABLE 234 MIDDLE EAST & AFRICA: CAR T-CELL THERAPY MARKET, BY END USER, 2022-2029 (USD MILLION)
- TABLE 235 OVERVIEW OF STRATEGIES DEPLOYED BY KEY MANUFACTURING COMPANIES
- TABLE 236 CAR T-CELL THERAPY MARKET: DEGREE OF COMPETITION
- TABLE 237 CAR T-CELL THERAPY MARKET: PRODUCT FOOTPRINT
- TABLE 238 CAR T-CELL THERAPY MARKET: TARGET FOOTPRINT
- TABLE 239 CAR T-CELL THERAPY MARKET: INDICATION FOOTPRINT
- TABLE 240 CAR T-CELL THERAPY MARKET: REGION FOOTPRINT
- TABLE 241 CAR T-CELL THERAPY MARKET: DETAILED LIST OF KEY STARTUP/SME PLAYERS
- TABLE 242 CAR T-CELL THERAPY MARKET: COMPETITIVE BENCHMARKING OF KEY EMERGING PLAYERS/STARTUPS
- TABLE 243 CAR T-CELL THERAPY MARKET: PRODUCT LAUNCHES & APPROVALS, JANUARY 2021-MARCH 2024
- TABLE 244 CAR T-CELL THERAPY MARKET: DEALS, JANUARY 2021-MARCH 2024
- TABLE 245 CAR T-CELL THERAPY MARKET: EXPANSIONS, JANUARY 2021-MARCH 2024
- TABLE 246 CAR T-CELL THERAPY MARKET: OTHER DEVELOPMENTS, JANUARY 2021-MARCH 2024
- TABLE 247 BRISTOL-MYERS SQUIBB COMPANY: COMPANY OVERVIEW
- TABLE 248 BRISTOL-MYERS SQUIBB COMPANY: PRODUCTS OFFERED
- TABLE 249 BRISTOL-MYERS SQUIBB COMPANY: PRODUCT APPROVALS, JANUARY 2021-MAY 2024
- TABLE 250 BRISTOL-MYERS SQUIBB COMPANY: DEALS, JANUARY 2021-MAY 2024
- TABLE 251 GILEAD SCIENCES, INC.: COMPANY OVERVIEW
- TABLE 252 GILEAD SCIENCES, INC.: PRODUCTS OFFERED
- TABLE 253 GILEAD SCIENCES, INC.: PRODUCT APPROVALS, JANUARY 2021-MAY 2024
- TABLE 254 GILEAD SCIENCES, INC.: DEALS, JANUARY 2021-MAY 2024
- TABLE 255 GILEAD SCIENCES, INC.: OTHER DEVELOPMENTS, JANUARY 2021-MAY 2024
- TABLE 256 NOVARTIS AG: COMPANY OVERVIEW
- TABLE 257 NOVARTIS AG: PRODUCTS OFFERED
- TABLE 258 NOVARTIS AG: PRODUCT LAUNCHES & APPROVALS, JANUARY 2021-MAY 2024
- TABLE 259 NOVARTIS AG: EXPANSIONS, JANUARY 2021-MAY 2024
- TABLE 260 NOVARTIS AG: OTHER DEVELOPMENTS, JANUARY 2021-MAY 2024
- TABLE 261 JOHNSON & JOHNSON: COMPANY OVERVIEW
- TABLE 262 JOHNSON & JOHNSON: PRODUCTS OFFERED
- TABLE 263 JOHNSON & JOHNSON: PRODUCT APPROVALS, JANUARY 2021-MAY 2024
- TABLE 264 JOHNSON & JOHNSON: DEALS, JANUARY 2021-MAY 2024
- TABLE 265 JW (CAYMAN) THERAPEUTICS CO. LTD.: COMPANY OVERVIEW
- TABLE 266 JW (CAYMAN) THERAPEUTICS CO. LTD.: PRODUCTS OFFERED
- TABLE 267 JW (CAYMAN) THERAPEUTICS CO. LTD.: PRODUCT APPROVALS, JANUARY 2021-MAY 2024
- TABLE 268 JW (CAYMAN) THERAPEUTICS CO. LTD.: DEALS, JANUARY 2021-MAY 2024
- TABLE 269 IMMUNOADOPTIVE CELL THERAPY PRIVATE LIMITED: COMPANY OVERVIEW
- TABLE 270 IMMUNOADOPTIVE CELL THERAPY PRIVATE LIMITED: PRODUCTS OFFERED
- TABLE 271 IMMUNOADOPTIVE CELL THERAPY PRIVATE LIMITED: DEALS, JANUARY 2021-MAY 2024
- TABLE 272 IMMUNOADOPTIVE CELL THERAPY PRIVATE LIMITED: OTHER DEVELOPMENTS, JANUARY 2021-MAY 2024
- TABLE 273 CARSGEN THERAPEUTICS HOLDINGS LIMITED: COMPANY OVERVIEW
- TABLE 274 CARSGEN THERAPEUTICS HOLDINGS LIMITED: PRODUCTS OFFERED
- TABLE 275 CARSGEN THERAPEUTICS HOLDINGS LIMITED: PRODUCT APPROVALS, JANUARY 2021-MAY 2024
- TABLE 276 CARSGEN THERAPEUTICS HOLDINGS LIMITED: DEALS, JANUARY 2021-MAY 2024
- TABLE 277 CARSGEN THERAPEUTICS HOLDINGS LIMITED: EXPANSIONS, JANUARY 2021-MAY 2024
- TABLE 278 IASO BIOTHERAPEUTICS: COMPANY OVERVIEW
- TABLE 279 IASO BIOTHERAPEUTICS: PRODUCTS OFFERED
- TABLE 280 IASO BIOTHERAPEUTICS: PRODUCT APPROVALS, JANUARY 2021-MAY 2024
- TABLE 281 IASO BIOTHERAPEUTICS: DEALS, JANUARY 2021-MAY 2024
List of Figures
- FIGURE 1 CAR T-CELL THERAPY MARKET SEGMENTATION
- FIGURE 2 CAR T-CELL THERAPY MARKET: REGIONAL SEGMENTATION
- FIGURE 3 RESEARCH DESIGN
- FIGURE 4 BREAKDOWN OF PRIMARIES: CAR T-CELL THERAPY MARKET
- FIGURE 5 CAR T-CELL THERAPY MARKET SIZE ESTIMATION (SUPPLY-SIDE ANALYSIS), 2023
- FIGURE 6 CAR T-CELL THERAPY MARKET SIZE ESTIMATION (PRODUCT-BASED ANALYSIS), 2023
- FIGURE 7 MARKET SIZE ESTIMATION: COMPANY REVENUE ANALYSIS-BASED ESTIMATION (PRODUCTS), 2023
- FIGURE 8 ILLUSTRATIVE EXAMPLE OF GILEAD SCIENCES, INC.: REVENUE SHARE ANALYSIS (2023)
- FIGURE 9 MARKET VALIDATION FROM PRIMARY SOURCES
- FIGURE 10 MARKET SIZE ESTIMATION METHODOLOGY: TOP-DOWN APPROACH
- FIGURE 11 CAR T-CELL THERAPY MARKET: CAGR PROJECTIONS, 2024-2029
- FIGURE 12 DATA TRIANGULATION METHODOLOGY
- FIGURE 13 CAR T-CELL THERAPY MARKET, BY PRODUCT, 2024 VS 2029 (USD MILLION)
- FIGURE 14 CAR T-CELL THERAPY MARKET, BY TARGET, 2024 VS. 2029 (USD MILLION)
- FIGURE 15 CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC, 2024 VS. 2029 (USD MILLION)
- FIGURE 16 CAR T-CELL THERAPY MARKET, BY INDICATION, 2024 VS. 2029 (USD MILLION)
- FIGURE 17 CAR T-CELL THERAPY MARKET, BY END USER, 2024 VS. 2029 (USD MILLION)
- FIGURE 18 GEOGRAPHICAL SNAPSHOT OF CAR T-CELL THERAPY MARKET
- FIGURE 19 GROWING PREVALENCE OF CANCER TO DRIVE MARKET
- FIGURE 20 US AND YESCARTA COMMANDED LARGEST SHARE IN NORTH AMERICAN CAR T-CELL THERAPY MARKET IN 2023
- FIGURE 21 YESCARTA HELD HIGHEST GLOBAL MARKET SHARE IN 2023
- FIGURE 22 HOSPITALS SEGMENT TO GROW AT HIGHEST CAGR DURING STUDY PERIOD
- FIGURE 23 CAR T-CELL THERAPY MARKET: DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES
- FIGURE 24 REVENUE SHIFT IN CAR T-CELL THERAPY MARKET
- FIGURE 25 VALUE CHAIN ANALYSIS: CAR T-CELL THERAPY PRODUCTS
- FIGURE 26 CAR T-CELL THERAPY MARKET: ECOSYSTEM
- FIGURE 27 TOTAL NUMBER OF PATENTS GRANTED IN CAR T-CELL THERAPY MARKET, 2014-2023
- FIGURE 28 TOP 13 PLAYERS WITH HIGHEST NUMBER OF PATENT APPLICATIONS, 2014-2024
- FIGURE 29 REGIONAL ANALYSIS OF PATENTS GRANTED, 2014-2024
- FIGURE 30 CAR T-CELL THERAPY MARKET
- FIGURE 31 KEY STAKEHOLDERS IN PRODUCT BUYING PROCESS
- FIGURE 32 KEY BUYING CRITERIA FOR PRODUCTS
- FIGURE 33 NORTH AMERICA: CAR T-CELL THERAPY MARKET SNAPSHOT
- FIGURE 34 ASIA PACIFIC: CAR T-CELL THERAPY MARKET SNAPSHOT
- FIGURE 35 REVENUE ANALYSIS OF KEY PLAYERS IN CAR T-CELL THERAPY MARKET (2021-2023)
- FIGURE 36 MARKET SHARE ANALYSIS OF KEY PLAYERS IN CAR T-CELL THERAPY MARKET (2023)
- FIGURE 37 RANKING OF KEY PLAYERS IN CAR T-CELL THERAPY MARKET, 2023
- FIGURE 38 CAR T-CELL THERAPY MARKET: COMPANY EVALUATION MATRIX (KEY PLAYERS), 2023
- FIGURE 39 CAR T-CELL MARKET: COMPANY FOOTPRINT
- FIGURE 40 CAR T-CELL THERAPY MARKET: COMPANY EVALUATION MATRIX (STARTUPS/SMES), 2023
- FIGURE 41 EV/EBITDA OF KEY VENDORS
- FIGURE 42 YEAR-TO-DATE (YTD) PRICE TOTAL RETURN AND 5-YEAR STOCK BETA OF KEY VENDORS
- FIGURE 43 CAR T-CELL THERAPY MARKET: BRAND/PRODUCT COMPARATIVE ANALYSIS
- FIGURE 44 BRISTOL-MYERS SQUIBB COMPANY: COMPANY SNAPSHOT (2023)
- FIGURE 45 GILEAD SCIENCES, INC.: COMPANY SNAPSHOT (2023)
- FIGURE 46 NOVARTIS AG: COMPANY SNAPSHOT (2023)
- FIGURE 47 JOHNSON & JOHNSON: COMPANY SNAPSHOT (2023)
- FIGURE 48 JW (CAYMAN) THERAPEUTICS CO. LTD.: COMPANY SNAPSHOT (2023)
The global CAR T-cell therapy market is estimated to reach USD 29.0 Billion by 2029 from USD 5.5 billion in 2024, at a CAGR of 39.6% during the forecast period of 2024 to 2029. Market growth is driven by the rise in the global cancer prevalence. Technological advancements in CAR T-cell therapies and rising therapy development investment and funding is likely to propel the market growth. However, adverse effects associated with CAR T-cell therapy and high treatment costs are some of the major factors expected to restrain the market growth during the forecast period.
| Scope of the Report | |
|---|---|
| Years Considered for the Study | 2022-2029 |
| Base Year | 2023 |
| Forecast Period | 2024-2029 |
| Units Considered | Value (USD Billion) |
| Segments | By Product, By Target, By Indication, By Demographic & By End User |
| Regions covered | North America, Europe, the Asia Pacific, Latin America and the Middle East & Africa |
"Yescarta segment accounted for the highest share of 2023."
Based on product, the CAR T-cell therapy market is broadly segmented into Abecma (idecabtagene vicleucel), Breyanzi (lisocabtagene maraleucel), Carvykti (ciltacabtagene autoleucel), Yescarta (axicabtagene ciloleucel), Tecartus (brexucabtagene autoleucel), Kymriah (tisagenlecleucel) and other products. The Yescarta segment held the largest market share in 2023. Market growth is attributed to high response rates and durable remissions of Yescarta in treating relapsed/refractory cancers leading to an increase in therapy adoption.
"The Hospitals End User segment held the dominant share in the CAR T-cell therapy market."
Based on end users, the global CAR T-cell therapy market is segmented into hospitals, long term care facilities and specialty centers. In 2023, the hospitals segment emerged as the primary growth driver in the global CAR T-cell therapy market, with the highest CAGR during the forecast period. Hospitals are incorporating CAR T-cell therapies into their standard oncology treatment protocols. This trend is boosting demand for CAR T-cell treatments as more hospitals embrace these therapies. Furthermore, hospitals play a key role in facilitating clinical trials for CAR T-cell therapies. By actively participating in research initiatives and collaborations with pharmaceutical companies, hospitals contribute to expanding the evidence base and accelerating regulatory approvals, which in turn stimulates market growth.
"Asia Pacific region estimated to show fastest growth rate."
The CAR T-cell therapy market is segmented into North America, Europe, Asia Pacific, Latin America and the Middle East & Africa. During the forecast period, Asia Pacific region is estimated to grow at the highest CAGR. The supportive regulatory frameworks, rich CAR T-cell therapy product pipeline and expedited approval processes facilitate quicker market entry for innovative therapies, driving growth in the Asia Pacific market. In addition, increasing collaborations between hospitals, universities, and industries in Chinaare expected to provide lucrative market growth. The rapid increase in clinical trials evaluating the safety and effectiveness of CAR T-cell therapy across diverse cancer types in China also signals significant advancements. These studies have demonstrated encouraging results in treating leukemia, lymphoma, and solid tumors, with specific patient cohorts showing substantial rates of complete remission and sustained responses. This trend highlights Asia Pacific's progress in developing and validating CAR T-cell treatments, positioning the region as a key player in advancing therapeutic outcomes in oncology in CAR T-cell therapy.
The primary interviews conducted for this report can be categorized as follows:
- By Respondent: Supply Side- 70%, and Demand Side - 30%
- By Designation (Supply Side): Managers - 45%, CXOs & Directors - 30%, Executives- 25%
- By Region: North America -40%, Europe -25%, Asia-Pacific -20%, Latin America -10%, MEA- 5%
List of Companies Profiled in the Report:
- Bristol-Myers Squibb Company (US)
- Gilead Sciences Inc. (US)
- Novartis AG (Switzerland)
- Johnson & Johnson (US)
- CARsgen Therapeutics Holdings Limited (China)
- IASO Biotherapeutics (China)
- JW (Cayman) Therapeutics Co. Ltd (China)
- ImmunoAct (India)
- CRISPR Therapeutics (Switzerland)
- Autolus Therapeutics (UK)
- Allogene Therapeutics (US)
- Cartesian Therapeutics Inc. (US)
- Guangzhou Bio-gene Technology Co. Ltd (China)
- Wugen (US).
Research Coverage:
This report provides a detailed picture of the CAR T-cell therapy market. It aims to estimate the size and future growth potential of the market across different segments, such as product, target, demographic, indications, end-users and region. The report also includes an in-depth competitive analysis of the key market players, along with their company profiles, recent developments, and key market strategies.
Key Benefits of Buying the Report:
The report will help market leaders/new entrants by providing them with the closest approximations of the revenue numbers for the overall CAR T-cell therapy market and its subsegments. It will also help stakeholders better understand the competitive landscape and gain more insights to better position their business and make suitable go-to-market strategies. This report will enable stakeholders to understand the market's pulse and provide them with information on the key market drivers, restraints, opportunities, and challenges.
The report provides insights on the following pointers:
- Analysis of key drivers (technological advancements in CAR T-cell therapies, growing cancer prevalence, and rising investment and funding for therapy development), restraints (high therapy costs and adverse effects), opportunities (expansion into solid tumors and collaborations and partnerships) and challenges (patient recruitment for trials and reimbursement issues) are influencing the growth of CAR T-cell therapy market.
- Product Development/Innovation: Detailed insights on newly launched products of the CAR T-cell therapy market.
- Market Development: Comprehensive information about lucrative markets - the report analyses the CAR T-cell therapy market across varied regions.
- Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the CAR T-cell therapy market.
- Competitive Assessment: In-depth assessment of market shares, growth strategies, and product offerings of leading players include Bristol-Myers Squibb Company (US), Gilead Sciences, Inc. (US), Novartis AG (Switzerland), Johnson & Johnson (US), JW (Cayman) Therapeutics Co. Ltd (China), ImmunoAct (India), among others in the CAR T-cell therapy market.
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 STUDY OBJECTIVES
- 1.2 MARKET DEFINITION
- 1.2.1 INCLUSIONS & EXCLUSIONS
- 1.3 MARKET SCOPE
- 1.3.1 MARKETS COVERED
- 1.3.2 REGIONS COVERED
- 1.3.3 YEARS CONSIDERED
- 1.3.4 CURRENCY CONSIDERED
- 1.4 STAKEHOLDERS
- 1.5 RECESSION IMPACT
2 RESEARCH METHODOLOGY
- 2.1 RESEARCH DATA
- 2.1.1 SECONDARY DATA
- 2.1.2 PRIMARY DATA
- 2.1.3 BREAKDOWN OF PRIMARIES
- 2.2 MARKET SIZE ESTIMATION
- 2.2.1 INSIGHTS FROM PRIMARY EXPERTS
- 2.2.2 SEGMENTAL MARKET SIZE ESTIMATION
- 2.3 GROWTH RATE ASSUMPTIONS
- 2.3.1 CAGR PROJECTIONS
- 2.3.2 IMPACT OF SUPPLY-SIDE AND DEMAND-SIDE FACTORS
- 2.4 VOLUME ESTIMATION
- 2.5 MARKET BREAKDOWN AND DATA TRIANGULATION
- 2.6 RESEARCH LIMITATIONS
- 2.7 STUDY ASSUMPTIONS
- 2.8 RISK ANALYSIS
- 2.9 RECESSION IMPACT ANALYSIS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
- 4.1 CAR T-CELL THERAPY MARKET OVERVIEW
- 4.2 NORTH AMERICA: CAR T-CELL THERAPY MARKET, BY PRODUCT AND COUNTRY (2023)
- 4.3 CAR T-CELL THERAPY MARKET SHARE, BY PRODUCT
- 4.4 CAR T-CELL THERAPY MARKET, BY END USER
5 MARKET OVERVIEW
- 5.1 INTRODUCTION
- 5.2 MARKET DYNAMICS
- 5.2.1 DRIVERS
- 5.2.1.1 Growing cancer prevalence
- 5.2.1.2 Technological advancements in CAR T-cell therapies
- 5.2.1.3 Rising investment and funding for therapy development
- 5.2.2 RESTRAINTS
- 5.2.2.1 High therapy costs
- 5.2.2.2 Adverse effects
- 5.2.3 OPPORTUNITIES
- 5.2.3.1 Expansion into solid tumors
- 5.2.3.2 Collaborations and partnerships
- 5.2.4 CHALLENGES
- 5.2.4.1 Patient recruitment for trials
- 5.2.4.2 Reimbursement issues
- 5.2.1 DRIVERS
- 5.3 TRENDS/DISRUPTIONS IMPACTING CUSTOMERS' BUSINESSES
- 5.4 VALUE CHAIN ANALYSIS
- 5.5 ECOSYSTEM ANALYSIS
- 5.5.1 PRODUCTS
- 5.5.2 END USERS
- 5.5.3 REGULATORY BODIES
- 5.6 TECHNOLOGY ANALYSIS
- 5.6.1 KEY TECHNOLOGIES
- 5.6.1.1 CAR DESIGN AND OPTIMIZATION
- 5.6.1.2 VIRAL VECTOR TECHNOLOGY
- 5.6.1.3 CELL CULTURE AND EXPANSION TECHNIQUES
- 5.6.2 COMPLIMENTARY TECHNOLOGIES
- 5.6.2.1 GENE EDITING TECHNOLOGY (CRISPR-CAS9-BASED GENOME EDITING)
- 5.6.3 ADJACENT TECHNOLOGIES
- 5.6.3.1 MONITORING AND IMAGING TECHNOLOGIES
- 5.6.1 KEY TECHNOLOGIES
- 5.7 PATENT ANALYSIS
- 5.7.1 METHODOLOGY
- 5.7.2 NUMBER OF PATENTS FILED
- 5.7.3 INNOVATION AND PATENT APPLICATIONS
- 5.7.4 TOP APPLICANTS
- 5.8 PRICING ANALYSIS
- 5.8.1 AVERAGE SELLING PRICE, BY TYPE
- 5.8.2 AVERAGE SELLING PRICE, BY REGION
- 5.8.3 AVERAGE SELLING PRICE TREND, BY PRODUCT
- 5.9 KEY CONFERENCES & EVENTS, 2024-2O25
- 5.10 REGULATORY LANDSCAPE
- 5.10.1 REGULATORY SCENARIO
- 5.10.2 REGULATORY BODIES, GOVERNMENT AGENCIES, AND OTHER ORGANIZATIONS
- 5.11 INVESTMENT & FUNDING SCENARIO
- 5.12 PORTER'S FIVE FORCES ANALYSIS
- 5.12.1 INTENSITY OF COMPETITIVE RIVALRY
- 5.12.2 BARGAINING POWER OF SUPPLIERS
- 5.12.3 BARGAINING POWER OF BUYERS
- 5.12.4 THREAT OF SUBSTITUTES
- 5.12.5 THREAT OF NEW ENTRANTS
- 5.13 KEY STAKEHOLDERS & BUYING CRITERIA
- 5.13.1 KEY BUYING CRITERIA
6 CAR T-CELL THERAPY MARKET, BY PRODUCT
- 6.1 INTRODUCTION
- 6.2 YESCARTA
- 6.2.1 INCREASING REACH TO DRIVE MARKET
- 6.3 KYMRIAH
- 6.3.1 APPROVAL FOR PEDIATRIC USE TO SUPPORT MARKET GROWTH
- 6.4 CARVYKTI
- 6.4.1 WIDESPREAD REACH TO ENSURE MARKET GROWTH
- 6.5 ABECMA
- 6.5.1 RISING RESEARCH FOR ALTERNATIVE INDICATIONS TO DRIVE MARKET
- 6.6 TECARTUS
- 6.6.1 GROWING FOCUS ON PATIENT-CENTRIC REIMBURSEMENT TO PROPEL MARKET
- 6.7 BREYANZI
- 6.7.1 MULTIPLE INDICATIONS TO PROPEL MARKET GROWTH
- 6.8 OTHER PRODUCTS
7 CAR T-CELL THERAPY MARKET, BY TARGET
- 7.1 INTRODUCTION
- 7.2 CD19
- 7.2.1 RISING INDICATIONS IN PIPELINE TO DRIVE MARKET
- 7.3 BCMA
- 7.3.1 TECHNOLOGICAL ADVANCEMENTS IN CAR T-CELL THERAPY TO PROPEL MARKET GROWTH
- 7.4 OTHER TARGETS
8 CAR T-CELL THERAPY MARKET, BY INDICATION
- 8.1 INTRODUCTION
- 8.2 MULTIPLE MYELOMA
- 8.2.1 RISING INCIDENCE OF MULTIPLE MYELOMA TO DRIVE MARKET
- 8.3 B-CELL LYMPHOMA
- 8.3.1 RISING PREVALENCE TO PROPEL MARKET GROWTH
- 8.4 ACUTE LYMPHOBLASTIC LEUKEMIA
- 8.4.1 FOCUS ON AFFORDABLE CAR T-CELL THERAPIES TO DRIVE MARKET
- 8.5 OTHER INDICATIONS
9 CAR T-CELL THERAPY MARKET, BY DEMOGRAPHIC
- 9.1 INTRODUCTION
- 9.2 ADULTS
- 9.2.1 INCREASING NUMBER OF LYMPHOMAS TO DRIVE MARKET
- 9.3 PEDIATRICS
- 9.3.1 RISING PREVALENCE OF TARGET DISEASES TO PROPEL MARKET GROWTH
10 CAR T-CELL THERAPY MARKET, BY END USER
- 10.1 INTRODUCTION
- 10.2 HOSPITALS
- 10.2.1 GROWING PREVALENCE OF CANCER TO DRIVE MARKET
- 10.3 SPECIALTY CENTERS
- 10.3.1 GROWING RESEARCH COLLABORATIONS TO PROPEL MARKET
- 10.4 LONG-TERM CARE FACILITIES
- 10.4.1 GROWING CASES OF CHRONIC DISORDERS TO DRIVE MARKET
11 CAR T-CELL THERAPY MARKET, BY REGION
- 11.1 INTRODUCTION
- 11.2 NORTH AMERICA
- 11.2.1 NORTH AMERICA: RECESSION IMPACT
- 11.2.2 US
- 11.2.2.1 Rising R&D activities for CAR T-cell therapies to drive market
- 11.2.3 CANADA
- 11.2.3.1 Government initiatives for regenerative medicine research to drive market
- 11.3 EUROPE
- 11.3.1 EUROPE: RECESSION IMPACT
- 11.3.2 GERMANY
- 11.3.2.1 Rising focus on clinical research to drive market
- 11.3.3 UK
- 11.3.3.1 Rising technological advancements in automation to drive market
- 11.3.4 FRANCE
- 11.3.4.1 Growing focus on cell & gene therapy initiatives to boost demand
- 11.3.5 ITALY
- 11.3.5.1 Growth in biotech sector to drive market
- 11.3.6 SPAIN
- 11.3.6.1 Rising focus on cell therapies to support market growth
- 11.3.7 REST OF EUROPE
- 11.4 ASIA PACIFIC
- 11.4.1 ASIA PACIFIC: RECESSION IMPACT
- 11.4.2 CHINA
- 11.4.2.1 Rising number of CAR T-cell clinical trials to support market growth
- 11.4.3 JAPAN
- 11.4.3.1 Increasing product approvals to drive market
- 11.4.4 INDIA
- 11.4.4.1 Rising incidence of cancer and growing focus on product commercialization to boost demand
- 11.4.5 AUSTRALIA
- 11.4.5.1 Robust infrastructure for clinical trials and well-developed healthcare sector to drive market
- 11.4.6 SOUTH KOREA
- 11.4.6.1 Rising growth in biopharmaceutical industry to drive market
- 11.4.7 REST OF ASIA PACIFIC
- 11.5 LATIN AMERICA
- 11.5.1 LATIN AMERICA: RECESSION IMPACT
- 11.5.2 BRAZIL
- 11.5.2.1 High expenditure on healthcare to support market growth
- 11.5.3 REST OF LATIN AMERICA
- 11.6 MIDDLE EAST & AFRICA
- 11.6.1 MEA TO GROW AT SLOWER PACE DURING FORECAST PERIOD
- 11.6.2 MIDDLE EAST & AFRICA: RECESSION IMPACT
12 COMPETITIVE LANDSCAPE
- 12.1 OVERVIEW
- 12.2 KEY PLAYER STRATEGY/RIGHT TO WIN
- 12.2.1 OVERVIEW OF STRATEGIES ADOPTED BY PLAYERS IN CAR T-CELL THERAPY MARKET
- 12.3 REVENUE ANALYSIS
- 12.4 MARKET SHARE ANALYSIS
- 12.4.1 RANKING OF KEY MARKET PLAYERS
- 12.5 COMPANY EVALUATION MATRIX: KEY PLAYERS, 2023
- 12.5.1 STARS
- 12.5.2 EMERGING LEADERS
- 12.5.3 PERVASIVE PLAYERS
- 12.5.4 PARTICIPANTS
- 12.5.5 COMPANY FOOTPRINT: KEY PLAYERS, 2023
- 12.5.5.1 Company footprint
- 12.5.5.2 Product footprint
- 12.5.5.3 Target footprint
- 12.5.5.4 Indication footprint
- 12.5.5.5 Region footprint
- 12.6 COMPANY EVALUATION MATRIX: STARTUPS/SMES, 2023
- 12.6.1 PROGRESSIVE COMPANIES
- 12.6.2 RESPONSIVE COMPANIES
- 12.6.3 DYNAMIC COMPANIES
- 12.6.4 STARTING BLOCKS
- 12.6.5 COMPETITIVE BENCHMARKING: STARTUPS/SMES, 2023
- 12.7 VALUATION & FINANCIAL METRICS
- 12.7.1 FINANCIAL METRICS
- 12.7.2 COMPANY VALUATION
- 12.8 BRAND/PRODUCT COMPARISON
- 12.9 COMPETITIVE SCENARIO
- 12.9.1 PRODUCT LAUNCHES & APPROVALS
- 12.9.2 DEALS
- 12.9.3 EXPANSIONS
- 12.9.4 OTHER DEVELOPMENTS
13 COMPANY PROFILES
- 13.1 KEY PLAYERS
- 13.1.1 BRISTOL-MYERS SQUIBB COMPANY
- 13.1.1.1 Business overview
- 13.1.1.2 Products offered
- 13.1.1.3 Recent developments
- 13.1.1.3.1 Product approvals
- 13.1.1.3.2 Deals
- 13.1.1.4 MnM view
- 13.1.1.4.1 Key strengths
- 13.1.1.4.2 Strategic choices
- 13.1.1.4.3 Weaknesses & competitive threats
- 13.1.2 GILEAD SCIENCES, INC.
- 13.1.2.1 Business overview
- 13.1.2.2 Products offered
- 13.1.2.3 Recent developments
- 13.1.2.3.1 Product approvals
- 13.1.2.3.2 Deals
- 13.1.2.3.3 Other developments
- 13.1.2.4 MnM view
- 13.1.2.4.1 Key strengths
- 13.1.2.4.2 Strategic choices
- 13.1.2.4.3 Weaknesses & competitive threats
- 13.1.3 NOVARTIS AG
- 13.1.3.1 Business overview
- 13.1.3.2 Products offered
- 13.1.3.3 Recent developments
- 13.1.3.3.1 Product launches & approvals
- 13.1.3.3.2 Expansions
- 13.1.3.3.3 Other developments
- 13.1.3.4 MnM view
- 13.1.3.4.1 Key strengths
- 13.1.3.4.2 Strategic choices
- 13.1.3.4.3 Weaknesses and competitive threats
- 13.1.4 JOHNSON & JOHNSON
- 13.1.4.1 Business overview
- 13.1.4.2 Products offered
- 13.1.4.3 Recent developments
- 13.1.4.3.1 Product approvals
- 13.1.4.3.2 Deals
- 13.1.5 JW (CAYMAN) THERAPEUTICS CO. LTD.
- 13.1.5.1 Business overview
- 13.1.5.2 Products offered
- 13.1.5.3 Recent developments
- 13.1.5.3.1 Product approvals
- 13.1.5.3.2 Deals
- 13.1.6 IMMUNOADOPTIVE CELL THERAPY PRIVATE LIMITED (IMMUNOACT)
- 13.1.6.1 Business overview
- 13.1.6.2 Products offered
- 13.1.6.3 Recent developments
- 13.1.6.3.1 Deals
- 13.1.6.3.2 Other developments
- 13.1.7 CARSGEN THERAPEUTICS HOLDINGS LIMITED
- 13.1.7.1 Business overview
- 13.1.7.2 Products offered
- 13.1.7.3 Recent developments
- 13.1.7.3.1 Product approvals
- 13.1.7.3.2 Deals
- 13.1.7.3.3 Expansions
- 13.1.8 IASO BIOTHERAPEUTICS
- 13.1.8.1 Business overview
- 13.1.8.2 Products offered
- 13.1.8.3 Recent developments
- 13.1.8.3.1 Product approvals
- 13.1.8.3.2 Deals
- 13.1.1 BRISTOL-MYERS SQUIBB COMPANY
- 13.2 OTHER PLAYERS
- 13.2.1 GUANGZHOU BIO-GENE TECHNOLOGY CO., LTD (HEDY GROUP CO., LTD.)
- 13.2.2 CARTESIAN THERAPEUTICS, INC.
- 13.2.3 AUTOLUS THERAPEUTICS
- 13.2.4 ALLOGENE THERAPEUTICS
- 13.2.5 CRISPR THERAPEUTICS
- 13.2.6 WUGEN
14 APPENDIX
- 14.1 DISCUSSION GUIDE
- 14.2 KNOWLEDGESTORE: MARKETSANDMARKETS' SUBSCRIPTION PORTAL
- 14.3 CUSTOMIZATION OPTIONS
- 14.4 RELATED REPORTS
- 14.5 AUTHOR DETAILS