|
|
市場調査レポート
商品コード
1266481
世界のドーパミン細胞置換療法の臨床試験、企業、市場動向:2023年Global Dopamine Cell Replacement Therapy Clinical Trials , Companies & Market Trends Insight 2023 |
||||||
| 世界のドーパミン細胞置換療法の臨床試験、企業、市場動向:2023年 |
|
出版日: 2023年04月01日
発行: KuicK Research
ページ情報: 英文 75 Pages
納期: 即日から翌営業日
|
- 全表示
- 概要
- 図表
- 目次
世界人口の高齢化に伴い、神経変性疾患の発症率は上昇傾向にあり、医療における大きなアンメットニーズとなっています。このことは、再生医療の開発・商業化に携わる製薬会社やバイオテクノロジー企業にとって、大きな商機となります。ドーパミン細胞補充療法は、パーキンソン病やその他の神経変性疾患に苦しむ患者さんに長期的かつ効果的な治療法を提供することができる、大きな可能性を秘めています。
当レポートでは、世界のドーパミン細胞置換療法市場について調査し、市場の概要と臨床試験について、企業別、適応症別、相別の動向、市場の課題、および市場に参入する企業の競合情勢などをまとめています。
目次
第1章 ドーパミン細胞療法のイントロダクション
第2章 ドーパミン細胞療法- 作用機序
- 幹細胞ベースのアプローチ
- 胎児細胞の置換
第3章 世界のドーパミン細胞置換療法の臨床試験の概要
- 企業別
- 国別
- 適応症別
- 相別
第4章 世界のドーパミン細胞置換療法の臨床試験の企業別、適応症別、相別
- 研究
- 前臨床
- 第I相
- 第I/II相
- 事前登録
第5章 現在のドーパミン細胞療法の市場シナリオ
- 規制指定
- 臨床スタンス
- 市場動向、コラボレーション、ライセンシングと資金調達
第6章 ドーパミン細胞置換治療アプローチ、企業別
第7章 ドーパミン細胞療法市場力学
- 市場の促進要因
- 市場の課題
第8章 ドーパミン細胞置換療法の今後の展望
第9章 競合情勢
- Aspen Neuroscience
- BlueRock Therapeutics
- Sumitomo Pharma
- Tel Aviv University
- The Scripps Research Institute
List of Figures
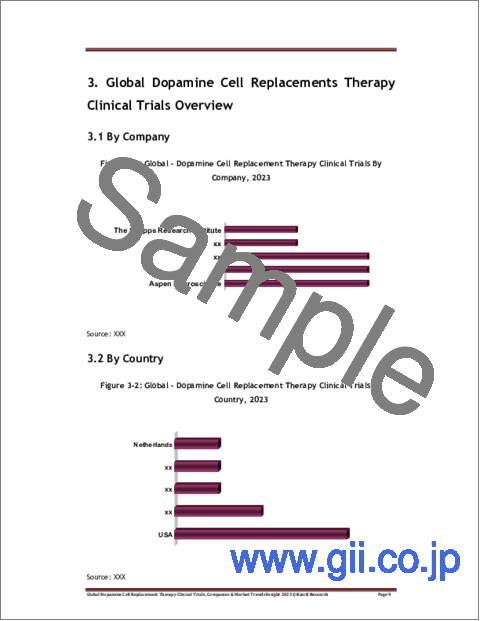
- Figure 3-1: Global - Dopamine Cell Replacement Therapy Clinical Trials By
Company, 2023
- Figure 3-2: Global - Dopamine Cell Replacement Therapy Clinical Trials By Country,
2023
- Figure 3-3: Global - Dopamine Cell Replacement Therapy Clinical Trials By
Indication, 2023
- Figure 3-4: Global - Dopamine Cell Replacement Therapy Clinical Trials By Phase,
2023
- Figure 5-1: FDA Drug Approval Pathways
- Figure 5-2: Clinical Development Strategy & Drug Approval By FDA
- Figure 5-3: Multidisciplinary Product Development Strategies
- Figure 6-1: Differentiation of Neural Precursor Cells in TED
- Figure 6-2: TED Technology Mechanism
- Figure 6-3: Expandability of TED Technology Platform
- Figure 6-4: TED Technology used to Develop TED-A9
- Figure 6-5: Clinical Pipeline of Aspen Neuroscience
- Figure 6-6: Aspen Neuroscience's Autologous Cell Replacement Therapy
- Figure 6-7: BlueRock Therapeutics - Authentic Cells
- Figure 6-8: Production of MSC-NTF Cells
- Figure 6-9: Multiple Applications of Innervace's Technology Platform
- Figure 6-10: Innervace - Bioengineered Live Active Neurons
- Figure 6-11: Advantages & Opportunities Associated with Innervace's Technology
- Figure 6-12: FUJIFILM Cellular Dynamics - Making Induced Pluripotent Stem Cells
- Figure 6-13: Sumitomo Pharma - iPS Drug Discovery Technology
- Figure 7-1: Dopamine Cell Replacement Therapy Market Drivers
- Figure 7-2: Dopamine Cell Replacement Therapy Market Challenges
- Figure 8-1: Dopamine Cell Replacement Therapy Future Outlook
“Global Dopamine Cell Replacement Therapy Clinical Trials, Companies & Market Trends Insight 2023” Report Highlights:
- Research Methodology
- Global Market Trends, Collaborations, Licensing & Financing
- Global Dopamine Cell Replacement Therapy Clinical Trials By Company, Indication & Phase
- Dopamine Cell Replacement Therapeutic Approach/Technology Platform By Company
- Dopamine Cell Replacement Therapy Future Outlook
- Insight On Key Companies Involved In Development Of Dopamine Cell Replacement Therapy
Dopamine cell replacement therapy has emerged as new innovative treatment methodology that has the potential to transform the way neurodegenerative diseases such as Parkinson's are treated. Dopamine is a neurotransmitter that plays a critical role in regulating movement, mood, and motivation. In Parkinson's disease, the dopamine-producing cells in the brain gradually die off, leading to a range of weakening symptoms such as tremors, rigidity, and difficulty with balance and coordination. This innovative therapy involves transplanting healthy dopamine-producing cells into the brain to replace the damaged or lost cells that are responsible for the symptoms of the disease.
Dopamine cell replacement approach has shown tremendous promise in preclinical studies and early clinical trials, with many patients experiencing significant improvements in their symptoms. In fact, some patients have been able to reduce or even eliminate their reliance on traditional Parkinson's medications, which can have significant side effects and may lose effectiveness over time. The potential of dopamine cell replacement therapy extends beyond Parkinson's disease, as dopamine dysfunction has been implicated in a range of other neurological and psychiatric disorders. For example, some researchers are investigating the use of dopamine cell replacement therapy in treating depression, ADHD, and even Alzheimer's disease.
As the global population ages, the incidence of neurodegenerative diseases is on the rise, leading to a significant unmet medical need. This presents a massive commercial opportunity for pharmaceutical and biotech companies involved in the development and commercialization of regenerative medicines. The potential of dopamine cell replacement therapy is huge, with the ability to offer a long-lasting and effective treatment for patients suffering from Parkinson's disease and other neurodegenerative diseases.
This innovative approach has gained significant attention from the pharmaceutical industry due to its potential to offer a cure for Parkinson's disease and other related conditions. As the therapy gains attention in the market, the competitive landscape for dopamine cell replacement therapy is slowly expanding. Many companies are investing heavily in research and development to advance the therapy, and the competition is driving innovation in the field. This competition is also encouraging strategic collaborations and partnerships, which are crucial for the advancement of the therapy.
Collaborations, licensing, and financing deals are essential factors for the successful commercialization of dopamine cell replacement therapy. Several pharmaceutical and biotech companies have already entered into partnerships to develop and commercialize dopamine cell replacement therapy. For instance, Ryne Biotechnology, a leading developer of cell therapies, has entered into a partnership with FUJIFILM Cellular Dynamics, a leading contract development and manufacturing organization, to develop and manufacture induced pluripotent stem cell-derived dopaminergic neurons for use in cell replacement therapy.
Even though the market of dopamine cell replacement therapy is at its initial stage, there are quite a few companies and research institutions working to bring this treatment in the market. These companies are conducting robust research to develop new and innovative approaches to dopamine cell replacement therapy. Despite the competition, there is ample room for growth and expansion in the dopamine cell replacement therapy market. As the therapy gains regulatory milestones and clinical acceptance, the market is poised for significant growth in the coming years. In addition, the therapy's potential to treat a range of neurodegenerative diseases beyond Parkinson's disease, including Alzheimer's disease, further increases its commercial potential.
Strategic collaborations and partnerships will also play a crucial role in driving the commercial success of the therapy. As the competitive landscape continues to evolve, companies will need to stay ahead of the curve to remain competitive in the market. The companies that are successful in developing innovative and effective dopamine cell replacement therapies will likely gain a significant advantage over their competitors. In conclusion, dopamine cell replacement therapy has the potential to become a game-changer in the treatment of neurodegenerative diseases. With the growing demand for effective treatments, significant market potential, and strong partnerships, dopamine cell replacement therapy is expected to become a commercially successful therapy in the near future.
Table of Contents
1. Introduction to Dopamine Cell Therapy
- 1.1 Utilizing Dopamine To Treat Neurodegenerative Diseases
- 1.2 Evolution of Dopamine Into Cell Replacement Therapy
2. Dopamine Cell Therapy - Mechanism Of Action
- 2.1 Stem Cell Based Approaches
- 2.2 Fetal Cell Replacement
3. Global Dopamine Cell Replacements Therapy Clinical Trials Overview
- 3.1 By Company
- 3.2 By Country
- 3.3 By Indication
- 3.4 By Phase
4. Global Dopamine Cell Replacement Therapy Clinical Trials By Company, Indication & Phase
- 4.1 Research
- 4.2 Preclinical
- 4.3 Phase-I
- 4.4 Phase-I/II
- 4.5 Preregistration
5. Current Dopamine Cell Therapy Market Scenario
- 5.1 Regulatory Designations
- 5.2 Clinical Stance
- 5.3 Market Trends, Collaborations, Licensing & Financing
6. Dopamine Cell Replacement Therapeutic Approach By Company
7. Dopamine Cell Therapy Market Dynamics
- 7.1 Market Drivers
- 7.2 Market Challenges
8. Dopamine Cell Replacement Therapy Future Outlook
9. Competitive Landscape
- 9.1 Aspen Neuroscience
- 9.2 BlueRock Therapeutics
- 9.3 Sumitomo Pharma
- 9.4 Tel Aviv University
- 9.5 The Scripps Research Institute