|
|
市場調査レポート
商品コード
1562715
急性冠症候群市場 - 競合情勢Acute coronary syndrome: Competitive Landscape |
||||||
カスタマイズ可能
適宜更新あり
|
|||||||
| 急性冠症候群市場 - 競合情勢 |
|
出版日: 2024年08月20日
発行: GlobalData
ページ情報: 英文 79 Pages
納期: 即納可能
|
全表示
- 概要
- 目次
概要
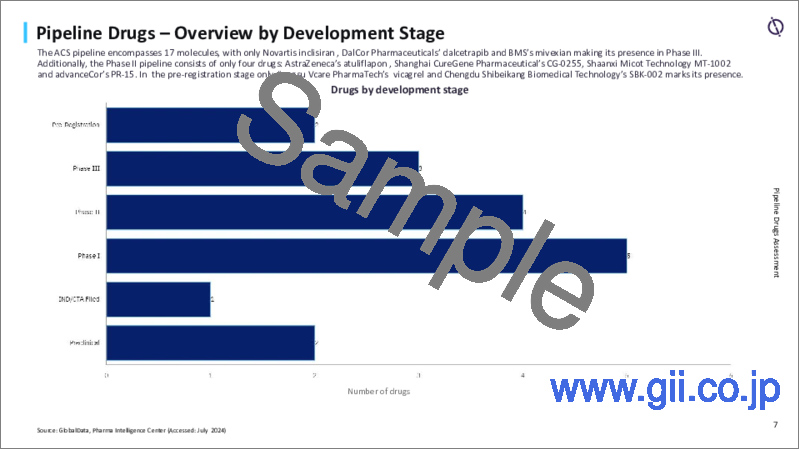
2024年には、世界16カ国で780万人以上の急性冠症候群(ACS)入院偶発症例が予測されています。ACS市場における有望な革新の開発には、新規の経口P2Yプリン受容体12(P2Y12)受容体阻害薬が含まれます。ACSのパイプラインには17の分子があります。第III相のパイプラインは3剤、次いで第II相の4つの薬剤です。
過去10年間で、ACSの治験施設数は米国が最も多く723施設、次いで中国が550施設以上となりました。過去10年間、北米とアジア太平洋では買収が最も一般的な取引形態でした。欧州ではライセンス契約が主流となりました。
当レポートでは、世界の急性冠症候群市場について調査し、疾患の概要とともに、治験動向、パイプライン概要、将来の見通しなどを提供しています。
目次
第1章 序文
第2章 主な調査結果
第3章 病気の情勢
- 疾患の概要
- 疫学の概要
- 治療の概要
第4章 上市済み薬剤の評価
- 主な市販薬
- 作用機序別概要
- 分子タイプ別概要
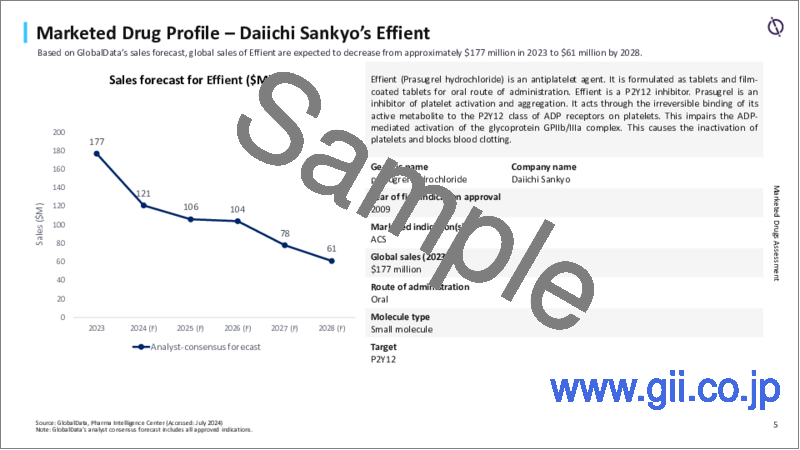
- 製品プロファイルと売上予測
第5章 価格設定と償還評価
- 年間治療費
- 価格設定と償還までの時間
第6章 パイプライン薬剤の評価
- 中期から後期段階のパイプライン薬剤
- 開発段階別概要
- 作用機序別概要
- 分子タイプ別概要
- 薬剤固有の相転移成功率(PTSR)と承認可能性(LoA)
- 治療領域と適応症別のPTSRとLoA
第7章 臨床試験の評価
- 実績の概要
- 相別概要
- ステータス別概要
- 進行中および計画中の試験の相別概要
- 仮想コンポーネントの試験
- 地域の試験概要
- 地域別の単一国および多国間試験
- 相別のスポンサー上位20社
- ステータス別のスポンサー上位20社
- エンドポイントステータス別概要
- 人種と民族別概要
- 登録データ
- 治験実施施設上位20カ国
- 世界のトップ20サイト
- 実現可能性分析- 地理的概要
- 実現可能性分析- ベンチマークモデル
第8章 取引の情勢
第9章 商業的評価
- 主要な市場参入企業
第10章 将来の市場促進要因
第11章 付録
目次
Product Code: GDHC168CL
This reports provides a data-driven overview of the current and future competitive landscape in Acute coronary syndrome therapeutics.
- In 2024, more than 7.8 million hospitalized incident cases of ACS are anticipated in the 16 countries covered in GlobalData's epidemiology forecast for ACS.
- Promising innovator developments in the ACS market include newer oral P2Y purinoceptor 12 (P2Y12)-receptor inhibitors.
- There are 17 molecules in the pipeline for ACS. The Phase III pipeline consists of three drugs, followed by four drugs in Phase II.
- Over the past 10 years, the US has hosted the highest number of trial sites for ACS, at 723 trial sites, followed by China with more than 550 trial sites.
- During the past decade, acquisitions were the most common deal type in North America and in Asia-Pacific. In Europe, licensing agreements were the predominant deal type.
Scope
GlobalData's Acute coronary syndrome: Competitive Landscape combines data from the Pharma Intelligence Center with in-house analyst expertise to provide a competitive assessment of the disease marketplace.
Components of the report include -
- Disease Landscape
- Disease Overview
- Epidemiology Overview
- Treatment Overview
- Marketed Products Assessment
- Breakdown by Mechanism of Action, Route of Administration
- Product Profiles with Sales Forecast
- Pricing and Reimbursement Assessment
- Annual Therapy Cost
- Time to Pricing and Time to Reimbursement
- Pipeline Assessment
- Breakdown by Development Stage, Mechanism of Action, Molecule Type, Route of Administration
- Product Profiles with Sales Forecast
- Late-to-mid-stage Pipeline Drugs
- Phase Transition Success Rate and Likelihood of Approval
- Clinical Trials Assessment
- Breakdown of Trials by Phase, Status, Virtual Components, Sponsors, Geography, and Endpoint Status
- Enrolment Analytics, Site Analytics, Feasibility Analysis
- Deals Landscape
- Mergers, Acquisitions, and Strategic Alliances by Region
- Overview of Recent Deals
- Commercial Assessment
- Key Market Players
- Future Market Catalysts
Reasons to Buy
- Develop and design your in-licensing and out-licensing strategies through a review of pipeline products and technologies, and by identifying the companies with the most robust pipeline.
- Develop business strategies by understanding the trends shaping and driving the Acute coronary syndrome market.
- Drive revenues by understanding the key trends, innovative products and technologies, and companies likely to impact the global Acute coronary syndrome market in the future.
- Formulate effective sales and marketing strategies by understanding the competitive landscape and analyzing the performance of various competitors.
- Identify emerging players with potentially strong product portfolios and create effective counter-strategies to gain a competitive advantage.
- Organize your sales and marketing efforts by identifying the market categories that present the maximum opportunities for consolidations, investments, and strategic partnerships.
Table of Contents
1 Preface
- 1.1 Contents
- 1.2 Report Scope
- 1.3 List of Tables and Figures
- 1.4 Abbreviations
2 Key Findings
3 Disease Landscape
- 3.1 Disease Overview
- 3.2 Epidemiology Overview
- 3.3 Treatment Overview
4 Marketed Drugs Assessment
- 4.1 Leading Marketed Drugs
- 4.2 Overview by Mechanism of Action
- 4.3 Overview by Molecule Type
- 4.4 Product Profiles and Sales Forecast
5 Pricing and Reimbursement Assessment
- 5.1 Annual Cost of Therapy
- 5.2 Time to Pricing and Reimbursement
6 Pipeline Drugs Assessment
- 6.1 Mid-to-late-stage Pipeline Drugs
- 6.2 Overview by Development Stage
- 6.3 Overview by Mechanism of Action
- 6.4 Overview by Molecule Type
- 6.5 Drug Specific Phase Transition Success Rate (PTSR) and Likelihood of Approval (LoA)
- 6.6 Therapy Area and Indication-specific PTSR and LoA
7 Clinical Trials Assessment
- 7.1 Historical Overview
- 7.2 Overview by Phase
- 7.3 Overview by Status
- 7.4 Overview by Phase for Ongoing and Planned Trials
- 7.5 Trials with Virtual Components
- 7.6 Overview of Trials by Geography
- 7.7 Single-Country and Multinational Trials by Region
- 7.8 Top 20 Sponsors with Breakdown by Phase
- 7.9 Top 20 Sponsors with Breakdown by Status
- 7.10 Overview by Endpoint Status
- 7.11 Overview by Race and Ethnicity
- 7.12 Enrollment Data
- 7.13 Top 20 countries for Trial Sites
- 7.14 Top 20 Sites Globally
- 7.15 Feasibility Analysis - Geographic Overview
- 7.16 Feasibility Analysis - Benchmark Models
8 Deals Landscape
- 8.1 Mergers, Acquisitions, and Strategic Alliances by Region
- 8.2 Recent Mergers, Acquisitions, and Strategic Alliances
9 Commercial Assessment
- 9.1 Key Market Players
10 Future Market Catalysts
11 Appendix
- 11.1 Methodology
- 11.2 Methodology - Sales Forecast
- 11.3 Methodology - Pricing and Reimbursement
- 11.4 Methodology - PTSR and LoA Analysis
- 11.5 About the Authors
- 11.6 Contact Us
- 11.7 Disclaimer