|
|
市場調査レポート
商品コード
1576950
眼科用レーザーの世界市場:洞察、競合情勢、市場予測:2030年Ophthalmic Lasers - Market Insights, Competitive Landscape, and Market Forecast - 2030 |
||||||
カスタマイズ可能
適宜更新あり
|
|||||||
| 眼科用レーザーの世界市場:洞察、競合情勢、市場予測:2030年 |
|
出版日: 2024年10月01日
発行: DelveInsight
ページ情報: 英文 150 Pages
納期: 2~10営業日
|
全表示
- 概要
- 図表
- 目次
世界の眼科用レーザーの市場規模は、2023年に12億5,000万米ドルなりました。同市場は、2024年から2030年までの予測期間に6.11%のCAGRで拡大し、2030年には17億8,000万米ドルに達すると予測されています。緑内障、糖尿病性網膜症、加齢黄斑変性(AMD)、白内障などの眼科疾患の有病率上昇は、眼科用レーザー市場の主要促進要因です。さらに、回復時間の短縮、合併症リスクの低減、痛みの軽減などの利点から、患者やヘルスケアプロバイダの間で低侵襲処置への強い嗜好があります。さらに、主な市場参入企業が革新的な眼科用レーザーを導入するために研究開発に継続的に投資していること、パートナーシップや共同研究の増加、製品の発売や承認は、2024年から2030年までの予測期間中に眼科用レーザー市場を押し上げる主な要因の一部です。
世界保健機関(WHO)が提供した最近のデータと統計(2023年)によると、世界全体で少なくとも22億人が近見または遠見の視力障害を経験しています。このうち、約10億人が未対処のままです。このグループの中で、遠方視力障害や失明の主な原因は、9,400万人が罹患している白内障、8,840万人が罹患している屈折異常、800万人が罹患している加齢黄斑変性症、770万人が罹患している緑内障、390万人が罹患している糖尿病性網膜症などです。老眼は近見視力障害の主な原因であり、8億2,600万人が罹患しています。
このように、糖尿病性網膜症、緑内障、加齢黄斑変性などの疾患の発生率が上昇するにつれて、効果的で低侵襲の治療に対する需要が高まっています。眼科用レーザーは、従来の方法よりも効率的にこれらの疾患を管理できる、正確で的を絞った治療を提供します。例えば、糖尿病網膜症や網膜静脈閉塞症に対するレーザー治療は、視力低下を防ぎ、予後を改善します。フェムト秒レーザやYAGレーザなどの革新的なレーザ技術の進歩により、治療可能な疾患の範囲が拡大し、処置の有効性が向上しました。
さらに、世界中の製品開発活動の増加が眼科用レーザー市場全体をさらに押し上げています。例えば、2024年1月、レーザベースの医療機器の大手プロバイダであるIRIDEX Corporationは、米国でIRIDEX 532とIRIDEX 577レーザを特徴とする次世代プラットフォームを発表しました。これらの先進的なレーザーは網膜疾患や緑内障の治療のために設計され、連続波やイリデックス独自のマイクロパルス技術を含む様々な治療モードを提供します。また、このプラットフォームは直感的なタッチスクリーンインターフェースを誇り、様々な臨床制御機能や設定へのアクセスを提供し、使いやすさと治療の精度を向上させました。
このように、上記の要因は予測期間中に眼科用レーザー市場を押し上げる可能性が高いです。
しかし、眼科用レーザー装置の使用後に感染症を発症するリスクや、製品承認のための厳しい規制上の懸念は、眼科用レーザーの将来の市場を妨げる可能性があります。
当レポートでは、世界の眼科用レーザー市場について調査し、市場の概要とともに、製品タイプ別、用途別、エンドユーザー別、地域別動向、および市場に参入する企業のプロファイルなどを提供しています。
目次
第1章 眼科用レーザー市場レポートのイントロダクション
第2章 眼科用レーザー市場のエグゼクティブサマリー
第3章 競合情勢
第4章 規制分析
- 米国
- 欧州
- 日本
- 中国
第5章 眼科用レーザー市場の主な要因分析
- 眼科用レーザーの市場促進要因
- 眼科用レーザーの市場抑制要因と課題
- 眼科用レーザーの市場機会
第6章 眼科用レーザー市場におけるポーターのファイブフォース分析
第7章 眼科用レーザー市場の評価
- 製品タイプ別
- 用途別
- エンドユーザー別
- 地域別
第8章 眼科用レーザー市場の企業と製品のプロファイル
- Alcon Inc.
- IRIDEX Corporation
- Alna-Medicalsystem AG & Co. KG
- BAUSCH + LOMB INCORPORATED
- Lumenis Be Ltd.
- Abbott
- NIDEK CO., LTD.
- MERIDIAN AG
- Johnson & Johnson Vision
- LIGHTMED
- ZEISS
- Navilas Academy
- Aeon Meditec
- Quantel Medical
- Lumibird Medical
- Ziemer Ophthalmic Systems AG
- Novartis AG
- Lumenis
- Canon Medical Systems
- Optovue
第9章 KOLの見解
第10章 プロジェクトのアプローチ
第11章 DelveInsightについて
第12章 免責事項とお問い合わせ
List of Tables
- Table 1: Competitive Analysis
- Table 2: Ophthalmic Lasers Market in Global (2021-2030)
- Table 3: Ophthalmic Lasers Market in Global by Product Type (2021-2030)
- Table 4: Ophthalmic Lasers Market in Global by Application (2021-2030)
- Table 5: Ophthalmic Lasers Market in Global by End-User (2021-2030)
- Table 6: Ophthalmic Lasers Market in Global by Geography (2021-2030)
- Table 7: Ophthalmic Lasers Market in North America (2021-2030)
- Table 8: Ophthalmic Lasers Market in the United States (2021-2030)
- Table 9: Ophthalmic Lasers Market in Canada (2021-2030)
- Table 10: Ophthalmic Lasers Market in Mexico (2021-2030)
- Table 11: Ophthalmic Lasers Market in Europe (2021-2030)
- Table 12: Ophthalmic Lasers Market in France (2021-2030)
- Table 13: Ophthalmic Lasers Market in Germany (2021-2030)
- Table 14: Ophthalmic Lasers Market in United Kingdom (2021-2030)
- Table 15: Ophthalmic Lasers Market in Italy (2021-2030)
- Table 16: Ophthalmic Lasers Market in Spain (2021-2030)
- Table 17: Ophthalmic Lasers Market in the Rest of Europe (2021-2030)
- Table 18: Ophthalmic Lasers Market in Asia-Pacific (2021-2030)
- Table 19: Ophthalmic Lasers Market in China (2021-2030)
- Table 20: Ophthalmic Lasers Market in Japan (2021-2030)
- Table 21: Ophthalmic Lasers Market in India (2021-2030)
- Table 22: Ophthalmic Lasers Market in Australia (2021-2030)
- Table 23: Ophthalmic Lasers Market in South Korea (2021-2030)
- Table 24: Ophthalmic Lasers Market in Rest of Asia-Pacific (2021-2030)
- Table 25: Ophthalmic Lasers Market in the Rest of the World (2021-2030)
- Table 26: Ophthalmic Lasers Market in the Middle East (2021-2030)
- Table 27: Ophthalmic Lasers Market in Africa (2021-2030)
- Table 28: Ophthalmic Lasers Market in South America (2021-2030)
List of Figures
- Figure 1: Competitive Analysis
- Figure 2: Ophthalmic Lasers Market in Global (2021-2030)
- Figure 3: Ophthalmic Lasers Market in Global by Product Type (2021-2030)
- Figure 4: Ophthalmic Lasers Market in Global by Application (2021-2030)
- Figure 5: Ophthalmic Lasers Market in Global by End-User (2021-2030)
- Figure 6: Ophthalmic Lasers Market in Global by Geography (2021-2030)
- Figure 7: Ophthalmic Lasers Market in North America (2021-2030)
- Figure 8: Ophthalmic Lasers Market in the United States (2021-2030)
- Figure 9: Ophthalmic Lasers Market in Canada (2021-2030)
- Figure 10: Ophthalmic Lasers Market in Mexico (2021-2030)
- Figure 11: Ophthalmic Lasers Market in Europe (2021-2030)
- Figure 12: Ophthalmic Lasers Market in France (2021-2030)
- Figure 13: Ophthalmic Lasers Market in Germany (2021-2030)
- Figure 14: Ophthalmic Lasers Market in United Kingdom (2021-2030)
- Figure 15: Ophthalmic Lasers Market in Italy (2021-2030)
- Figure 16: Ophthalmic Lasers Market in Spain (2021-2030)
- Figure 17: Ophthalmic Lasers Market in the Rest of Europe (2021-2030)
- Figure 18: Ophthalmic Lasers Market in Asia-Pacific (2021-2030)
- Figure 19: Ophthalmic Lasers Market in China (2021-2030)
- Figure 20: Ophthalmic Lasers Market in Japan (2021-2030)
- Figure 21: Ophthalmic Lasers Market in India (2021-2030)
- Figure 22: Ophthalmic Lasers Market in Australia (2021-2030)
- Figure 23: Ophthalmic Lasers Market in South Korea (2021-2030)
- Figure 24: Ophthalmic Lasers Market in Rest of Asia-Pacific (2021-2030)
- Figure 25: Ophthalmic Lasers Market in the Rest of the World (2021-2030)
- Figure 26: Ophthalmic Lasers Market in the Middle East (2021-2030)
- Figure 27: Ophthalmic Lasers Market in Africa (2021-2030)
- Figure 28: Ophthalmic Lasers Market in South America (2021-2030)
- Figure 29: Market Drivers
- Figure 30: Market Barriers
- Figure 31: Market Opportunities
- Figure 31: PORTER'S Five Force Analysis
Ophthalmic Lasers Market by Product Type (Argon Blue-Green Laser, ND: Yag Laser, Femtosecond Lasers, Krypton Red Laser, and Others), Application (Glaucoma, Diabetic Retinopathy, Cataracts, and Others), End-User (Hospitals, Ambulatory Surgical Centers, Ophthalmic Clinics, and Others), and Geography (North America, Europe, Asia-Pacific, and Rest of the World) is expected to grow at a steady CAGR forecast till 2030 owing to the surge in the prevalence of ophthalmic diseases such as glaucoma, diabetic retinopathy, and others, growing preference for minimally invasive procedures, and increase in product launches and approvals by key market players across the globe.
The global ophthalmic laser devices market was valued at USD 1.25 billion in 2023, growing at a CAGR of 6.11% during the forecast period from 2024 to 2030, to reach USD 1.78 billion by 2030. The rising prevalence of ophthalmic diseases such as glaucoma, diabetic retinopathy, age-related macular degeneration (AMD), and cataracts are the major drivers for the ophthalmic lasers market. Additionally, a strong preference among patients and healthcare providers for minimally invasive procedures due to their benefits, including shorter recovery times, reduced risk of complications, and less pain. Furthermore, key market players continuously investing in research and development to introduce innovative ophthalmic lasers, increased partnerships and collaborations, product launches and approvals are some of the key factors boosting the market for ophthalmic lasers, during the forecast period from 2024 to 2030.
Ophthalmic Lasers Market Dynamics:
According to the recent data and stats provided by the World Health Organization (2023), globally, at least 2.2 billion people experienced near or distance vision impairment. Of these, approximately 1 billion cases remained unaddressed. Among this group, the leading causes of distance vision impairment or blindness included cataracts, affecting 94 million people; refractive errors, impacting 88.4 million; age-related macular degeneration, which affected 8 million; glaucoma, with 7.7 million cases; and diabetic retinopathy, affected 3.9 million. Presbyopia was the primary condition causing near vision impairment, affecting 826 million people.
Thus, as the incidence of conditions like diabetic retinopathy, glaucoma, and age-related macular degeneration rises, there is a growing demand for effective, minimally invasive treatments. Ophthalmic lasers offer precise, targeted therapies that can manage these disorders more efficiently than traditional methods. For instance, laser treatments for diabetic retinopathy and retinal vein occlusions can prevent vision loss and improve outcomes. The advancement of laser technology, with innovations such as femtosecond and YAG lasers, expanded the range of treatable conditions and enhanced procedural efficacy, driving further adoption in clinical settings and hence driving the market growth of ophthalmic laser across the globe.
Additionally, the increase in product development activities across the globe is further boosting the overall market of ophthalmic lasers. For instance, in January 2024, IRIDEX Corporation, a leading provider of laser-based medical equipment, unveiled its next-generation platform featuring the IRIDEX 532 and IRIDEX 577 lasers in the United States. These advanced lasers were designed for the treatment of retinal diseases and glaucoma, offering a range of treatment modes, including continuous-wave and IRIDEX's exclusive MicroPulse technology. The platform also boasts an intuitive touchscreen interface that provided the access to various clinical control features and settings, enhancing ease of use and precision in treatment.
Thus, the factors mentioned above are likely to boost the market of ophthalmic lasers during the forecasted period.
However, the risk of developing infection after the use of an ophthalmic laser device and stringent regulatory concerns for product approval may hinder the future market of ophthalmic lasers.
Ophthalmic Lasers Market Segment Analysis:
Ophthalmic Lasers Market by Product Type (Argon Blue-Green Laser, ND: Yag Laser, Femtosecond Lasers, Krypton Red Laser, and Others), Application (Glaucoma, Diabetic Retinopathy, Cataracts, and Others), End-User (Hospitals, Ambulatory Surgical Centers, Ophthalmic Clinics, and Others), and Geography (North America, Europe, Asia-Pacific, and Rest of the World)
In the product type segment of the ophthalmic lasers market, femtosecond lasers is projected to hold a considerable market share in 2023. Femtosecond lasers can significantly boost the ophthalmic laser market through their advanced capabilities and the benefits they offer over traditional methods. These lasers operate at incredibly short pulse durations-femtoseconds (one quadrillionth of a second)-allowing for precise and controlled tissue disruption with minimal thermal damage. This precision are particularly advantageous in delicate eye surgeries, such as cataract surgery, where femtosecond lasers are used to perform tasks like corneal incision, lens fragmentation, and capsulotomy with high accuracy. By enhancing surgical outcomes and reducing complications, femtosecond lasers contribute to better patient experiences and quicker recoveries, which in turn increases their adoption among both surgeons and patients. The technology also allows for customized treatments, as surgeons can tailor the laser's parameters to each patient's specific anatomy and condition, further improving efficacy and safety. As a result, femtosecond lasers drive market growth by expanding the range of treatable conditions, enhancing procedural success rates, and encouraging investment in advanced ophthalmic technologies. This growth is further supported by ongoing advancements in laser technology and increasing patient demand for minimally invasive, precise, and effective treatment options.
Additionally, the increasing product approval across the globe are further propelling the market of the segment. For instance, In April 2023, Johnson & Johnson Vision, a global leader in eye health and part of Johnson & Johnson MedTech received U.S Food and Drug Administration FDA 510(k) approval on their ELITA Femtosecond Laser.
Therefore, owing to the above-mentioned factors, the femtosecond lasers category is expected to generate considerable revenue thereby pushing the overall growth of the global ophthalmic lasers market during the forecast period.
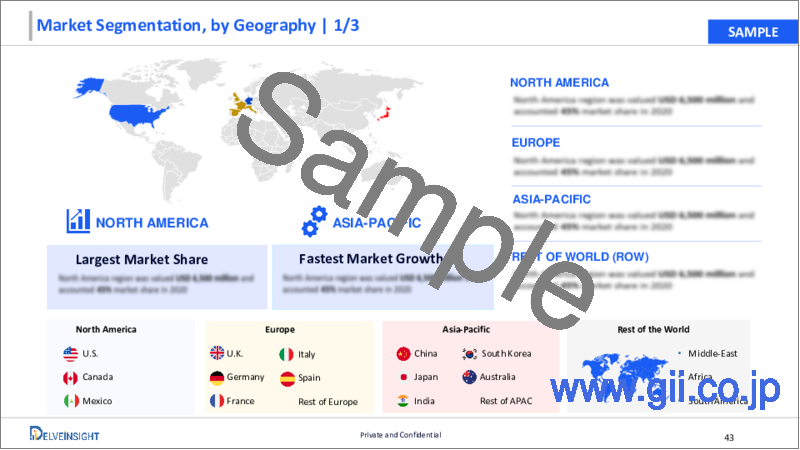
North America is expected to dominate the overall Ophthalmic Lasers market:
Among all the regions, North America is expected to hold the largest share in the global ophthalmic lasers market in 2023. The ophthalmic lasers market in North America is witnessing growth due to several factors like rising incidence of eye disorders like diabetic retinopathy and glaucoma across the region. Additionally, the market is benefited from the presence of prominent industry players and ongoing product development initiatives, including new launches and regulatory approvals. These factors collectively contribute to the expansion of the ophthalmic lasers market in North America, catering to the increasing demand for advanced treatments and technologies in eye care.
According to the data provided by the Centers for Disease Control and Prevention (CDC) (2023), in 2021 it was estimated that in the United States, around 9.6 million people with diabetes, or 2.6%, have diabetic retinopathy (DR), and 1.84 million, or 5.1% of those with diabetes, had vision-threatening diabetic retinopathy (VTDR). Among people over the age of 40 years with diabetes, approximately 1 in 4 had diabetic retinopathy. The prevalence of DR is highest (28.4%) in the 65-79 age group and lowest (13.0%) in those under 25. Overall, there were 8.94 million cases of diabetic retinopathy and 1.71 million cases of vision-threatening diabetic retinopathy among people aged 40 and above. Thus, the increasing prevalence of diabetic retinopathy (DR) in North America, is driving demand for ophthalmic lasers.
Furthermore, recent merger, acquisitions, and approvals among key market players in the region further propels the market across the region. For example, in September 2023, MERIDIAN AG got the approval from the U.S. Food and Drug Administration (FDA) for their innovative Merilas product family which was developed by MERIDIAN AG for the treatment of retinal disorder and glaucoma. Therefore, increasing product approvals, and launches of the product along with the presence of key players, among others is expected to boost the growth of the ophthalmic lasers market in the North American region during the forecast period from 2024 to 2030.
Ophthalmic Lasers Market Key Players:
Some of the key market players operating in the ophthalmic lasers market include Alcon Inc., IRIDEX Corporation, Alna-Medicalsystem AG & Co. KG, BAUSCH + LOMB INCORPORATED, Lumenis Be Ltd., Abbott, NIDEK CO., LTD, MERIDIAN AG, Johnson & Johnson Vision, LIGHTMED, ZEISS, Navilas Academy, Aeon Meditec, Quantel Medical, Lumibird Medical, Ziemer Ophthalmic Systems AG, Novartis, Lumenis, Canon Medical Systems, Optovue, and others.
Recent Developmental Activities in the Ophthalmic Lasers Market:
- In January 2024, MERIDIAN AG received the U.S. Food and Drug Administration (FDA) approval for their MR Q series, this product provided a high-quality and inventive healthcare solution that required the needs of patients and physicians around the world.
- In March 2021, IRIDEX Corporation, announced a collaboration with Topcon Corporation a Japanese manufacturer and distributor of the eye care business to expand their market of ophthalmic lasers in Asia-Pacific.
Key Takeaways From the Ophthalmic Lasers Market Report Study:
- Market size analysis for current ophthalmic lasers size (2023), and market forecast for 6 years (2024 to 2030)
- Top key product/technology developments, mergers, acquisitions, partnerships, and joint ventures happened for the last 3 years
- Key companies dominating the ophthalmic lasers market.
- Various opportunities available for the other competitors in the ophthalmic lasers market space.
- What are the top-performing segments in 2023? How these segments will perform in 2030?
- Which are the top-performing regions and countries in the current ophthalmic lasers market scenario?
- Which are the regions and countries where companies should have concentrated on opportunities for ophthalmic lasers market growth in the coming future?
Target Audience who can be Benefited From This Ophthalmic Lasers Market Report Study:
- Ophthalmic lasers product providers
- Research organizations and consulting companies
- Ophthalmic lasers -related organizations, associations, forums, and other alliances
- Government and corporate offices
- Start-up companies, venture capitalists, and private equity firms
- Distributors and traders dealing in ophthalmic lasers
- Various end-users who want to know more about the ophthalmic lasers market and the latest technological developments in the ophthalmic lasers market.
Frequently Asked Questions for the Ophthalmic Lasers Market:
1. What are ophthalmic lasers?
- An ophthalmic laser is a medical device used in eye care to perform precise and controlled procedures on the eye. It utilizes focused light beams to treat various eye conditions, including cataracts, glaucoma, and retinal disorders, by either cutting, reshaping, or stimulating tissues with minimal invasiveness.
2. What is the market for ophthalmic lasers?
- The global ophthalmic laser devices market was valued at USD 1.25 billion in 2023, growing at a CAGR of 6.11% during the forecast period from 2024 to 2030, to reach USD 1.78 billion by 2030.
3. What are the drivers for the ophthalmic lasers market?
- The rising prevalence of ophthalmic diseases such as glaucoma, diabetic retinopathy, age-related macular degeneration (AMD), and cataracts is a major driver of the ophthalmic laser market. Additionally, a strong preference among patients and healthcare providers for minimally invasive procedures due to their benefits, including shorter recovery times, reduced risk of complications, and less pain. Further, key market players continuously investing in research and development to introduce innovative ophthalmic lasers, increased partnerships and collaborations, product launches and approvals ensures that market remains dynamic and innovative, during the forecast period from 2024 to 2030.
4. Who are the key players operating in the ophthalmic lasers market?
- Some of the key market players operating in the ophthalmic lasers market include Alcon Inc., IRIDEX Corporation, Alna-Medicalsystem AG & Co. KG, BAUSCH + LOMB INCORPORATED, Lumenis Be Ltd., Abbott, NIDEK CO., LTD, MERIDIAN AG, Johnson & Johnson Vision, LIGHTMED, ZEISS, Navilas Academy, Aeon Meditec, Quantel Medical, Lumibird Medical, Ziemer Ophthalmic Systems AG, Novartis, Lumenis, Canon Medical Systems, Optovue, and others.
5. Which region has the highest share in the ophthalmic lasers market?
- North America is expected to hold the largest share in the global ophthalmic lasers market in 2023. This can be attributed to the rising incidence of eye disorders such as diabetic retinopathy and glaucoma across the region. Additionally, the market is benefited from the presence of prominent industry players and ongoing product development initiatives, including new launches and regulatory approvals. These factors collectively contribute to the expansion of the ophthalmic lasers market in North America, catering to the increasing demand for advanced treatments and technologies in eye care.
Table of Contents
1. Ophthalmic Lasers Market Report Introduction
- 1.1. Scope of the Study
- 1.2. Market Segmentation
- 1.3. Market Assumption
2. Ophthalmic Lasers Market Executive Summary
- 2.1. Market at Glance
3. Competitive Landscape
4. Regulatory Analysis
- 4.1. The United States
- 4.2. Europe
- 4.3. Japan
- 4.4. China
5. Ophthalmic Lasers Market Key Factors Analysis
- 5.1. Ophthalmic Lasers Market Drivers
- 5.1.1. Growing prevalence of ophthalmic diseases such as glaucoma, diabetic retinopathy, and others
- 5.1.2. Growing preference for minimally invasive procedures
- 5.1.3. Increase in product launches and approvals by key market players across the globe.
- 5.2. Ophthalmic Lasers Market Restraints and Challenges
- 5.2.1. Risk of developing infection after the use of ophthalmic lasers
- 5.2.2. Stringent regulatory concerns for product approval
- 5.3. Ophthalmic Lasers Market Opportunities
- 5.3.1. Integration of artificial intelligence in the field of ophthalmology lasers
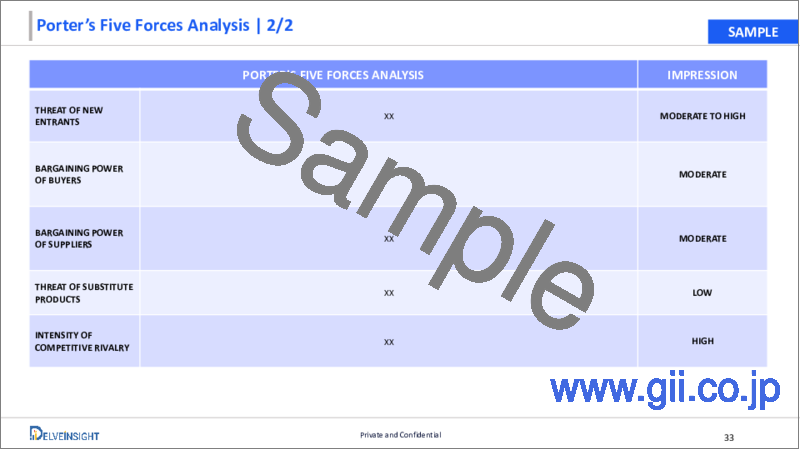
6. Ophthalmic Lasers Market Porter's Five Forces Analysis
- 6.1. Bargaining Power of Suppliers
- 6.2. Bargaining Power of Consumers
- 6.3. Threat of New Entrants
- 6.4. Threat of Substitutes
- 6.5. Competitive Rivalry
7. Ophthalmic Lasers Market Assessment
- 7.1. By Product Type
- 7.1.1. Argon blue-green laser
- 7.1.2. ND: YAG Laser
- 7.1.3. Femtosecond Lasers
- 7.1.4. Krypton Red lasers
- 7.1.5. Others
- 7.2. By Application
- 7.2.1. Glaucoma
- 7.2.2. Diabetic Retinopathy
- 7.2.3. Cataracts
- 7.2.4. Others
- 7.3. By End-User
- 7.3.1. Hospitals
- 7.3.2. Ophthalmic Clinics
- 7.3.3. Ambulatory Surgical Centers
- 7.3.4. Others
- 7.4. By Geography
- 7.4.1. North America
- 7.4.1.1. United States Ophthalmic Lasers Market Size in USD Million (2021-2030)
- 7.4.1.2. Canada Ophthalmic Lasers Market Size in USD Million (2021-2030)
- 7.4.1.3. Mexico Ophthalmic Lasers Market Size in USD Million (2021-2030)
- 7.4.2. Europe
- 7.4.2.1. France Ophthalmic Lasers Market Size in USD Million (2021-2030)
- 7.4.2.2. Germany Ophthalmic Lasers Market Size in USD Million (2021-2030)
- 7.4.2.3. United Kingdom Ophthalmic Lasers Market Size in USD Million (2021-2030)
- 7.4.2.4. Italy Ophthalmic Lasers Market Size in USD Million (2021-2030)
- 7.4.2.5. Spain Ophthalmic Lasers Market Size in USD Million (2021-2030)
- 7.4.2.6. Rest of Europe Ophthalmic Lasers Market Size in USD Million (2021-2030)
- 7.4.3. Asia-Pacific
- 7.4.3.1. China Ophthalmic Lasers Market Size in USD Million (2021-2030)
- 7.4.3.2. Japan Ophthalmic Lasers Market Size in USD Million (2021-2030)
- 7.4.3.3. India Ophthalmic Lasers Market Size in USD Million (2021-2030)
- 7.4.3.4. Australia Ophthalmic Lasers Market Size in USD Million (2021-2030)
- 7.4.3.5. South Korea Ophthalmic Lasers Market Size in USD Million (2021-2030)
- 7.4.3.6. Rest of Asia-Pacific Ophthalmic Lasers Market Size in USD Million (2021-2030)
- 7.4.4. Rest of the World (RoW)
- 7.4.4.1. Middle East Ophthalmic Lasers Market Size in USD Million (2021-2030)
- 7.4.4.2. Africa Ophthalmic Lasers Market Size in USD Million (2021-2030)
- 7.4.4.3. South America Ophthalmic Lasers Market Size in USD Million (2021-2030)
- 7.4.1. North America
8. Ophthalmic Lasers Market Company and Product Profiles
- 8.1. Alcon Inc.
- 8.1.1. Company Overview
- 8.1.2. Company Snapshot
- 8.1.3. Financial Overview
- 8.1.4. Product Listing
- 8.1.5. Entropy
- 8.2. IRIDEX Corporation
- 8.2.1. Company Overview
- 8.2.2. Company Snapshot
- 8.2.3. Financial Overview
- 8.2.4. Product Listing
- 8.2.5. Entropy
- 8.3. Alna-Medicalsystem AG & Co. KG
- 8.3.1. Company Overview
- 8.3.2. Company Snapshot
- 8.3.3. Financial Overview
- 8.3.4. Product Listing
- 8.3.5. Entropy
- 8.4. BAUSCH + LOMB INCORPORATED
- 8.4.1. Company Overview
- 8.4.2. Company Snapshot
- 8.4.3. Financial Overview
- 8.4.4. Product Listing
- 8.4.5. Entropy
- 8.5. Lumenis Be Ltd.
- 8.5.1. Company Overview
- 8.5.2. Company Snapshot
- 8.5.3. Financial Overview
- 8.5.4. Product Listing
- 8.5.5. Entropy
- 8.6. Abbott
- 8.6.1. Company Overview
- 8.6.2. Company Snapshot
- 8.6.3. Financial Overview
- 8.6.4. Product Listing
- 8.6.5. Entropy
- 8.7. NIDEK CO., LTD.
- 8.7.1. Company Overview
- 8.7.2. Company Snapshot
- 8.7.3. Financial Overview
- 8.7.4. Product Listing
- 8.7.5. Entropy
- 8.8. MERIDIAN AG
- 8.8.1. Company Overview
- 8.8.2. Company Snapshot
- 8.8.3. Financial Overview
- 8.8.4. Product Listing
- 8.8.5. Entropy
- 8.9. Johnson & Johnson Vision
- 8.9.1. Company Overview
- 8.9.2. Company Snapshot
- 8.9.3. Financial Overview
- 8.9.4. Product Listing
- 8.9.5. Entropy
- 8.10. LIGHTMED
- 8.10.1. Company Overview
- 8.10.2. Company Snapshot
- 8.10.3. Financial Overview
- 8.10.4. Product Listing
- 8.10.5. Entropy
- 8.11. ZEISS
- 8.11.1. Company Overview
- 8.11.2. Company Snapshot
- 8.11.3. Financial Overview
- 8.11.4. Product Listing
- 8.11.5. Entropy
- 8.12. Navilas Academy
- 8.12.1. Company Overview
- 8.12.2. Company Snapshot
- 8.12.3. Financial Overview
- 8.12.4. Product Listing
- 8.12.5. Entropy
- 8.13. Aeon Meditec
- 8.13.1. Company Overview
- 8.13.2. Company Snapshot
- 8.13.3. Financial Overview
- 8.13.4. Product Listing
- 8.13.5. Entropy
- 8.14. Quantel Medical
- 8.14.1. Company Overview
- 8.14.2. Company Snapshot
- 8.14.3. Financial Overview
- 8.14.4. Product Listing
- 8.14.5. Entropy
- 8.15. Lumibird Medical
- 8.15.1. Company Overview
- 8.15.2. Company Snapshot
- 8.15.3. Financial Overview
- 8.15.4. Product Listing
- 8.15.5. Entropy
- 8.16. Ziemer Ophthalmic Systems AG
- 8.16.1. Company Overview
- 8.16.2. Company Snapshot
- 8.16.3. Financial Overview
- 8.16.4. Product Listing
- 8.16.5. Entropy
- 8.17. Novartis AG
- 8.17.1. Company Overview
- 8.17.2. Company Snapshot
- 8.17.3. Financial Overview
- 8.17.4. Product Listing
- 8.17.5. Entropy
- 8.18. Lumenis
- 8.18.1. Company Overview
- 8.18.2. Company Snapshot
- 8.18.3. Financial Overview
- 8.18.4. Product Listing
- 8.18.5. Entropy
- 8.19. Canon Medical Systems
- 8.19.1. Company Overview
- 8.19.2. Company Snapshot
- 8.19.3. Financial Overview
- 8.19.4. Product Listing
- 8.19.5. Entropy
- 8.20. Optovue
- 8.20.1. Company Overview
- 8.20.2. Company Snapshot
- 8.20.3. Financial Overview
- 8.20.4. Product Listing
- 8.20.5. Entropy