|
|
市場調査レポート
商品コード
1632597
核酸治療薬CDMOの世界市場:市場規模・シェア・動向分析 (種類別・サービス別・最終用途別・用途別・地域別・セグメント別、2025年~2030年)Nucleic Acid Therapeutics CDMO Market Size, Share & Trends Analysis Report By Type (Gene Therapy, RNA-based Therapies), By Service (Process Development and Optimization), By End-use, By Application, By Region, And Segment Forecasts, 2025 - 2030 |
||||||
カスタマイズ可能
|
|||||||
| 核酸治療薬CDMOの世界市場:市場規模・シェア・動向分析 (種類別・サービス別・最終用途別・用途別・地域別・セグメント別、2025年~2030年) |
|
出版日: 2024年12月23日
発行: Grand View Research
ページ情報: 英文 170 Pages
納期: 2~10営業日
|
全表示
- 概要
- 図表
- 目次
核酸治療薬CDMO市場の成長と動向:
Grand View Research, Inc.の最新レポートによると、世界の核酸治療薬CDMOの市場規模は2030年までに338億6,000万米ドルに達すると予測されています。
同市場は2025年から2030年にかけてCAGR 14.20%で成長すると予測されています。市場開拓の主な要因は、核酸治療薬に対する需要の増加、核酸技術の進歩、核酸治療薬のFDA承認の増加などであり、費用対効果の高い受託開発・製造サービスのニーズが高まっています。
バイオテクノロジー企業、製薬会社、学術機関による遺伝子治療研究開発への投資と関心の高まりが、CDMOサービスの需要を促進しています。CDMOは遺伝子治療製造において重要な役割を果たしています。例えば、キャタレント社はサレプタ・セラピューティクス社の商業製造パートナーとして、遺伝子治療ソリューションを推進するための主要な役割を担っています。
COVID-19パンデミックへの対応におけるmRNAワクチンの成功は、核酸治療薬の可能性を示し、この分野でのさらなる研究と投資を刺激しました。例えば、サノフィは2022年3月、フランスにおけるメッセンジャーRNAワクチンの開発に今後数年間で10億2,000万米ドル (9億3,500万ユーロ) を充てる予定であると発表しました。サノフィは、2021年6月に発表した20億ユーロ規模の国際投資イニシアチブの一環として、2026年までこの投資を行う予定です。このイニシアチブは、同社のmRNA戦略を促進するためのものです。このような資金増加シナリオは市場成長を促進します。
さらに、CDMOの買収や提携の増加は、今後数年間、核酸治療薬CDMOの市場成長を加速させると予想されます。例えば、2022年5月、eureKAREは細胞・遺伝子治療開発製造受託機関 (CDMO) を設立するため、1億6,098万米ドル (1億5,000万ユーロ) 相当の合併を開始しました。
同社は、欧州の3つの開発・製造受託機関 (CDMO) を統合する目的で、特別目的買収会社 (SPAC) であるeureKINGを設立しました。また、富士フイルムは2022年4月、カリフォルニア州にあるAtara Biotherapeutics, Inc.から細胞治療に特化した製造施設を取得することで、先端治療薬の開発・製造受託機関 (CDMO) のポートフォリオを拡大しました。このような取り組みが市場の成長を後押しすると期待されています。
核酸治療薬CDMO市場:分析概要
- RNAベース治療薬のセグメントは2024年に最大の収益シェアを占めました。RNAベース治療薬 (主にmRNA) は、様々な疾患に対応する汎用性を提供します。さまざまな治療用タンパク質を産生するように設計できるため、多様な治療領域に応用できます。
- 遺伝子治療薬分野は、今後数年間で大きな市場成長が見込まれます。これは、遺伝子治療薬の臨床的成功に起因しており、遺伝子治療薬の適応拡大に役立っています。
- 2024年には、製造サービス部門が市場を独占し、総売上シェアの38.37%以上を占めました。同分野の成長に寄与する主な課題には、複雑な製造プロセス、規模拡張と生産に関する課題、コスト効率などがあります。
- バイオテクノロジー企業は、革新的な治療法の研究開発に多額の投資を行っているため、2024年には58%以上の主要収益シェアを占めました。
- 2024年の核酸治療CDMO市場では、北米が40.24%の最大収益シェアを占めました。大規模な投資と資金援助、強力な学術研究機関、CDMOの世界の拡大がこの地域の成長を牽引しています。
目次
第1章 分析方法・範囲
第2章 エグゼクティブサマリー
第3章 核酸治療薬CDMO市場:変動要因・傾向・範囲
- 市場連関の見通し
- 親市場の見通し
- 付随市場の見通し
- 市場力学
- 市場促進要因の分析
- 市場抑制要因の分析
- 治験件数の分析 (2024年)
- 治験の総数:地域別 (2024年)
- 治験の総数:フェーズ別 (2024年)
- 治験の総数:研究デザイン別 (2024年)
- 治験の総数:主な治療領域別 (2024年)
- 市場分析ツール
- ポーターのファイブフォース分析
- SWOT分析によるPESTEL
- COVID-19の影響分析
第4章 核酸治療薬CDMO市場:種類別の推定・動向分析
- セグメントダッシュボード
- 世界の核酸治療薬CDMO市場:変動分析
- 世界の核酸治療薬CDMO市場:市場規模と動向分析、種類別 (2018~2030年)
- 遺伝子治療薬
- RNAベース治療薬
第5章 核酸治療薬CDMO市場:サービス別の推定・動向分析
- セグメントダッシュボード
- 世界の核酸治療薬CDMO市場:変動分析
- 世界の核酸治療薬CDMO市場:市場規模と動向分析、サービス別 (2018~2030年)
- プロセス開発・最適化
- 製造サービス
- 分析・品質管理サービス
第6章 核酸治療薬CDMO市場:最終用途別の推定・動向分析
- セグメントダッシュボード
- 世界の核酸治療薬CDMO市場:変動分析
- 世界の核酸治療薬CDMO市場:市場規模と動向分析、最終用途別 (2018~2030年)
- 製薬企業
- 政府・学術研究機関
- バイオテクノロジー企業
第7章 核酸治療薬CDMO市場:用途別の推定・動向分析
- セグメントダッシュボード
- 世界の核酸治療薬CDMO市場:変動分析
- 世界の核酸治療薬CDMO市場:市場規模と動向分析、用途別 (2018~2030年)
- 希少疾患
- 遺伝性疾患
- 感染症
- その他
第8章 核酸治療薬CDMO市場:地域別の推定・動向分析
- 地域別の市場ダッシュボード
- 世界市場のスナップショット:地域別
- 市場規模の予測と動向分析 (2018~2030年)
- 北米
- 米国
- カナダ
- メキシコ
- 欧州
- 英国
- ドイツ
- フランス
- イタリア
- スペイン
- デンマーク
- スウェーデン
- ノルウェー
- アジア太平洋
- 中国
- 日本
- インド
- オーストラリア
- 韓国
- タイ
- ラテンアメリカ
- ブラジル
- アルゼンチン
- 中東・アフリカ
- 南アフリカ
- サウジアラビア
- アラブ首長国連邦
- クウェート
第9章 競合情勢
- 市場参入企業の分類
- 市場リーダー
- 新興企業
- 競争市場シェア/評価分析 (2024年)
- 企業プロファイル
- Agilent Technologies, Inc
- Curia Global, Inc.
- Ajinomoto Co., Inc.
- Danaher Corporation
- KNC Laboratories Co., Ltd.
- LGC Limited
- Merck KGaA
- WuXi AppTec
- BIOSPRING
- Exothera
List of Tables
- Table 1 List of Secondary Sources
- Table 2 List of Abbreviations
- Table 3 Global Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 4 Global Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 5 Global Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 6 Global Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 7 Global Nucleic Acid Therapeutics CDMO, by Region, 2018 - 2030 (USD Million)
- Table 8 North America Nucleic Acid Therapeutics CDMO, by Country, 2018 - 2030 (USD Million)
- Table 9 North America Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 10 North America Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 11 North America Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 12 North America Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 13 U.S. Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 14 U.S. Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 15 U.S. Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 16 U.S. Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 17 Canada Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 18 Canada Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 19 Canada Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 20 Canada Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 21 Mexico Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 22 Mexico Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 23 Mexico Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 24 Mexico Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 25 Europe Nucleic Acid Therapeutics CDMO, by Country, 2018 - 2030 (USD Million)
- Table 26 Europe Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 27 Europe Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 28 Europe Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 29 Europe Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 30 Germany Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 31 Germany Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 32 Germany Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 33 Germany Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 34 UK Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 35 UK Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 36 UK Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 37 UK Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 38 France Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 39 France Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 40 France Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 41 France Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 42 Italy Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 43 Italy Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 44 Italy Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 45 Italy Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 46 Spain Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 47 Spain Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 48 Spain Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 49 Spain Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 50 Denmark Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 51 Denmark Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 52 Denmark Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 53 Denmark Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 54 Sweden Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 55 Sweden Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 56 Sweden Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 57 Sweden Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 58 Norway Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 59 Norway Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 60 Norway Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 61 Norway Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 62 Asia Pacific Nucleic Acid Therapeutics CDMO, by Country, 2018 - 2030 (USD Million)
- Table 63 Asia Pacific Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 64 Asia Pacific Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 65 Asia Pacific Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 66 Asia Pacific Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 67 China Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 68 China Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 69 China Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 70 China Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 71 Japan Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 72 Japan Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 73 Japan Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 74 Japan Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 75 India Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 76 India Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 77 India Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 78 India Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 79 South Korea Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 80 South Korea Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 81 South Korea Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 82 South Korea Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 83 Australia Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 84 Australia Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 85 Australia Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 86 Australia Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 87 Thailand Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 88 Thailand Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 89 Thailand Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 90 Thailand Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 91 Latin America Nucleic Acid Therapeutics CDMO, by Country, 2018 - 2030 (USD Million)
- Table 92 Latin America Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 93 Latin America Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 94 Latin America Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 95 Latin America Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 96 Brazil Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 97 Brazil Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 98 Brazil Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 99 Brazil Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 100 Argentina Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 101 Argentina Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 102 Argentina Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 103 Argentina Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 104 Middle East & Africa Nucleic Acid Therapeutics CDMO, by Country, 2018 - 2030 (USD Million)
- Table 105 Middle East & Africa Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 106 Middle East & Africa Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 107 Middle East & Africa Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 108 Middle East & Africa Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 109 South Africa Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 110 South Africa Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 111 South Africa Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 112 South Africa Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 113 Saudi Arabia Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 114 Saudi Arabia Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 115 Saudi Arabia Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 116 Saudi Arabia Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 117 UAE Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 118 UAE Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 119 UAE Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 120 UAE Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
- Table 121 Kuwait Nucleic Acid Therapeutics CDMO, by Type, 2018 - 2030 (USD Million)
- Table 122 Kuwait Nucleic Acid Therapeutics CDMO, by Service, 2018 - 2030 (USD Million)
- Table 123 Kuwait Nucleic Acid Therapeutics CDMO, by End Use, 2018 - 2030 (USD Million)
- Table 124 Kuwait Nucleic Acid Therapeutics CDMO, by Application, 2018 - 2030 (USD Million)
List of Figures
- Fig. 1 Information Procurement
- Fig. 2 Primary Research Pattern
- Fig. 3 Market Research Approaches
- Fig. 4 Value Chain-Based Sizing & Forecasting
- Fig. 5 Market Formulation & Validation
- Fig. 6 Nucleic Acid Therapeutics CDMO, Market Segmentation
- Fig. 7 Market Driver Relevance Analysis (Current & Future Impact)
- Fig. 8 Market Restraint Relevance Analysis (Current & Future Impact)
- Fig. 9 SWOT Analysis, By Factor (Political & Legal, Economic and Technological)
- Fig. 10 Porter's Five Forces Analysis
- Fig. 11 Regional Marketplace: Key Takeaways
- Fig. 12 Global Nucleic Acid Therapeutics CDMO, for Gene Therapy, 2018 - 2030 (USD Million)
- Fig. 13 Global Nucleic Acid Therapeutics CDMO, for RNA-based Therapies, 2018 - 2030 (USD Million)
- Fig. 14 Global Nucleic Acid Therapeutics CDMO, for Process Development and Optimization, 2018 - 2030 (USD Million)
- Fig. 15 Global Nucleic Acid Therapeutics CDMO, for Manufacturing Services, 2018 - 2030 (USD Million)
- Fig. 16 Global Nucleic Acid Therapeutics CDMO, for Analytical and Quality Control Services, 2018 - 2030 (USD Million)
- Fig. 17 Global Nucleic Acid Therapeutics CDMO, for Others, 2018 - 2030 (USD Million)
- Fig. 18 Global Nucleic Acid Therapeutics CDMO, for Pharmaceutical Companies, 2018 - 2030 (USD Million)
- Fig. 19 Global Nucleic Acid Therapeutics CDMO, for Government & Academic Research Institutes, 2018 - 2030 (USD Million)
- Fig. 20 Global Nucleic Acid Therapeutics CDMO, for Biotech Companies, 2018 - 2030 (USD Million)
- Fig. 21 Global Nucleic Acid Therapeutics CDMO, for Rare Diseases, 2018 - 2030 (USD Million)
- Fig. 22 Global Nucleic Acid Therapeutics CDMO, for Genetic Disorders, 2018 - 2030 (USD Million)
- Fig. 23 Global Nucleic Acid Therapeutics CDMO, for Infectious Diseases, 2018 - 2030 (USD Million)
- Fig. 24 Global Nucleic Acid Therapeutics CDMO, for Others, 2018 - 2030 (USD Million)
- Fig. 25 Regional Outlook, 2024 & 2030
- Fig. 26 North America Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 27 U.S. Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 28 Canada Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 29 Mexico Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 30 Europe Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 31 Germany Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 32 UK Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 33 France Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 34 Italy Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 35 Spain Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 36 Denmark Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 37 Sweden Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 38 Norway Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 39 Asia Pacific Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 40 Japan Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 41 China Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 42 India Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 43 Australia Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 44 South Korea Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 45 Thailand Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 46 Latin America Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 47 Brazil Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 48 Argentina Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 49 Middle East and Africa Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 50 South Africa Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 51 Saudi Arabia Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 52 UAE Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
- Fig. 53 Kuwait Nucleic Acid Therapeutics CDMO Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Nucleic Acid Therapeutics CDMO Market Growth & Trends:
The global nucleic acid therapeutics CDMO market size is anticipated to reach USD 33.86 billion by 2030, according to a new report by Grand View Research, Inc. The market is projected to grow at a CAGR of 14.20% from 2025 to 2030. The market's growth is primarily attributed to factors such as increasing demand for nucleic acid therapeutics, advancements in nucleic acid technologies, and rising FDA approvals of nucleic acid therapeutics, propelling the need for cost-effective contract development and manufacturing services.
Growing investment and interest in gene therapy research and development by biotechnology companies, pharmaceutical firms, and academic institutions are driving the demand for CDMO services. CDMOs have played a crucial role in gene therapy manufacturing. For instance, Catalent has taken on the primary role as the commercial manufacturing partner for Sarepta Therapeutics in their efforts to advance gene therapy solutions.
The success of mRNA vaccines in responding to the COVID-19 pandemic has showcased the potential of nucleic acid therapeutics, stimulating further research and investment in this field. For instance, in March 2022, Sanofi announced that it plans to allocate USD 1.02 billion (935 million euros) for the development of messenger RNA vaccines in France over the coming years. Sanofi intends to allocate this investment until 2026 as a part of a 2-billion-euro global investment initiative unveiled in June 2021. This initiative is designed to expedite the company's mRNA strategy. Such increased funding scenarios drive market growth.
Furthermore, increasing incidences of CDMO acquisitions and partnerships are expected to accelerate the market growth for nucleic acid therapeutics CDMO in the coming years. For instance, in May 2022, eureKARE initiated a merger worth USD 160.98 million (€150 million) to create a cell & gene therapy contract development and manufacturing organization (CDMO).
The company has established eureKING, a Special Purpose Acquisition Company (SPAC), with the aim of merging three European Contract Development and Manufacturing Organizations (CDMOs) into a unified entity. In another instance, in April 2022, Fujifilm broadened its Advanced Therapies Contract Development and Manufacturing Organization (CDMO) portfolio by acquiring a specialized cell therapy manufacturing facility from Atara Biotherapeutics, Inc. located in California. Such initiatives are expected to boost market growth.
Nucleic Acid Therapeutics CDMO Market Report Highlights:
- The RNA-based therapies segment held the largest revenue share in 2024. RNA-based therapies, mainly mRNA, offer versatility in addressing various diseases. They can be designed to produce a variety of therapeutic proteins, making them applicable to diverse therapeutic areas.
- The gene therapy segment is expected to show significant market growth in the coming years. This is attributed to the clinical success of gene therapies, which have helped in expanding indications of gene therapies.
- In 2024, the manufacturing services segment dominated the market, accounting for over 38.37% of the total revenue share. Key factors contributing to segment growth include complex manufacturing processes, scale-up & production challenges, and cost efficiency.
- The biotechnology companies held a major revenue share of over 58% in 2024 due to substantial investments in the research and development of innovative therapies.
- North America held the largest revenue share of 40.24% in the nucleic acid therapeutic CDMO market in 2024. Substantial investments and funding support, strong academic and research institutions, and global expansion of CDMOs drive regional growth.
Table of Contents
Chapter 1. Methodology and Scope
- 1.1. Market Segmentation & Scope
- 1.1.1. Regional Scope
- 1.1.2. Estimates and Forecast Timeline
- 1.2. Market Definitions
- 1.3. Research Methodology
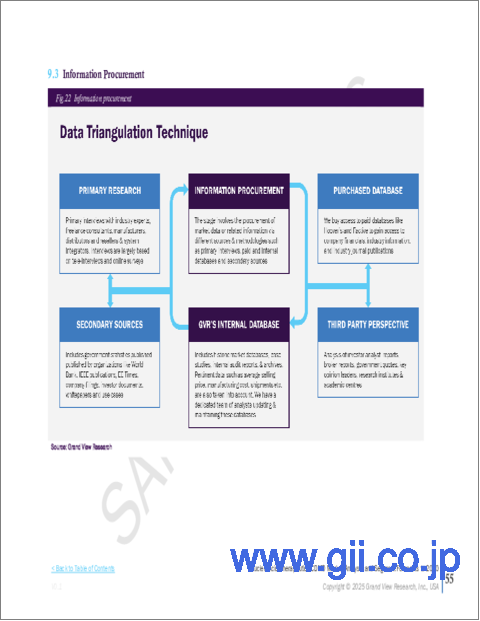
- 1.3.1. Information Procurement
- 1.3.2. Purchased Database
- 1.3.3. GVR's Internal Database
- 1.3.4. Secondary Sources
- 1.3.5. Primary Research
- 1.4. Information or Data Analysis
- 1.4.1. Data Analysis Models
- 1.5. Market Formulation & Validation
- 1.5.1. Region Wise Market: Base Estimates
- 1.5.2. Global Market: CAGR Calculation
- 1.6. Model Details
- 1.6.1. Commodity Flow Analysis (Model 1)
- 1.6.2. Value-Chain-Based Sizing & Forecasting (Model 2)
- 1.6.3. QFD Model Sizing & Forecasting (Model 3)
- 1.6.4. Bottom-Up Approach (Model 4)
- 1.7. List of Secondary Sources
- 1.8. List of Abbreviations
- 1.9. Objectives
- 1.9.1. Objective - 1
- 1.9.2. Objective - 2
- 1.9.3. Objective - 3
- 1.9.4. Objective - 4
Chapter 2. Executive Summary
- 2.1. Market Outlook
- 2.2. Segment Outlook
- 2.3. Competitive Insights
Chapter 3. Nucleic Acid Therapeutics CDMO Market Variables, Trends & Scope
- 3.1. Market Lineage Outlook
- 3.1.1. Parent Market Outlook
- 3.1.2. Ancillary Market Outlook
- 3.2. Market Dynamics
- 3.2.1. Market Driver Analysis
- 3.2.1.1. Rising incidence of genetic disorders across the globe
- 3.2.1.2. Robust expansion of the genomics research sector and strong traction gained by custom oligonucleotides
- 3.2.1.3. Increasing demand for one-stop-shop CDMOs and growing foreign direct investments in nucleic acid therapeutics
- 3.2.2. Market Restraint Analysis
- 3.2.2.1. Limited outsourcing by big pharmaceutical and biopharmaceutical companies
- 3.2.2.2. Stringent regulatory framework
- 3.2.1. Market Driver Analysis
- 3.3. Clinical Trials Volume Analysis, 2024
- 3.3.1. Total Number of Clinical Trials, by Region (2024)
- 3.3.2. Total Number of Clinical Trials, by Phase (2024)
- 3.3.3. Total Number of Clinical Trials, by Study Design (2024)
- 3.3.4. Total Number of Clinical Trials, by Key Therapeutic Area (2024)
- 3.4. Market Analysis Tools
- 3.4.1. Porter's Five Forces Analysis
- 3.4.2. PESTEL by SWOT Analysis
- 3.4.3. COVID-19 Impact Analysis
Chapter 4. Nucleic Acid Therapeutics CDMO Market: Type Estimates & Trend Analysis
- 4.1. Segment Dashboard
- 4.2. Global Nucleic Acid Therapeutics CDMO Market Movement Analysis
- 4.3. Global Nucleic Acid Therapeutics CDMO Market Size & Trend Analysis, by Type, 2018 to 2030 (USD Million)
- 4.4. Gene Therapy
- 4.4.1. Gene Therapy Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 4.5. RNA-based Therapies
- 4.5.1. RNA-based Therapies Market Estimates and Forecasts, 2018 to 2030 (USD Million)
Chapter 5. Nucleic Acid Therapeutics CDMO Market: Service Estimates & Trend Analysis
- 5.1. Segment Dashboard
- 5.2. Global Nucleic Acid Therapeutics CDMO Market Movement Analysis
- 5.3. Global Nucleic Acid Therapeutics CDMO Market Size & Trend Analysis, by Service, 2018 to 2030 (USD Million)
- 5.4. Process Development and Optimization
- 5.4.1. Process Development and Optimization Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 5.5. Manufacturing Services
- 5.5.1. Manufacturing Services Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 5.6. Analytical and Quality Control Services
- 5.6.1. Analytical and Quality Control Services Market Estimates and Forecasts, 2018 to 2030 (USD Million)
Chapter 6. Nucleic Acid Therapeutics CDMO Market: End Use Estimates & Trend Analysis
- 6.1. Segment Dashboard
- 6.2. Global Nucleic Acid Therapeutics CDMO Market Movement Analysis
- 6.3. Global Nucleic Acid Therapeutics CDMO Market Size & Trend Analysis, by End Use, 2018 to 2030 (USD Million)
- 6.4. Pharmaceutical Companies
- 6.4.1. Pharmaceutical Companies Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 6.5. Government & Academic Research Institutes
- 6.5.1. Government & Academics Research Institutes Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 6.6. Biotech Companies
- 6.6.1. Biotech Companies Market Estimates and Forecasts, 2018 to 2030 (USD Million)
Chapter 7. Nucleic Acid Therapeutics CDMO Market: Application Estimates & Trend Analysis
- 7.1. Segment Dashboard
- 7.2. Global Nucleic Acid Therapeutics CDMO Market Movement Analysis
- 7.3. Global Nucleic Acid Therapeutics CDMO Market Size & Trend Analysis, Application, 2018 to 2030 (USD Million)
- 7.4. Rare Diseases
- 7.4.1. Rare Diseases Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 7.5. Genetic Disorders
- 7.5.1. Genetic Disorders Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 7.6. Infectious Diseases
- 7.6.1. Infectious Diseases Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 7.7. Others
- 7.7.1. Others Market Estimates and Forecasts, 2018 to 2030 (USD Million)
Chapter 8. Nucleic Acid Therapeutics CDMO Market: Regional Estimates & Trend Analysis
- 8.1. Regional Market Dashboard
- 8.2. Global Regional Market Snapshot
- 8.3. Market Size & Forecasts Trend Analysis, 2018 to 2030:
- 8.4. North America
- 8.4.1. North America Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.4.2. U.S.
- 8.4.2.1. Key Country Dynamics
- 8.4.2.2. Competitive Scenario
- 8.4.2.3. Regulatory Framework
- 8.4.2.4. U.S. Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.4.3. Canada
- 8.4.3.1. Key Country Dynamics
- 8.4.3.2. Competitive Scenario
- 8.4.3.3. Regulatory Framework
- 8.4.3.4. Canada Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.4.4. Mexico
- 8.4.4.1. Key Country Dynamics
- 8.4.4.2. Competitive Scenario
- 8.4.4.3. Regulatory Framework
- 8.4.4.4. Mexico Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.5. Europe
- 8.5.1. Europe Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.5.2. UK
- 8.5.2.1. Key Country Dynamics
- 8.5.2.2. Competitive Scenario
- 8.5.2.3. Regulatory Framework
- 8.5.2.4. UK Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.5.3. Germany
- 8.5.3.1. Key Country Dynamics
- 8.5.3.2. Competitive Scenario
- 8.5.3.3. Regulatory Framework
- 8.5.3.4. Germany Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.5.4. France
- 8.5.4.1. Key Country Dynamics
- 8.5.4.2. Competitive Scenario
- 8.5.4.3. Regulatory Framework
- 8.5.4.4. France Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.5.5. Italy
- 8.5.5.1. Key Country Dynamics
- 8.5.5.2. Competitive Scenario
- 8.5.5.3. Regulatory Framework
- 8.5.5.4. Italy Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.5.6. Spain
- 8.5.6.1. Key Country Dynamics
- 8.5.6.2. Competitive Scenario
- 8.5.6.3. Regulatory Framework
- 8.5.6.4. Spain Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.5.7. Denmark
- 8.5.7.1. Key Country Dynamics
- 8.5.7.2. Competitive Scenario
- 8.5.7.3. Regulatory Framework
- 8.5.7.4. Denmark Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.5.8. Sweden
- 8.5.8.1. Key Country Dynamics
- 8.5.8.2. Competitive Scenario
- 8.5.8.3. Regulatory Framework
- 8.5.8.4. Sweden Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.5.9. Norway
- 8.5.9.1. Key Country Dynamics
- 8.5.9.2. Competitive Scenario
- 8.5.9.3. Regulatory Framework
- 8.5.9.4. Norway Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.6. Asia Pacific
- 8.6.1. Asia Pacific Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.6.2. China
- 8.6.2.1. Key Country Dynamics
- 8.6.2.2. Competitive Scenario
- 8.6.2.3. Regulatory Framework
- 8.6.2.4. China Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.6.3. Japan
- 8.6.3.1. Key Country Dynamics
- 8.6.3.2. Competitive Scenario
- 8.6.3.3. Regulatory Framework
- 8.6.3.4. Japan Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.6.4. India
- 8.6.4.1. Key Country Dynamics
- 8.6.4.2. Competitive Scenario
- 8.6.4.3. Regulatory Framework
- 8.6.4.4. India Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.6.5. Australia
- 8.6.5.1. Key Country Dynamics
- 8.6.5.2. Competitive Scenario
- 8.6.5.3. Regulatory Framework
- 8.6.5.4. Australia Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.6.6. South Korea
- 8.6.6.1. Key Country Dynamics
- 8.6.6.2. Competitive Scenario
- 8.6.6.3. Regulatory Framework
- 8.6.6.4. South Korea Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.6.7. Thailand
- 8.6.7.1. Key Country Dynamics
- 8.6.7.2. Competitive Scenario
- 8.6.7.3. Regulatory Framework
- 8.6.7.4. Thailand Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.7. Latin America
- 8.7.1. Latin America Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.7.2. Brazil
- 8.7.2.1. Key Country Dynamics
- 8.7.2.2. Competitive Scenario
- 8.7.2.3. Regulatory Framework
- 8.7.2.4. Brazil Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.7.3. Argentina
- 8.7.3.1. Key Country Dynamics
- 8.7.3.2. Competitive Scenario
- 8.7.3.3. Regulatory Framework
- 8.7.3.4. Argentina Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.8. MEA
- 8.8.1. MEA Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.8.2. South Africa
- 8.8.2.1. Key Country Dynamics
- 8.8.2.2. Competitive Scenario
- 8.8.2.3. Regulatory Framework
- 8.8.2.4. South Africa Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.8.3. Saudi Arabia
- 8.8.3.1. Key Country Dynamics
- 8.8.3.2. Competitive Scenario
- 8.8.3.3. Regulatory Framework
- 8.8.3.4. Saudi Arabia Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.8.4. UAE
- 8.8.4.1. Key Country Dynamics
- 8.8.4.2. Competitive Scenario
- 8.8.4.3. Regulatory Framework
- 8.8.4.4. UAE Market Estimates and Forecasts, 2018 to 2030 (USD Million)
- 8.8.5. Kuwait
- 8.8.5.1. Key Country Dynamics
- 8.8.5.2. Competitive Scenario
- 8.8.5.3. Regulatory Framework
- 8.8.5.4. Kuwait Market Estimates and Forecasts, 2018 to 2030 (USD Million)
Chapter 9. Competitive Landscape
- 9.1. Market Participant Categorization
- 9.1.1. Market Leaders
- 9.1.2. Emerging Players
- 9.2. Competitive Market Share/Assessment Analysis, 2024
- 9.3. Company Profiles
- 9.3.1. Agilent Technologies, Inc
- 9.3.1.1. Company Overview
- 9.3.1.2. Financial Performance
- 9.3.1.3. Service Benchmarking
- 9.3.1.4. Strategic Initiatives
- 9.3.2. Curia Global, Inc.
- 9.3.2.1. Company Overview
- 9.3.2.2. Financial Performance
- 9.3.2.3. Service Benchmarking
- 9.3.2.4. Strategic Initiatives
- 9.3.3. Ajinomoto Co., Inc.
- 9.3.3.1. Company Overview
- 9.3.3.2. Financial Performance
- 9.3.3.3. Service Benchmarking
- 9.3.3.4. Strategic Initiatives
- 9.3.4. Danaher Corporation
- 9.3.4.1. Company Overview
- 9.3.4.2. Financial Performance
- 9.3.4.3. Service Benchmarking
- 9.3.4.4. Strategic Initiatives
- 9.3.5. KNC Laboratories Co., Ltd.
- 9.3.5.1. Company Overview
- 9.3.5.2. Financial Performance
- 9.3.5.3. Service Benchmarking
- 9.3.5.4. Strategic Initiatives
- 9.3.6. LGC Limited
- 9.3.6.1. Company Overview
- 9.3.6.2. Financial Performance
- 9.3.6.3. Service Benchmarking
- 9.3.6.4. Strategic Initiatives
- 9.3.7. Merck KGaA
- 9.3.7.1. Company Overview
- 9.3.7.2. Financial Performance
- 9.3.7.3. Service Benchmarking
- 9.3.7.4. Strategic Initiatives
- 9.3.8. WuXi AppTec
- 9.3.8.1. Company Overview
- 9.3.8.2. Financial Performance
- 9.3.8.3. Service Benchmarking
- 9.3.8.4. Strategic Initiatives
- 9.3.9. BIOSPRING
- 9.3.9.1. Company Overview
- 9.3.9.2. Financial Performance
- 9.3.9.3. Service Benchmarking
- 9.3.9.4. Strategic Initiatives
- 9.3.10. Exothera
- 9.3.10.1. Company Overview
- 9.3.10.2. Financial Performance
- 9.3.10.3. Service Benchmarking
- 9.3.10.4. Strategic Initiatives
- 9.3.1. Agilent Technologies, Inc