|
|
市場調査レポート
商品コード
1241324
四重特異性抗体市場:臨床試験、独自技術、企業、市場動向(2023年)Tetraspecific Antibodies Clinical Trials, Proprietary Technologies, Companies & Market Trends Insight 2023 |
||||||
| 四重特異性抗体市場:臨床試験、独自技術、企業、市場動向(2023年) |
|
出版日: 2023年03月01日
発行: KuicK Research
ページ情報: 英文 78 Pages
納期: 即日から翌営業日
|
- 全表示
- 概要
- 図表
- 目次
四重特異性抗体の開発により、抗体医薬の分野では競合が激しくなっています。様々な疾患や症状に対して、より的を絞った効果的な治療法を提供できる可能性があるため、多くの企業がこれらの新世代抗体の研究開発に多額の投資を行っています。世界の四重特異性抗体市場は、より効果的で個別化された治療法に対する需要の高まりにより、今後数年間で大きく成長すると予測されています。
当レポートでは、世界の四重特異性抗体市場について調査し、市場の概要とともに、四重特異性抗体の作用機序、癌治療における役割、臨床試験動向、および市場に参入する企業の競合動向などを提供しています。
目次
第1章 四重特異性抗体のイントロダクション
第2章 四重特異性抗体の作用機序
第3章 世界の四重特異性抗体の臨床試験:企業別、国別、適応症別、相別
- 企業別
- 国別
- 適応症別
- 相別
第4章 世界の四重特異性抗体臨床試験の洞察
- 研究
- 前臨床
- 第I相
- 第Ⅱ相
第5章 企業別独自技術
第6章 現在の臨床開発と将来の商業化の見通し
- 現在のシナリオ
- 今後の市場商品化の見通し
第7章 四重特異性抗体の治療アプローチ
- 単剤療法としての四特異性抗体
- 併用療法としての四特異性抗体
- 標的アプローチとしての四特異性抗体
第8章 四重特異性抗体の癌治療への応用
第9章 競合情勢
- Innate Pharma
- ModeX Therapeutics (OPKO Health)
- Ruijin Hospital
- Sichuan Baili Pharmaceutical
- SystImmune
List of Figures
- Figure 1-1: FL518 - Structure
- Figure 1-2: CRTB6 - Structure
- Figure 1-3: LegoBody Tetraspecific Antibody Structure
- Figure 1-4: LegoBody Tetra-N-Fab Post Thrombin Cleavage
- Figure 2-1: Tetraspecific Antibody Structure
- Figure 2-2: Tetraspecific Antibody Bringing T Cells & NK Cells In Close Proximity
- Figure 3-1: Global - Tetraspecific Antibodies In Clinical Trials By Company, 2023
- Figure 3-2: Global - Tetraspecific Antibodies In Clinical Trials By Country, 2023
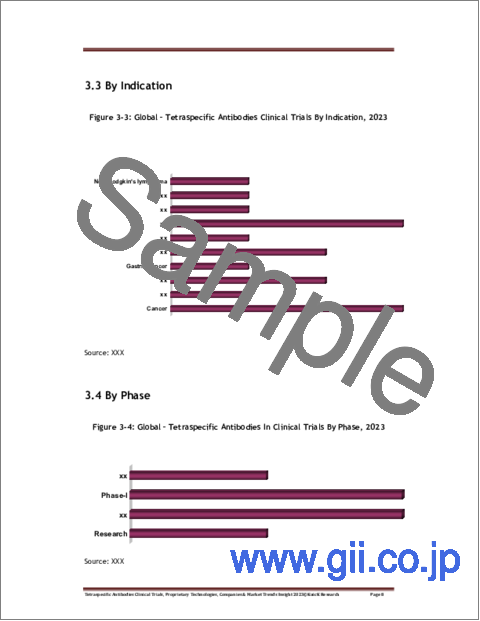
- Figure 3-3: Global - Tetraspecific Antibodies Clinical Trials By Indication, 2023
- Figure 3-4: Global - Tetraspecific Antibodies In Clinical Trials By Phase, 2023
- Figure 5-1: ANKET® Platform - Innate Pharma
- Figure 5-2: GNC Antibodies - Sichuan Baili Pharmaceutical/SystImmune
- Figure 5-3: STEALTH - ModeX Therapeutics
- Figure 5-4: MSTAR - ModeX Therapeutics
- Figure 5-5: Features of MSTAR
- Figure 5-6: MATCH - Numab
- Figure 5-7: Features of BiXAb
- Figure 5-8: Zyngenia Technology Approach
- Figure 5-1: Tetraspecific Antibody GNC035 Targeting Cell Surface Antigen CD3
- Figure 5-2: Tetraspecific Antibody Engaging CD3 T cells
- Figure 5-3: Tetraspecific Antibody GNC039 Targeting Co-Stimulatory Receptor 4-1BB
- Figure 5-4: IPH6501 - Tetra-Specific NK Cell Engager
- Figure 5-5: Tetraspecific Antibody GNC038 Targeting Immune Checkpoint Inhibitor PDL-1
- Figure 5-6: Regulation of CD47 Signal From Cancer Cells By Tetraspecific Antibody
- Figure 5-7: Novel Tetraspecific Format Developed By The Roche Pharma Research & Early Development
- Figure 8-1: GNC-038 Phase I/II Study (NCT04606433) - Initiation & Completion Years
- Figure 8-2: GNC-038 Phase I/II Study (NCT05192486) - Initiation & Completion Years
- Figure 8-3: GNC-038 Phase I/II Study (NCT05485753) - Initiation & Completion Years
- Figure 8-4: GNC-038 Phase I/II Study (NCT05623982) - Initiation & Completion Years
- Figure 8-5: GNC-038 Phase I/II Study (NCT05627856) - Initiation & Completion Years
- Figure 8-6: GNC-039 Phase I/II Study (NCT04794972) - Initiation & Completion Years
- Figure 8-7: GNC-035 Phase I Study (NCT05039931) - Initiation & Completion Years
“Tetraspecific Antibodies Clinical Trials, Proprietary Technologies, Companies & Market Trends Insight 2023” Report Highlights:
- Global Tetraspecific Clinical Trials Landscape Insight
- Global Tetraspecific Ongoing Clinical Trials By Company, Country, Indication & Phase
- Tetraspecific Antibodies Proprietary Technologies By Company
- Current Clinical Development & Future Commercialization Outlook
- Tetraspecific Antibodies Mono & Combination Therapeutic Approaches
- Competitive Landscape
The field of antibody therapeutics has been rapidly evolving in recent years, and one of the most exciting developments has been the transition from monoclonal to bispecific to the now emerging tetraspecific antibodies. These new generation antibodies have the potential to offer more targeted and effective therapies for a range of diseases and conditions. Traditionally, monoclonal antibodies have been used in the development of therapeutics. These are antibodies that are designed to target a single antigen or protein, and have been effective in treating a range of diseases, including cancer, autoimmune disorders, and infectious diseases.
However, tetraspecific antibodies offer a new level of selectivity and flexibility in targeted therapy approach. These multispecific antibodies are designed to target four different antigens or proteins, making them more targeted and effective in treating complex diseases and conditions. Tetraspecific antibodies can be used to target multiple pathways or receptors that are involved in disease progression, allowing for a more comprehensive and personalized approach to treatment. The potential of tetraspecific antibodies has been demonstrated in preclinical and clinical studies, with promising results in the treatment of cancer, autoimmune disorders, and infectious diseases. These antibodies have shown improved efficacy and safety compared to monoclonal antibodies, and have the potential to revolutionize the field of antibody therapeutics.
The swift change in the treatment landscape to tetraspecific antibodies represents an exciting new chapter in the field of antibody based therapies. With their increased specificity and versatility, tetraspecific antibodies have the potential to offer more targeted and effective therapies for a range of diseases and conditions. There are a number of companies that are currently developing tetraspecific antibodies, including some of the largest pharmaceutical companies in the world. These companies are investing heavily in research and development, and are working to bring these new therapies to market as quickly as possible. As research and development in this area continues, we can expect to see more exciting developments in the years to come.
The development of tetraspecific antibodies has created a highly competitive landscape in the field of antibody therapeutics. With their potential to offer more targeted and effective therapies for a range of diseases and conditions, many companies are investing heavily in the research and development of these new generation antibodies. The global market for tetraspecific antibodies is expected to grow significantly in the coming years, driven by increasing demand for more effective and personalized therapies.
For now, China, in particular, is poised to play a key role in the growth of the tetraspecific antibody market. Becoming the first country to step forward into the idea has given China an exciting advantage. Even though the notion is now adopted by US based pharmaceuticals companiesas well, China might still continue to lead the clinical development for a long time. The country's biotech industry has seen significant investment in recent years, with the government providing favorable policies and regulatory support to encourage innovation and growth. Chinese biotech companies have already made significant progress in the development of monoclonal antibodies and other innovative therapies, and are well-positioned to capitalize on the growing demand for tetraspecific antibodies. As such, the demand for tetraspecific antibodies is likely to increase in the coming years, as researchers and pharmaceutical companies work to develop more targeted and personalized treatments.
As per Tetraspecific Antibodies Clinical Trials, Proprietary Technologies, Companies & Market Trends Insight 2023 report findings, the future market outlook for tetraspecific antibodies looks promising. As the clinical research for tetraspecific antibodies continues to grow, we can expect to see more exciting developments in the years to come. With their potential to offer more targeted and effective therapies for a range of diseases and conditions, these new generation antibodies have the potential to revolutionize the field of antibody therapeutics and improve the lives of millions of people around the world. With its supportive regulatory environment and growing biotech industry, China along with its western counterparts is well-positioned to play a key role in the growth of this innovative and exciting sector. As such, investors and stakeholders should keep a close eye on the emerging opportunities in the tetraspecific antibody market.
Table of Contents
1. Introduction to Tetraspecific Antibodies
- 1.1 Clinical Overview
- 1.2 History & Development
2. Tetraspecific Antibodies Mechanism Of Action
- 2.1 Multispecific Formats
- 2.2 Tetraspecific Antibodies Bringing Immune Cells & Target Cells Closer
3. Global Tetraspecific Ongoing Clinical Trials By Company, Country, Indication & Phase
- 3.1 By Company
- 3.2 By Country
- 3.3 By Indication
- 3.4 By Phase
4. Global Tetraspecific Ongoing Clinical Trials Insight
- 4.1 Research
- 4.2 Preclinical
- 4.3 Phase-I
- 4.4 Phase-II
5. Tetraspecific Antibodies Proprietary Technologies By Company
- 5.1 Available Proprietary Platforms
- 5.2 Potential Proprietary Platforms
6. Current Clinical Development & Future Commercialization Outlook
- 6.1 Current Scenario
- 6.2 Future Market Commercialization Outlook
7. Tetraspecific Antibodies Therapeutic Approaches
- 7.1 Tetraspecific Antibodies As Monotherapy
- 7.2 Tetraspecific Antibodies As Combinational Therapy
- 7.3 Tetraspecific Antibodies As Targeted Approach
8. Tetraspecific Antibodies Therapeutic Application In Cancer
- 8.1 Overview
- 8.2 Ongoing Research & Development
9. Competitive Landscape
- 9.1 Innate Pharma
- 9.2 ModeX Therapeutics (OPKO Health)
- 9.3 Ruijin Hospital
- 9.4 Sichuan Baili Pharmaceutical
- 9.5 SystImmune