|
|
市場調査レポート
商品コード
1544151
重症筋無力症 - 市場の洞察、疫学、市場予測:2034年Myasthenia Gravis - Market Insight, Epidemiology And Market Forecast - 2034 |
||||||
カスタマイズ可能
|
|||||||
| 重症筋無力症 - 市場の洞察、疫学、市場予測:2034年 |
|
出版日: 2024年08月01日
発行: DelveInsight
ページ情報: 英文 287 Pages
納期: 1~3営業日
|
全表示
- 概要
- 図表
- 目次
主要7ヶ国における重症筋無力症の総市場規模は、2023年に約49億5,000万米ドルに達しました。予測期間中は大幅な成長が見込まれます。主要7ヶ国のうち、米国が2023年に市場を独占し、約76%の最大シェアを占めました。2023年には、EU4ヶ国と英国が推定8億9,300万米ドルを獲得し、これは大幅なCAGRで増加すると予測されています。欧州諸国の中では、ドイツが2023年に最大の市場シェアを占め、英国、フランス、イタリアがこれに続いた。スペインは同年の市場シェアが最も低くなっています。
重症筋無力症治療市場全体の市場規模は、ニポカリマブ、バトクリマブ、Descartes-08などの新しく効果的な治療法の出現により、予測期間中に成長が見込まれます。
筋ジストロフィー協会によると、重症筋無力症は、免疫系が身体の組織を攻撃する自己免疫疾患です。重症筋無力症では、その攻撃によって神経と筋肉の結合-神経筋接合部-が阻害されます。
重症筋無力症における最も一般的な自己抗体の標的はニコチン性アセチルコリン受容体(AChR)であり、次いで筋特異的キナーゼ(MuSK)およびリポ蛋白受容体関連蛋白4(LRP4)です。重症筋無力症は、特定の筋群を含む無痛、変動性、疲労性の筋力低下を呈します。最も典型的な初発症状は、非対称性眼瞼下垂および両眼複視を伴う眼筋脱力です。
重症筋無力症の臨床診断は、筋電図検査、薬理学的検査、および血清Ab測定によって確定されます。筋電図検査で陽性であればNMTのシナプス後障害、コリンエステラーゼ阻害薬(ChE-Is)に対する臨床反応は重症筋無力症の診断を支持し、特異的Absの検出は重症筋無力症を確定し、Ab関連サブグループを同定します。標準的アッセイでAChRもMuSKも検出されない患者では、筋電図による確認が重要です。
現在、治療法は確立されていません。利用可能な治療法は症状をコントロールすることができ、多くの場合、比較的高いQOLを保つことができます。
重症筋無力症の主な治療法としては、コリンエステラーゼ酵素阻害薬、免疫抑制薬および生物学的療法が一般的です。これらの標準的な治療で症状が改善しない場合や、早急な症状緩和が必要な場合には、プラズマフェレーシスや免疫グロブリン静注療法が用いられます。これらの治療は、筋無力症の危機的状況において特に有用です。
当レポートでは、主要7ヶ国における重症筋無力症市場について調査し、市場の概要とともに、疫学、患者動向、新たな治療法、2034年までの市場規模予測、および医療のアンメットニーズなどを提供しています。
目次
第1章 重要な洞察
第2章 レポートのイントロダクション
第3章 重症筋無力症市場概要
第4章 疫学と市場予測の調査手法
第5章 エグゼクティブサマリー
第6章 主な出来事
第7章 疾患の背景と概要
- イントロダクション
- 重症筋無力症の種類
- 重症筋無力症の臨床分類
- 病因
- リスク要因
- 臨床症状と徴候
- 病態生理学
- バイオマーカー
- 診断
- 治療と管理
第8章 疫学と患者人口
- 主な調査結果
- 仮定と根拠
- 主要7ヶ国における重症筋無力症の診断された有病症例の総数
- 米国
- EU4ヶ国と英国
- 日本
第9章 患者動向
第10章 上市済み薬剤
第11章 新興治療薬
第11章 新たな治療法
第12章 重症筋無力症:市場分析
- 主な調査結果
- 主要な市場予測の前提条件
- 市場見通し
- コンジョイント分析
- 主要7ヶ国における重症筋無力症の総市場規模
- 主要7ヶ国における重症筋無力症の治療法別市場規模
- 米国における重症筋無力症の市場規模
- EU4ヶ国と英国における重症筋無力症の市場規模
- 日本における重症筋無力症の市場規模
第13章 主要オピニオンリーダーの見解
第14章 SWOT分析
第15章 アンメットニーズ
第16章 市場アクセスと償還
- 米国
- EU4ヶ国と英国
- 日本
第17章 付録
第18章 DelveInsightのサービス内容
第19章 免責事項
第20章 DelveInsightについて
List of Tables
- Table 1: Summary of Myasthenia Gravis Epidemiology and Market (2020-2034)
- Table 2: Key Events
- Table 3: Classification of myasthenia gravis Subgroups
- Table 4: Proposed Diagnostic Criteria for Myasthenia Gravis - Japanese Treatment Guidelines
- Table 5: Treatment Options for Myasthenia Gravis German Neurological Society Guidelines (1/3)
- Table 6: Treatment Options for Myasthenia Gravis German Neurological Society Guidelines (2/3)
- Table 7: Treatment Options for Myasthenia Gravis German Neurological Society Guidelines (3/3)
- Table 8: Total Diagnosed Prevalent Cases of Myasthenia Gravis in the 7MM (2020-2034)
- Table 9: Total Diagnosed Prevalent Cases of Myasthenia Gravis in the US (2020-2034)
- Table 10: Gender-specific Diagnosed Prevalent Cases of Myasthenia Gravis in the US (2020-2034)
- Table 11: Age-specific Diagnosed Prevalent Cases of Myasthenia Gravis in the US (2020-2034)
- Table 12: Diagnosed Prevalent Cases of Myasthenia Gravis by MGFA Classification in the US (2020-2034)
- Table 13: Diagnosed Prevalent Cases of Generalized Myasthenia Gravis by Antibody Serology in the US (2020-2034)
- Table 14: Total Diagnosed Prevalent Cases of Myasthenia Gravis in EU4 and the UK (2020-2034)
- Table 15: Gender-specific Diagnosed Prevalent Cases of Myasthenia Gravis in EU4 and the UK (2020-2034)
- Table 16: Age-specific Diagnosed Prevalent Cases of Myasthenia Gravis in Germany (2020-2034)
- Table 17: Age-specific Diagnosed Prevalent Cases of Myasthenia Gravis in France (2020-2034)
- Table 18: Age-specific Diagnosed Prevalent Cases of Myasthenia Gravis in Italy (2020-2034)
- Table 19: Age-specific Diagnosed Prevalent Cases of Myasthenia Gravis in Spain (2020-2034)
- Table 20: Age-specific Diagnosed Prevalent Cases of Myasthenia Gravis in the UK (2020-2034)
- Table 21: Age-specific Diagnosed Prevalent Cases of Myasthenia Gravis in EU4 and the UK (2020-2034)
- Table 22: Diagnosed Prevalent Cases of Myasthenia Gravis by MGFA Classification in Germany (2020-2034)
- Table 23: Diagnosed Prevalent Cases of Myasthenia Gravis by MGFA Classification in France (2020-2034)
- Table 24: Diagnosed Prevalent Cases of Myasthenia Gravis by MGFA Classification in Italy (2020-2034)
- Table 25: Diagnosed Prevalent Cases of Myasthenia Gravis by MGFA Classification in Spain (2020-2034)
- Table 26: Diagnosed Prevalent Cases of Myasthenia Gravis by MGFA Classification in the UK (2020-2034)
- Table 27: Diagnosed Prevalent Cases of Myasthenia Gravis by MGFA Classification in EU4 and the UK (2020-2034)
- Table 28: Diagnosed Prevalent Cases of Generalized Myasthenia Gravis by Antibody Serology in Germany (2020-2034)
- Table 29: Diagnosed Prevalent Cases of Generalized Myasthenia Gravis by Antibody Serology in France (2020-2034)
- Table 30: Diagnosed Prevalent Cases of Generalized Myasthenia Gravis by Antibody Serology in Italy (2020-2034)
- Table 31: Diagnosed Prevalent Cases of Generalized Myasthenia Gravis by Antibody Serology in Spain (2020-2034)
- Table 32: Diagnosed Prevalent Cases of Generalized Myasthenia Gravis by Antibody Serology in the UK (2020-2034)
- Table 33: Diagnosed Prevalent Cases of Generalized Myasthenia Gravis by Antibody Serology in EU4 and the UK (2020-2034)
- Table 34: Total Diagnosed Prevalent Cases of Myasthenia Gravis in Japan (2020-2034)
- Table 35: Gender-specific Diagnosed Prevalent Cases of Myasthenia Gravis in Japan (2020-2034)
- Table 36: Age-specific Diagnosed Prevalent Cases of Myasthenia Gravis in Japan (2020-2034)
- Table 37: Diagnosed Prevalent Cases of Myasthenia Gravis by MGFA Classification in Japan (2020-2034)
- Table 38: Diagnosed Prevalent Cases of Generalized Myasthenia Gravis by Antibody Serology in Japan (2020-2034)
- Table 39: Key Cross Competition of Marketed Drugs
- Table 40: SOLIRIS (eculizumab), Clinical Trial Description, 2024
- Table 41: ULTOMIRIS (ravulizumab), Clinical Trial Description, 2024
- Table 42: RYSTIGGO (rozanolixizumab-noli), Clinical Trial Description, 2024
- Table 43: ZILBRYSQ (zilucoplan), Clinical Trial Description, 2024
- Table 44: VYVGART (efgartigimod alfa-fcab), Clinical Trial Description, 2024
- Table 45: VYVGART HYTRULO/ VYVDURA (efgartigimod alfa and hyaluronidase-qvfc), Clinical Trial Description, 2024
- Table 46: Comparison of Emerging Drugs
- Table 47: UPLIZNA (inebilizumab), Clinical Trial Description, 2024
- Table 48: Nipocalimab; Clinical Trial Description, 2024
- Table 49: ENSPRYNG (satralizumab); Clinical Trial Description, 2024
- Table 50: Batoclimab; Clinical Trial Description, 2024
- Table 51: Gefurulimab (ALXN-1720); Clinical Trial Description, 2024
- Table 52: Pozelimab + Cemdisiran; Clinical Trial Description, 2024
- Table 53: KYV-101; Clinical Trial Description, 2024
- Table 54: Descartes-08, Clinical Trial Description, 2024
- Table 55: DNTH103, Clinical Trial Description, 2024
- Table 56: Vemircopan (ALXN2050); Clinical Trial Description, 2024
- Table 57: Mezagitamab (TAK-079), Clinical Trial Description, 2024
- Table 58: CNP-106, Clinical Trial Description, 2024
- Table 59: Key Market Forecast Assumptions for RYSTIGGO
- Table 60: Key Market Forecast Assumptions for ZILBRYSQ
- Table 61: Key Market Forecast Assumptions for VYVGART HYTRULO
- Table 62: Key Market Forecast Assumptions for Nipocalimab
- Table 63: Key Market Forecast Assumptions for Batoclimab
- Table 64: Key Market Forecast Assumptions for Descartes-08
- Table 65: Total Market Size of Myasthenia Gravis in the 7MM, in USD million (2020-2034)
- Table 66: Market Size of Myasthenia Gravis by Therapies in the 7MM, in USD million (2020-2034)
- Table 67: Total Market Size of Myasthenia Gravis in the US, in USD million (2020-2034)
- Table 68: Market Size of Myasthenia Gravis by Therapies in the US, in USD million (2020-2034)
- Table 69: Total Market Size of Myasthenia Gravis in EU4 and the UK, in USD million (2020-2034)
- Table 70: Market Size of Myasthenia Gravis by Therapies in Germany, in USD million (2020-2034)
- Table 71: Market Size of Myasthenia Gravis by Therapies in France, in USD million (2020-2034)
- Table 72: Market Size of Myasthenia Gravis by Therapies in Italy, in USD million (2020-2034)
- Table 73: Market Size of Myasthenia Gravis by Therapies in Spain, in USD million (2020-2034)
- Table 74: Market Size of Myasthenia Gravis by Therapies in the UK, in USD million (2020-2034)
- Table 75: Market Size of Myasthenia Gravis by Therapies in EU4 and the UK, in USD million (2020-2034)
- Table 76: Total Market Size of Myasthenia Gravis in Japan, in USD million (2020-2034)
- Table 77: Market Size of Myasthenia Gravis by Therapies in Japan, in USD million (2020-2034)
List of Figures
- Figure 1: Types of Myasthenia Gravis
- Figure 2: Risk Factors of myasthenia gravis
- Figure 3: Speculative Mechanisms of AChR Myasthenia Gravis Immunopathology
- Figure 4: Speculative Mechanisms of MuSK Myasthenia Gravis Immunopathology
- Figure 5: Diagnostic Algorithm for Myasthenia Gravis
- Figure 6: Treatment Algorithm for Myasthenia Gravis
- Figure 7: Total Diagnosed Prevalent Cases of Myasthenia Gravis in the 7MM (2020-2034)
- Figure 8: Total Diagnosed Prevalent Cases of Myasthenia Gravis in the US (2020-2034)
- Figure 9: Gender-specific Diagnosed Prevalent Cases of Myasthenia Gravis in the US (2020-2034)
- Figure 10: Age-specific Diagnosed Prevalent Cases of Myasthenia Gravis in the US (2020-2034)
- Figure 11: Diagnosed Prevalent Cases of Myasthenia Gravis by MGFA Classification in the US (2020-2034)
- Figure 12: Diagnosed Prevalent Cases of Generalized Myasthenia Gravis by Antibody Serology in the US (2020-2034)
- Figure 13: Total Diagnosed Prevalent Cases of Myasthenia Gravis in EU4 and the UK (2020-2034)
- Figure 14: Gender-specific Diagnosed Prevalent Cases of Myasthenia Gravis in EU4 and the UK (2020-2034)
- Figure 15: Age-specific Diagnosed Prevalent Cases of Myasthenia Gravis in EU4 and the UK (2020-2034)
- Figure 16: Diagnosed Prevalent Cases of Myasthenia Gravis by MGFA Classification in EU4 and the UK (2020-2034)
- Figure 17: Diagnosed Prevalent Cases of Generalized Myasthenia Gravis by Antibody Serology in EU4 and the UK (2020-2034)
- Figure 18: Total Diagnosed Prevalent Cases of Myasthenia Gravis in Japan (2020-2034)
- Figure 19: Gender-specific Diagnosed Prevalent Cases of Myasthenia Gravis in Japan (2020-2034)
- Figure 20: Age-specific Diagnosed Prevalent Cases of Myasthenia Gravis in Japan (2020-2034)
- Figure 21: Diagnosed Prevalent Cases of Myasthenia Gravis by MGFA Classification in Japan (2020-2034)
- Figure 22: Diagnosed Prevalent Cases of Generalized Myasthenia Gravis by Antibody Serology in Japan (2020-2034)
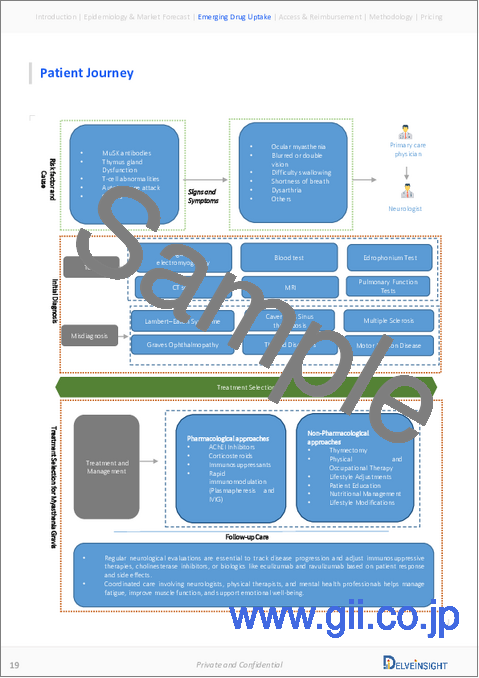
- Figure 23: Patient Journey of Myasthenia Gravis
- Figure 24: Total Market Size of Myasthenia Gravis in the 7MM, in USD million (2020-2034)
- Figure 25: Market Size of Myasthenia Gravis by Therapies in the 7MM (2020-2034)
- Figure 26: Total Market Size of Myasthenia Gravis in the US, in USD million (2020-2034)
- Figure 27: Market Size of Myasthenia Gravis by Therapies in the US (2020-2034)
- Figure 28: Total Market Size of Myasthenia Gravis in EU4 and the UK, in USD million (2020-2034)
- Figure 29: Market Size of Myasthenia Gravis by Therapies in EU4 and the UK (2020-2034)
- Figure 30: Total Market Size of Myasthenia Gravis in Japan, in USD million (2020-2034)
- Figure 31: Market Size of Myasthenia Gravis by Therapies in Japan, in USD million (2020-2034)
- Figure 32: Unmet Needs
- Figure 33: Health Technology Assessment
- Figure 34: Reimbursement Process in Germany
- Figure 35: Reimbursement Process in France
- Figure 36: Reimbursement Process in Spain
- Figure 37: Reimbursement Process in the United Kingdom
- Figure 38: Reimbursement Process in Japan
Key Highlights:
- The Myasthenia Gravis market is projected to witness consistent growth throughout the forecast period (2024-2034). The market size of Myasthenia gravis in the 7MM is expected to increase, driven by the launch of emerging therapies.
- DelveInsight's analyst projects that among the total diagnosed prevalent cases of myasthenia gravis in 7MM approximately 45% of cases were from the US. As per our estimations, in 2023, the EU4 and the UK accounted for nearly 125 thousand diagnosed prevalent cases of myasthenia gravis.
- In the 7MM, the market mainly consisted of refractory treatment, which generated nearly USD 4,950 million in 2023.
- The total market size of the Myasthenia gravis treatment market is anticipated to experience growth during the forecast period due to the emergence of new and effective treatments, namely, Nipocalimab, Batoclimab, Descartes-08, and others.
DelveInsight's "Myasthenia Gravis - Market Insights, Epidemiology, and Market Forecast - 2034" report delivers an in-depth understanding of the myasthenia gravis, historical and forecasted epidemiology and the Myasthenia Gravis market trends in the United States, EU4 (Germany, France, Italy, and Spain) and the United Kingdom, and Japan.
The myasthenia gravis market report provides current treatment practices, emerging drugs, market share of individual therapies, and current and forecasted 7MM Myasthenia Gravis market size from 2020 to 2034. The report also covers current Myasthenia Gravis treatment practices/algorithms and unmet medical needs to curate the best of the opportunities and assesses the underlying potential of the market.
Geography Covered:
- The United States
- EU4 (Germany, France, Italy, and Spain) and the United Kingdom
- Japan
Study Period: 2020-2034
Disease Understanding and Treatment Algorithm
Myasthenia Gravis Overview
According to the Muscular Dystrophy Association, myasthenia gravis is an autoimmune disease when the immune system attacks the body's tissues. In myasthenia gravis, that attack interrupts the connection between nerve and muscle-the neuromuscular junction.
The most common target of pathogenic autoantibodies in myasthenia gravis is the nicotinic acetylcholine receptor (AChR), followed by a muscle-specific kinase (MuSK) and lipoprotein receptor-related protein 4 (LRP4). Myasthenia gravis presents with painless, fluctuating, fatigable weakness involving specific muscle groups. The most typical initial presentation includes ocular weakness with asymmetric ptosis and binocular diplopia, while the less common presentation includes early or isolated oropharyngeal or limb weakness.
Myasthenia Gravis Diagnosis
The clinical diagnosis of Myasthenia gravis is confirmed by electromyography (EMG) studies, pharmacologic testing, and serum Ab assay. Positive results on EMG confirm a postsynaptic defect of the NMT, the clinical response to cholinesterase inhibitors (ChE-Is) supports myasthenia gravis diagnosis, and detection of specific Abs confirms myasthenia gravis and identifies Ab-related subgroups. EMG confirmation is crucial in patients with neither AChR nor MuSK Abs on the standard assay.
Myasthenia Gravis Treatment
Currently, there is no known cure. Available treatments can control symptoms and often allow you to have a relatively high quality of life.
The primary approach for managing myasthenia gravis typically includes the use of cholinesterase enzyme inhibitors, immunosuppressive medications, and biological therapies. In cases where symptoms do not respond well to these standard treatments or when there is a need for quick symptom relief, options such as plasmapheresis or intravenous immunoglobulins may be employed. These interventions are particularly useful during myasthenic crises.
Several emerging treatments for myasthenia gravis are currently in the process of development. These encompass Fc receptor antagonists, Anti C5, Interleukin-6 receptor antagonists, T lymphocyte replacements, and other options.
Myasthenia Gravis Epidemiology
As the market is derived using the patient-based model, the Myasthenia Gravis epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by Total Diagnosed Prevalent Cases of Myasthenia Gravis, Gender-specific Diagnosed Prevalent cases of Myasthenia Gravis, Age-specific Diagnosed Prevalent cases of Myasthenia Gravis, Diagnosed Prevalent Cases of Myasthenia Gravis by MGFA classification, Diagnosed Prevalent Cases of Generalized Myasthenia Gravis by Antibody Serology in the 7MM covering the United States, EU4 countries (Germany, France, Italy, and Spain) and the United Kingdom, and Japan, from 2020 to 2034. As per DelveInsight's estimations, the total diagnosed prevalent cases of Myasthenia Gravis in the 7MM were approximately 287 thousand cases in 2023 and are projected to increase during the forecast period.
- The overall count of individuals diagnosed with myasthenia gravis in the United States was approximately 129 thousand in 2023, and it is expected to increase at an estimated CAGR throughout the study period (2020-2034).
- Among the 7MM, EU4 and the UK accounted for nearly 125 thousand diagnosed prevalent cases of myasthenia gravis, and these cases are expected to increase during the forecast period (2023-2034).
- Among EU4 and the UK, Germany had the highest diagnosed prevalent population of myasthenia gravis, with 39 thousand cases, followed by the UK and France in 2023. On the other hand, Spain had the lowest diagnosed prevalent population in EU4 and the UK in 2023.
- In Japan, there were around 32 thousand diagnosed prevalent cases of myasthenia gravis in 2023. These cases are expected to increase at a significant CAGR.
- Gender-specific diagnosed prevalent cases of myasthenia gravis showed that females were more affected by myasthenia gravis than males in the 7MM in 2023.
- The highest proportion of myasthenia gravis cases was estimated in the 65 years and above age group in the 7MM, while the least cases were in the age group 0-17 years.
- Based on MGFA classification, diagnosed prevalent cases of myasthenia gravis were categorized into five classes: Class I (ocular), Class II (mild generalized), Class III (moderate generalized), Class IV (severe generalized), and Class V (intubated). A higher number of cases were estimated in Class II, with 56 thousand cases in 2023 in the US.
- The antibody serology-specific cases of generalized myasthenia gravis were divided into anti-AchR Ab (+ve), anti-MuSK Ab (+ve), and double seronegative (anti-LRP4-ab and anti-Argin-ab) and others. In 2023, there were more cases estimated in the AchR Ab (+ve) category, with nearly 73 thousand cases reported in the United States.
Myasthenia Gravis Drug Chapters
The drug chapter segment of the Myasthenia gravis report encloses a detailed analysis of myasthenia gravis marketed drugs and late-stage (Phase III and Phase II) pipeline drugs. It also understands myasthenia gravis clinical trial details, expressive pharmacological action, agreements and collaborations, approval, and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Marketed Drugs
SOLIRIS (eculizumab): Alexion, AstraZeneca Rare Disease
SOLIRIS is a first-in-class complement inhibitor that works by inhibiting the terminal part of the complement cascade, a part of the immune system that, when activated in an uncontrolled manner, plays a role in ultrarare severe disorders like anti-acetylcholine receptor (AchR) antibody-positive myasthenia gravis. The drug is designed to have an IV infusion route of administration.
VYVGART (efgartigimod alfa-fcab): Argenx
VYVGART (efgartigimod alfa-fcab) is a human IgG1 antibody fragment that binds to the neonatal Fc receptor (FcRn), resulting in the reduction of circulating immunoglobulin G (IgG) autoantibodies. It is the first and only approved FcRn blocker. VYVGART is approved in the United States and Europe for the treatment of adults with gMG who are anti-acetylcholine receptor (AChR) antibody-positive and in Japan for the treatment of adults with gMG who do not have a sufficient response to steroids or nonsteroidal immunosuppressive therapies (ISTs). VYVGART has IV infusion route of administration.
Emerging Drugs
Nipocalimab: Janssen Research & Development, LLC
Nipocalimab (M281) is a high affinity, fully human, aglycosylated, effectors IgG1 anti-FcRn monoclonal antibody. In patients with gMG, nipocalimab is expected to improve nerve-to-muscle signals and muscle function, thus alleviating the clinical signs and symptoms of gMG. The drug is currently being evaluated in a Phase III trial to assess the safety and efficacy of nipocalimab in patients with myasthenia gravis at multiple locations around the globe.
Batoclimab: Immunovant Sciences GmbH
Batoclimab is a novel, fully humanized mAB that inhibits neonatal Fc receptors by blocking FcRn-IgG interactions and accelerating the degradation of autoantibodies. The drug is currently being evaluated in a Phase III trial to assess the efficacy and safety of batoclimab as induction and maintenance therapy in adult participants with gMG.
DESCARTES-08: Cartesian Therapeutics
DESCARTES-08 is the first RNA CAR T-cell (rCAR-T) therapy for autoimmune diseases. DESCARTES-08 contains killer T-cells engineered to hunt down pathogenic plasma cells that secrete autoantibodies. Descartes-08 is a T-cell therapy custom-made from a patient's blood. Cartesian Therapeutics is enrolling patients with gMG in a Phase IIb randomized controlled trial (RCT).
Myasthenia Gravis Market Outlook
Myasthenia gravis has a diverse treatment classification associated with the disease landscape. The management of myasthenia gravis primarily revolves around the utilization of cholinesterase enzyme inhibitors, immunosuppressive agents, biological therapies, and thymectomy as needed.
Refractory treatment options (VYVGART [efgartigimod alfa-fcab]; SOLIRIS; ULTOMIRIS [Ravulizumab (genetical recombination)], Plasma exchange/IVIG, and other therapies) are major revenue generators in the current treatment landscape.
The market for myasthenia gravis is expected to experience positive growth with the approval of potential drugs like Nipocalimab, Batoclimab, Descartes-08, and others.
- The Myasthenia Gravis market's total size in the 7MM reached approximately USD 4,950 million in 2023. Projections indicate a substantial growth during the forecast period.
- Out of the 7MM, the United States dominated the market in 2023, representing the largest share at nearly 76%.
- In 2023, EU4 and the UK captured an estimated USD 893 million, which is anticipated to increase at a substantial CAGR. Among the European countries, Germany covered the largest market share in 2023, followed by the UK, France, and Italy. Spain accounted for the least market in the same year.
- Japan alone represented approximately 6% of the total myasthenia gravis market in 2023, projected to increase at a substantial CAGR during the study period.
- The total market size of the myasthenia gravis treatment market is anticipated to experience growth during the forecast period due to the emergence of new and effective treatments.
Myasthenia Gravis Drugs Uptake
This section focuses on the uptake rate of potential drugs expected to launch in the market during 2020-2034. For example, Nipocalimab in the US is expected to be launched by 2025.
Further detailed analysis of emerging therapies drug uptake in the report...
Myasthenia Gravis Pipeline Development Activities
The report provides insights into different therapeutic candidates in Phase III, Phase II, and Phase I stage. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The report covers information on collaborations, acquisitions and mergers, licensing, and patent details for Myasthenia Gravis emerging therapies.
KOL Views
To keep up with current market trends, we take KOLs and SMEs' opinions working in the domain through primary research to fill the data gaps and validate the secondary research. Industry Experts were contacted for insights on myasthenia gravis evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including KOL from University of California, UCSF Medical Center, San Francisco; Department of Neurology, University of Virginia, Charlottesville, Virginia, USA; Department of Neurology, Kamillus-Hospital, Asbach, Germany; Dipartimento di Neuroscienze, Universita Cattolica del Sacro Cuore, Rome, Italy; Department of Neurology, National Hospital Organization, Nagasaki Kawatana Medical Center, Nagasaki, Japan, and others.
Delveinsight's analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapies, treatment patterns, or myasthenia gravis market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst's discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies' safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the global drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment.
The report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Report:
- The report covers a segment of key events, an executive summary, descriptive overview of Myasthenia Gravis, explaining its causes, signs and symptoms, pathogenesis, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Myasthenia Gravis market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind the approach is included in the report covering the 7MM drug outreach.
- The report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Myasthenia Gravis market.
Myasthenia Gravis Report Insights
- Patient Population
- Therapeutic Approaches
- Myasthenia Gravis Pipeline Analysis
- Myasthenia Gravis Market Size and Trends
- Existing and Future Market Opportunity
Myasthenia Gravis Report Key Strengths
- 11 years Forecast
- The 7MM Coverage
- Myasthenia Gravis Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
- Drugs Uptake and Key Market Forecast Assumptions
Myasthenia Gravis Report Assessment
- Current Treatment Practices
- Unmet Needs
- Pipeline Product Profiles
- Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
Key Questions:
Market Insights
- What was the Myasthenia Gravis total market size, the market size by therapies, and market share (%) distribution in 2020, and how would it all look in 2034? What are the contributing factors for this growth?
- What unmet needs are associated with the current treatment market of Myasthenia Gravis?
- What are the patents of emerging therapies for myasthenia gravis?
- Which drug is going to be the largest contributor by 2034?
- What are the pricing variations among different geographies for approved and off-label therapies?
- How would the market drivers, barriers, and future opportunities affect the market dynamics and subsequent analysis of the associated trends?
Epidemiology Insights
- What are the disease risks, burdens, and unmet needs of Myasthenia Gravis? What will be the growth opportunities across the 7MM concerning the patient population of Myasthenia Gravis?
- What is the historical and forecasted Myasthenia Gravis patient pool in the United States, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan?
- Why do only limited patients appear for diagnosis?
- Which country is more prevalent for Myasthenia Gravis and why?
- What factors are affecting the diagnosis of the indication?
Current Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for treating Myasthenia Gravis? What are the current guidelines for treating Myasthenia Gravis in the US and Europe?
- How many companies are developing therapies for treating Myasthenia Gravis?
- How many emerging therapies are in the mid-stage and late stage of development for treating Myasthenia Gravis?
- What are the recent novel therapies, targets, mechanisms of action, and technologies developed to overcome the limitations of existing therapies?
- What are the key designations that have been granted for the emerging therapies for Myasthenia Gravis?
- Patient acceptability in terms of preferred treatment options as per real-world scenarios?
- What are the country-specific accessibility issues of expensive, recently approved therapies? Focus on reimbursement policies.
- What are the 7MM historical and forecasted markets of Myasthenia Gravis?
Reasons to Buy:
- The report will help develop business strategies by understanding the latest trends and changing treatment dynamics driving the Myasthenia Gravis Market.
- Insights on patient burden/disease prevalence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identifying strong upcoming players in the market will help devise strategies that will help get ahead of competitors.
- Detailed analysis and ranking of potential current and emerging therapies under the conjoint analysis section to provide visibility around leading classes.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in the future.
- Detailed insights on the unmet needs of the existing market so that the upcoming players can strengthen their development and launch strategy.
Frequently Asked Questions
1. What is the forecast period covered in the report?
The Myasthenia Gravis Epidemiology and Market Insight report for the 7MM covers the forecast period from 2023 to 2034, providing a projection of market dynamics and trends during this timeframe.
2. Who are the key players in the myasthenia gravis market?
The myasthenia gravis market is quite robust. The major players are Janssen Research & Development, LLC; Immunovant Sciences GmbH; Cartesian Therapeutics, and others which are currently developing drugs for the treatment of myasthenia gravis.
3. How is the market size estimated in the forecast report?
The market size is estimated through data analysis, statistical modeling, and expert opinions. It may consider factors such as incident cases, treatment costs, revenue generated, and market trends.
4. What is the key driver of the myasthenia gravis market?
The increase in diagnosed prevalent cases of myasthenia gravis and the launch of emerging therapies are attributed to be the key drivers for increasing myasthenia gravis market.
5. What is the expected impact of emerging therapies or advancements in myasthenia gravis treatment on the market?
Introducing new therapies, advancements in diagnostic techniques, and innovations in treatment approaches can significantly impact the myasthenia gravis treatment market. Market forecast reports may provide analysis and predictions regarding the potential impact of these developments.
6. Does the report provide insights into the competitive landscape of the market?
The market forecast report may include information on the competitive landscape, profiling key market players, their product offerings, partnerships, and strategies, and helping stakeholders understand the competitive dynamics of the Myasthenia Gravis market.
Table of Contents
1. Key Insights
2. Report Introduction
3. Myasthenia Gravis Market Overview at a Glance
- 3.1. Market Share (%) Distribution of Myasthenia Gravis in 2020 by Therapies
- 3.2. Market Share (%) Distribution of Myasthenia Gravis in 2034 by Therapies
4. Epidemiology and Market Forecast Methodology
5. Executive Summary
6. Key Events
7. Disease Background and Overview
- 7.1. Introduction
- 7.2. Types of myasthenia gravis
- 7.3. Clinical Classification of myasthenia gravis
- 7.4. Etiology
- 7.5. Risk Factors
- 7.6. Clinical Manifestations and Symptoms
- 7.7. Pathophysiology
- 7.8. Biomarkers
- 7.9. Diagnosis
- 7.9.1. Differential Diagnosis
- 7.9.2. Diagnostic Algorithm
- 7.9.3. Diagnostic Guidelines
- 7.9.3.1. Association of British Neurologists' Management Guidelines for Myasthenia Gravis
- 7.9.3.2. Japanese Diagnostic Criteria for Myasthenia Gravis
- 7.1. Treatment and Management
- 7.10.1. Treatment Algorithm
- 7.10.2. Treatment Guidelines
- 7.10.2.1. The Japanese Clinical Guidelines 2022 for Myasthenia Gravis
- 7.10.2.2. Association of British Neurologists' Management Guidelines for Myasthenia Gravis
- 7.10.2.3. International Consensus Guidance for Management of Myasthenia Gravis
- 7.10.2.4. Italian Recommendations to Treat Myasthenia Gravis
- 7.10.2.5. German Neurological Society Guidelines for Myasthenia Gravis
8. Epidemiology and Patient Population
- 8.1. Key Findings
- 8.2. Assumptions and Rationale
- 8.2.1. Diagnosed Prevalent Cases of Myasthenia Gravis
- 8.2.2. Gender-specific Diagnosed Prevalent Cases of Myasthenia Gravis
- 8.2.3. Age-specific Diagnosed Prevalent Cases of Myasthenia Gravis
- 8.2.4. Diagnosed Prevalent Cases of Myasthenia Gravis by MGFA classification
- 8.2.5. Diagnosed Prevalent Cases of Generalized Myasthenia Gravis by Antibody Serology
- 8.3. Total Diagnosed Prevalent Cases of Myasthenia Gravis in the 7MM
- 8.4. The United States
- 8.4.1. Total Diagnosed Prevalent Cases of Myasthenia Gravis in the US
- 8.4.2. Gender-specific Diagnosed Prevalent Cases of Myasthenia Gravis in the US
- 8.4.3. Age-specific Diagnosed Prevalent Cases of Myasthenia Gravis in the US
- 8.4.4. Diagnosed Prevalent Cases of Myasthenia Gravis by MGFA Classification in the US
- 8.4.5. Diagnosed Prevalent Cases of Generalized Myasthenia Gravis by Antibody Serology in the US
- 8.5. EU4 and the UK
- 8.5.1. Total Diagnosed Prevalent Cases of Myasthenia Gravis in EU4 and the UK
- 8.5.2. Gender-specific Diagnosed Prevalent Cases of Myasthenia Gravis in EU4 and the UK
- 8.5.3. Age-specific Cases of Myasthenia Gravis in EU4 and the UK
- 8.5.4. Diagnosed Prevalent Cases of Myasthenia Gravis by MGFA Classification in EU4 and the UK
- 8.5.5. Diagnosed Prevalent Cases of Generalized Myasthenia Gravis by Antibody Serology in EU4 and the UK
- 8.6. Japan
- 8.6.1. Total Diagnosed Prevalent Cases of Myasthenia Gravis in Japan
- 8.6.2. Gender-specific Diagnosed Prevalent Cases of Myasthenia Gravis in Japan
- 8.6.3. Age-specific Diagnosed Prevalent Cases of Myasthenia Gravis in Japan
- 8.6.4. Diagnosed Prevalent Cases of Myasthenia Gravis by MGFA Classification in Japan
- 8.6.5. Diagnosed Prevalent Cases of Generalized Myasthenia Gravis by Antibody Serology in Japan
9. Patient Journey
10. Marketed Therapies
- 10.1. Key Cross Competition of Marketed Drugs
- 10.2. SOLIRIS (eculizumab): Alexion: AstraZeneca Rare Disease
- 10.2.1. Product Description
- 10.2.2. Regulatory Milestones
- 10.2.3. Other Development Activities
- 10.2.4. Clinical Development
- 10.2.5. Clinical Trials Information
- 10.2.6. Safety and Efficacy
- 10.2.7. Product Profile
- 10.3. ULTOMIRIS (ravulizumab): Alexion: AstraZeneca Rare Disease
- 10.3.1. Product Description
- 10.3.2. Regulatory Milestones
- 10.3.3. Other Development Activities
- 10.3.4. Clinical Development
- 10.3.5. Clinical Trials Information
- 10.3.6. Safety and Efficacy
- 10.3.7. Product Profile
- 10.4. RYSTIGGO (rozanolixizumab-noli): UCB Biopharma
- 10.4.1. Product Description
- 10.4.2. Regulatory Milestones
- 10.4.3. Other development activities
- 10.4.4. Clinical Development
- 10.4.5. Clinical Trials Information
- 10.4.6. Safety and Efficacy
- 10.4.7. Product Profile
- 10.5. ZILBRYSQ (zilucoplan): UCB Biopharma
- 10.5.1. Product Description
- 10.5.2. Regulatory Milestones
- 10.5.3. Other Development Activities
- 10.5.4. Clinical Development
- 10.5.5. Clinical Trials Information
- 10.5.6. Safety and Efficacy
- 10.5.7. Product Profile
- 10.6. VYVGART (efgartigimod alfa-fcab): Argenx
- 10.6.1. Product Description
- 10.6.2. Regulatory Milestones
- 10.6.3. Other Development Activities
- 10.6.4. Clinical Development
- 10.6.5. Clinical Trials Information
- 10.6.6. Safety and Efficacy
- 10.6.7. Product Profile
- 10.7. VYVGART HYTRULO/VYVDURA (efgartigimod alfa and hyaluronidase-qvfc): Argenx
- 10.7.1. Product Description
- 10.7.2. Regulatory Milestones
- 10.7.3. Other Development Activities
- 10.7.4. Clinical Development
- 10.7.5. Clinical Trials Information
- 10.7.6. Safety and Efficacy
- 10.7.7. Product Profile
11. Emerging Therapies
- 11.1. Key Cross Competition
- 11.2. UPLIZNA (Inebilizumab): Horizon Therapeutics/Amgen
- 11.2.1. Product Description
- 11.2.2. Other Development Activities
- 11.2.3. Clinical Development
- 11.2.4. Clinical Trials Information
- 11.2.5. Product Profile
- 11.2.6. Analyst Views
- 11.3. Nipocalimab: Janssen Research & Development, LLC
- 11.3.1. Product Description
- 11.3.2. Other Development Activities
- 11.3.3. Clinical Development
- 11.3.4. Clinical Trials Information
- 11.3.5. Safety and Efficacy
- 11.3.6. Product Profile
- 11.3.7. Analysts' Views
- 11.4. ENSPRYNG (satralizumab): Hoffmann-La Roche
- 11.4.1. Product Description
- 11.4.2. Other Development Activities
- 11.4.3. Clinical Development
- 11.4.4. Clinical Trials Information
- 11.4.5. Safety and Efficacy
- 11.4.6. Product Profile
- 11.4.7. Analysts' Views
- 11.5. Batoclimab: Immunovant Sciences GmbH
- 11.5.1. Product Description
- 11.5.2. Other Development Activities
- 11.5.3. Clinical Development
- 11.5.4. Clinical Trials Information
- 11.5.5. Safety and Efficacy
- 11.5.6. Product Profile
- 11.5.7. Analysts' Views
- 11.6. Gefurulimab (ALXN-1720): Alexion, AstraZeneca Rare Disease
- 11.6.1. Product Description
- 11.6.2. Other Development Activities
- 11.6.3. Clinical Development
- 11.6.4. Clinical Trials Information
- 11.6.5. Product Profile
- 11.6.6. Analysts' Views
- 11.7. Pozelimab + Cemdisiran: Regeneron Pharmaceuticals
- 11.7.1. Product Description
- 11.7.2. Other Development Activities
- 11.7.3. Clinical Development
- 11.7.4. Clinical Trials Information
- 11.7.5. Product profile
- 11.7.6. Analysts' Views
- 11.8. KYV-101: Kyverna Therapeutics
- 11.8.1. Product Description
- 10.7.3. Other Development Activities
- 10.7.4. Clinical Development
- 10.7.5. Clinical Trials Information
- 10.7.6. Safety and Efficacy
- 10.7.7. Product Profile
11. Emerging Therapies
- 11.1. Key Cross Competition
- 11.2. UPLIZNA (Inebilizumab): Horizon Therapeutics/Amgen
- 11.2.1. Product Description
- 11.2.2. Other Development Activities
- 11.2.3. Clinical Development
- 11.2.4. Clinical Trials Information
- 11.2.5. Product Profile
- 11.2.6. Analyst Views
- 11.3. Nipocalimab: Janssen Research & Development, LLC
- 11.3.1. Product Description
- 11.3.2. Other Development Activities
- 11.3.3. Clinical Development
- 11.3.4. Clinical Trials Information
- 11.3.5. Safety and Efficacy
- 11.3.6. Product Profile
- 11.3.7. Analysts' Views
- 11.4. ENSPRYNG (satralizumab): Hoffmann-La Roche
- 11.4.1. Product Description
- 11.4.2. Other Development Activities
- 11.4.3. Clinical Development
- 11.4.4. Clinical Trials Information
- 11.4.5. Safety and Efficacy
- 11.4.6. Product Profile
- 11.4.7. Analysts' Views
- 11.5. Batoclimab: Immunovant Sciences GmbH
- 11.5.1. Product Description
- 11.5.2. Other Development Activities
- 11.5.3. Clinical Development
- 11.5.4. Clinical Trials Information
- 11.5.5. Safety and Efficacy
- 11.5.6. Product Profile
- 11.5.7. Analysts' Views
- 11.6. Gefurulimab (ALXN-1720): Alexion, AstraZeneca Rare Disease
- 11.6.1. Product Description
- 11.6.2. Other Development Activities
- 11.6.3. Clinical Development
- 11.6.4. Clinical Trials Information
- 11.6.5. Product Profile
- 11.6.6. Analysts' Views
- 11.7. Pozelimab + Cemdisiran: Regeneron Pharmaceuticals
- 11.7.1. Product Description
- 11.7.2. Other Development Activities
- 11.7.3. Clinical Development
- 11.7.4. Clinical Trials Information
- 11.7.5. Product profile
- 11.7.6. Analysts' Views
- 11.8. KYV-101: Kyverna Therapeutics
- 11.8.1. Product Description
- 11.8.2. Other Development Activities
- 11.8.3. Clinical Development
- 11.8.4. Clinical Trials Information
- 11.8.5. Product profile
- 11.8.6. Analysts' Views
- 11.9. Descartes-08: Cartesian Therapeutics
- 11.9.1. Product Profile
- 11.9.2. Other Developmental Activities
- 11.9.3. Clinical Development
- 11.9.4. Clinical Trials Information
- 11.9.5. Safety and Efficacy
- 11.9.6. Product Profile
- 11.9.7. Analyst Views
- 11.1. DNTH103: Dianthus Therapeutics
- 11.10.1. Product Description
- 11.10.2. Other Developmental Activities
- 11.10.3. Clinical Development
- 11.10.4. Clinical Trials Information
- 11.10.5. Safety and Efficacy
- 11.10.6. Product Profile
- 11.10.7. Analyst Views
- 11.11. Vemircopan (ALXN2050): Alexion, AstraZeneca Rare Disease
- 11.11.1. Product Description
- 11.11.2. Other Development Activities
- 11.11.3. Clinical Development
- 11.11.4. Clinical Trials Information
- 11.11.5. Product Profile
- 11.11.6. Analysts' Views
- 11.12. Mezagitamab (TAK-079): Takeda
- 11.12.1. Product Description
- 11.12.2. Other Development Activities
- 11.12.3. Clinical Development
- 11.12.4. Clinical Trials Information
- 11.12.5. Safety and Efficacy
- 11.12.6. Product Profile
- 11.12.7. Analysts' Views
- 11.13. CNP-106: COUR Pharmaceuticals
- 11.13.1. Product Description
- 11.13.2. Other Developmental Activities
- 11.13.3. Clinical Development
- 11.13.4. Clinical Trials Information
- 11.13.5. Product Profile
- 11.13.6. Analyst Views
12. Myasthenia Gravis: Market Analysis
- 12.1. Key Findings
- 12.2. Key Market Forecast Assumptions
- 12.3. Market Outlook
- 12.4. Conjoint Analysis
- 12.5. Total Market Size of Myasthenia Gravis in the 7MM
- 12.6. Market Size of Myasthenia Gravis by Therapies in the 7MM
- 12.7. Market Size of Myasthenia Gravis in the US
- 12.7.1. Total Market Size of Myasthenia Gravis
- 12.7.2. Market Size of Myasthenia Gravis by Therapies
- 12.8. Market Size of Myasthenia Gravis in EU4 and the UK
- 12.8.1. Germany
- 12.8.1.1. Total Market Size of Myasthenia Gravis
- 12.8.1.2. Market Size of Myasthenia Gravis by Therapies
- 12.8.2. France
- 12.8.2.1. Total Market Size of Myasthenia Gravis
- 12.8.2.2. Market Size of Myasthenia Gravis by Therapies
- 12.8.3. Italy
- 12.8.3.1. Market Size of Myasthenia Gravis by Therapies
- 12.8.3.2. Total Market Size of Myasthenia Gravis
- 12.8.4. Spain
- 12.8.4.1. Market Size of Myasthenia Gravis by Therapies
- 12.8.4.2. Total Market Size of Myasthenia Gravis
- 12.8.5. The UK
- 12.8.5.1. Market Size of Myasthenia Gravis by Therapies
- 12.8.5.2. Total Market Size of Myasthenia Gravis
- 12.8.1. Germany
- 12.9. Market Size of Myasthenia Gravis in Japan
- 12.9.1. Total Market Size of Myasthenia Gravis
- 12.9.2. Market Size of Myasthenia Gravis by Therapies
13. Key Opinion Leaders' Views
14. SWOT Analysis
15. Unmet Needs
16. Market Access and Reimbursement
- 16.1. The United States
- 16.1.1. Center for Medicare and Medicaid Services (CMS)
- 16.2. In EU4 and the UK
- 16.2.1. Germany
- 16.2.2. France
- 16.2.3. Italy
- 16.2.4. Spain
- 16.2.5. The United Kingdom
- 16.3. Japan
- 16.3.1. MHLW
17. Appendix
- 17.1. Bibliography
- 17.2. Acronyms and Abbreviations
- 17.3. Report Methodology