|
|
市場調査レポート
商品コード
1632487
アレルギー性鼻炎:市場洞察・疫学・市場予測 (~2034年)Allergic Rhinitis - Market Insight, Epidemiology, and Market Forecast - 2034 |
||||||
カスタマイズ可能
適宜更新あり
|
|||||||
| アレルギー性鼻炎:市場洞察・疫学・市場予測 (~2034年) |
|
出版日: 2025年01月01日
発行: DelveInsight
ページ情報: 英文 200 Pages
納期: 2~10営業日
|
全表示
- 概要
- 図表
- 目次
主なハイライト
- 2023年のアレルギー性鼻炎の市場規模は、主要7カ国の中で米国が最も大きく、約36億米ドルを占め、2034年までにさらに拡大すると予測されています。
- 2023年のアレルギー性鼻炎の有病者数は、主要7カ国の中で日本が最も多く、約6,500万人を占め、2034年までにさらに増加すると予想されています。
- 新薬REGN5713-5714-5715は、2025年までにEU4カ国と英国で、2026年までに日本で上市される見込みであり、予測される数年間でアレルギー性鼻炎の疾病負担を軽減する可能性があります。
当レポートでは、米国、EU4カ国 (ドイツ、フランス、イタリア、スペイン)、英国、日本のアレルギー性鼻炎の市場を調査し、疾患の背景・概要、疫学、治療と管理の概要、市場促進要因と障壁、アンメットニーズ、上市薬およびパイプライン薬のプロファイル、主要国の市場規模の推移・予測、競合情勢などをまとめ、最良の機会を発掘し、市場の潜在力を評価します。
目次
第1章 重要な洞察
第2章 レポート概要
第3章 アレルギー性鼻炎:市場概要
- アレルギー性鼻炎の市場シェア (%) 分布:2020年
- アレルギー性鼻炎の市場シェア (%) 分布:2034年
第4章 疫学と市場予測手法
第5章 重要な出来事
第6章 アレルギー性鼻炎:エグゼクティブサマリー
第7章 アレルギー性鼻炎:疾患背景と概要
- 鼻炎の分類
- 病因
- 発症機序
- 兆候と症状
- 合併症
- 診断
- 治療
第8章 アレルギー性鼻炎:疫学と患者人口
- 主な調査結果
- 前提と根拠:主要7カ国
- 主要7カ国の有病者数
- 主要7カ国の診断有病者数
- 米国
- EU4カ国・英国
- 日本
- 有病者数
- 診断有病者数
- 有病者数:年齢別
- 有病者数:重症度別
- 有病者数:アレルゲン別
第9章 ペイシェントジャーニー
第10章 上市済み治療薬
- キークロス
- RYALTRIS (オロパタジン塩酸塩およびモメタゾンフランカルボン酸エステル一水和物点鼻薬):Glenmark Pharmaceuticals Inc.
- 製品説明
- 規制上のマイルストーン
- 臨床開発
- 臨床試験情報
- 安全性と有効性
- 製品プロファイル
第11章 新興医薬品
- キークロスコンペティション
- REGN5713-5714-5715:Regeneron Pharmaceuticals
- 製品説明
- その他の開発活動
- 臨床開発
- 臨床試験情報
- 製品プロファイル
- アナリストの見解
- Grass MATA MPL: Allergy Therapeutics
- 臨床開発活動
- 臨床試験情報
第12章 アレルギー性鼻炎:主要7カ国市場の分析
- 主な調査結果
- 市場見通し
- 主要な前提条件
- コンジョイント分析
- 主要7カ国の市場規模
- 主要7カ国の市場規模:治療法別
- 米国
- EU4カ国・英国
- 日本
第13章 主要オピニオンリーダーの見解
第14章 SWOT分析
第15章 アンメットニーズ
第16章 市場アクセスと償還
- 米国
- EU4カ国・英国
- 日本
第17章 付録
第18章 DelveInsightのサービス内容
第19章 免責事項
List of Tables
- Table 1 Key Events
- Table 2 Summary of Allergic Rhinitis Market and Epidemiology (2020-2034)
- Table 3 Classification of Rhinitis
- Table 4 Key Recommendations for Practice
- Table 5 Evidence Levels for Grades of Evidence
- Table 6 Guideline Definitions for Evidence-based Statements
- Table 7 Correspondence Between The Evaluation of the Literature and the Grade of the Recommendations
- Table 8 Allergen Avoidance Measures and Their Effectiveness
- Table 9 Correspondence Between The Evaluation of the Literature and the Grade of the Recommendations
- Table 10 Treatment of Allergic Rhinitis
- Table 11 Total Prevalent Cases of Allergic Rhinitis in the 7MM in '000s (2020-2034)
- Table 12 Diagnosed Prevalent Cases of Allergic Rhinitis in the 7MM in '000s (2020-2034)
- Table 13 Prevalent Cases of Allergic Rhinitis in the United States in '000s (2020-2034)
- Table 14 Diagnosed Prevalent Cases of Allergic Rhinitis in the United States in '000s (2020-2034)
- Table 15 Age-specific Diagnosed Prevalent Cases of Allergic Rhinitis in the United States in '000s (2020-2034)
- Table 16 Severity-specific Diagnosed Prevalent Cases of Allergic Rhinitis in the United States in '000s (2020-2034)
- Table 17 Allergen-specific Diagnosed Prevalent Cases of Allergic Rhinitis in the United States in '000s (2020-2034)
- Table 18 Prevalent Cases of Allergic Rhinitis in Germany in '000s (2020-2034)
- Table 19 Diagnosed Prevalent Cases of Allergic Rhinitis in Germany in '000s (2020-2034)
- Table 20 Age-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Germany in '000s (2020-2034)
- Table 21 Severity-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Germany in '000s (2020-2034)
- Table 22 Allergen-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Germany in '000s (2020-2034)
- Table 23 Prevalent Cases of Allergic Rhinitis in France in '000s (2020-2034)
- Table 24 Diagnosed Prevalent Cases of Allergic Rhinitis in France in '000s (2020-2034)
- Table 25 Age-specific Diagnosed Prevalent Cases of Allergic Rhinitis in France in '000s (2020-2034)
- Table 26 Severity-specific Diagnosed Prevalent Cases of Allergic Rhinitis in France in '000s (2020-2034)
- Table 27 Allergen-specific Diagnosed Prevalent Cases of Allergic Rhinitis in France in '000s (2020-2034)
- Table 28 Prevalent Cases of Allergic Rhinitis in Italy in '000s (2020-2034)
- Table 29 Diagnosed Prevalent Cases of Allergic Rhinitis in Italy in '000s (2020-2034)
- Table 30 Age-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Italy in '000s (2020-2034)
- Table 31 Severity-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Italy in '000s (2020-2034)
- Table 32 Allergen-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Italy in '000s (2020-2034)
- Table 33 Prevalent Cases of Allergic Rhinitis in Spain in '000s (2020-2034)
- Table 34 Diagnosed Prevalent Cases of Allergic Rhinitis in Spain in '000s (2020-2034)
- Table 35 Age-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Spain in '000s (2020-2034)
- Table 36 Severity-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Spain in '000s (2020-2034)
- Table 37 Allergen-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Spain in '000s (2020-2034)
- Table 38 Prevalent Cases of Allergic Rhinitis in the United Kingdom in '000s (2020-2034)
- Table 39 Diagnosed Prevalent Cases of Allergic Rhinitis in the United Kingdom in '000s (2020-2034)
- Table 40 Age-specific Diagnosed Prevalent Cases of Allergic Rhinitis in the United Kingdom in '000s (2020-2034)
- Table 41 Severity-specific Diagnosed Prevalent Cases of Allergic Rhinitis in the United Kingdom in '000s (2020-2034)
- Table 42 Allergen-specific Diagnosed Prevalent Cases of Allergic Rhinitis in the United Kingdom in '000s (2020-2034)
- Table 43 Prevalent Cases of Allergic Rhinitis in EU4 and the UK in '000s (2020-2034)
- Table 44 Diagnosed Prevalent Cases of Allergic Rhinitis in EU4 and the UK in '000s (2020-2034)
- Table 45 Age-specific Diagnosed Prevalent Cases of Allergic Rhinitis in EU4 and the UK, in '000' (2020-2034)
- Table 46 Severity-specific Diagnosed Prevalent Cases of Allergic Rhinitis in EU4 and the UK, in '000' (2020-2034)
- Table 47 Allergen-specific Diagnosed Prevalent Cases of Allergic Rhinitis in EU4 and the UK, in '000' (2020-2034)
- Table 48 Prevalent Cases of Allergic Rhinitis in Japan in '000s (2020-2034)
- Table 49 Diagnosed Prevalent Cases of Allergic Rhinitis in Japan in '000s (2020-2034)
- Table 50 Age-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Japan in '000s (2020-2034)
- Table 51 Severity-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Japan in '000s (2020-2034)
- Table 52 Allergen-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Japan in '000s (2020-2034)
- Table 53 Marketed Drugs KeyCross
- Table 54 Ryaltris (Olopatadine Hydrochloride and Mometasone Furoate Monohydrate Nasal Spray), Clinical Trial Description, 2024
- Table 55 Comparison of Emerging Drugs for Treatment
- Table 56 Grass MATA MPL, Clinical Trial Description, 2024
- Table 57 REGN 5713-5714-5715, Clinical Trial Description, 2024
- Table 58 Key Market Forecast Assumptions for REGN5713-5714-5715
- Table 59 Key Market Forecast Assumptions for Grass MATA MPL
- Table 60 Total Allergic rhinitis Market Size in the 7MM in USD million (2020-2034)
- Table 61 Allergic rhinitis Market Size by Therapies in the 7MM in USD million (2020-2034)
- Table 62 The United States Total Allergic rhinitis Market Size in USD million (2020-2034)
- Table 63 Allergic rhinitis Market Size by Therapies in the US in USD million (2020-2034)
- Table 64 The EU4 and the UK Total Allergic rhinitis Market Size, in USD million (2020-2034)
- Table 65 Germany Total Allergic rhinitis Market Size, in USD million (2020-2034)
- Table 66 France Total Allergic rhinitis Market Size, in USD million (2020-2034)
- Table 67 Italy Total Allergic rhinitis Market Size, in USD million (2020-2034)
- Table 68 Spain Total Allergic rhinitis Market Size, in USD million (2020-2034)
- Table 69 The UK Total Allergic rhinitis Market Size, in USD million (2020-2034)
- Table 70 Allergic rhinitis Market Size by Therapies in Germany in USD million (2020-2034)
- Table 71 Allergic rhinitis Market Size by Therapies in France in USD million (2020-2034)
- Table 72 Allergic rhinitis Market Size by Therapies in Italy in USD million (2020-2034)
- Table 73 Allergic rhinitis Market Size by Therapies in Spain in USD million (2020-2034)
- Table 74 Allergic rhinitis Market Size by Therapies in the UK in USD million (2020-2034)
- Table 75 Allergic rhinitis Market Size by Therapies in the EU4 and the UK in USD million (2020-2034)
- Table 76 Total Allergic rhinitis Market Size in Japan, in USD million (2020-2034)
- Table 77 Allergic rhinitis Market Size by Therapies in Japan in USD million (2020-2034)
List of Figures
- Figure 1 Total Prevalent Cases of Allergic Rhinitis in the 7MM in '000s (2020-2034)
- Figure 2 Diagnosed Prevalent Cases of Allergic Rhinitis in the 7MM in '000s (2020-2034)
- Figure 3 Prevalent Cases of Allergic Rhinitis in the United States in '000s (2020-2034)
- Figure 4 Diagnosed Prevalent Cases of Allergic Rhinitis in the United States in '000s (2020-2034)
- Figure 5 Age-specific Diagnosed Prevalent Cases of Allergic Rhinitis in the United States in '000s (2020-2034)
- Figure 6 Severity-specific Diagnosed Prevalent Cases of Allergic Rhinitis in the United States in '000s (2020-2034)
- Figure 7 Allergen-specific Diagnosed Prevalent Cases of Allergic Rhinitis in the United States in '000s (2020-2034)
- Figure 8 Prevalent Cases of Allergic Rhinitis in Germany in '000s (2020-2034)
- Figure 9 Diagnosed Prevalent Cases of Allergic Rhinitis in Germany in '000s (2020-2034)
- Figure 10 Age-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Germany in '000s (2020-2034)
- Figure 11 Severity-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Germany in '000s (2020-2034)
- Figure 12 Allergen-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Germany in '000s (2020-2034)
- Figure 13 Prevalent Cases of Allergic Rhinitis in France in '000s (2020-2034)
- Figure 14 Diagnosed Prevalent Cases of Allergic Rhinitis in France in '000s (2020-2034)
- Figure 15 Age-specific Diagnosed Prevalent Cases of Allergic Rhinitis in France in '000s (2020-2034)
- Figure 16 Severity-specific Diagnosed Prevalent Cases of Allergic Rhinitis in France in '000s (2020-2034)
- Figure 17 Allergen-specific Diagnosed Prevalent Cases of Allergic Rhinitis in France in '000s (2020-2034)
- Figure 18 Prevalent Cases of Allergic Rhinitis in Italy in '000s (2020-2034)
- Figure 19 Diagnosed Prevalent Cases of Allergic Rhinitis in Italy in '000s (2020-2034)
- Figure 20 Age-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Italy in '000s (2020-2034)
- Figure 21 Severity-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Italy in '000s (2020-2034)
- Figure 22 Allergen-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Italy in '000s (2020-2034)
- Figure 23 Prevalent Cases of Allergic Rhinitis in Spain in '000s (2020-2034)
- Figure 24 Diagnosed Prevalent Cases of Allergic Rhinitis in Spain in '000s (2020-2034)
- Figure 25 Age-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Spain in '000s (2020-2034)
- Figure 26 Severity-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Spain in '000s (2020-2034)
- Figure 27 Allergen-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Spain in '000s (2020-2034)
- Figure 28 Prevalent Cases of Allergic Rhinitis in the United Kingdom in '000s (2020-2034)
- Figure 29 Diagnosed Prevalent Cases of Allergic Rhinitis in the United Kingdom in '000s (2020-2034)
- Figure 30 Age-specific Diagnosed Prevalent Cases of Allergic Rhinitis in the United Kingdom in '000s (2020-2034)
- Figure 31 Severity-specific Diagnosed Prevalent Cases of Allergic Rhinitis in the United Kingdom in '000s (2020-2034)
- Figure 32 Allergen-specific Diagnosed Prevalent Cases of Allergic Rhinitis in the United Kingdom in '000s (2020-2034)
- Figure 33 Prevalent Cases of Allergic Rhinitis in EU4 and the UK in '000s (2020-2034)
- Figure 34 Diagnosed Prevalent Cases of Allergic Rhinitis in EU4 and the UK in '000s (2020-2034)
- Figure 35 Age-specific Diagnosed Prevalent Cases of Allergic Rhinitis in EU4 and the UK, in '000' (2020-2034)
- Figure 36 Severity-specific Diagnosed Prevalent Cases of Allergic Rhinitis in EU4 and the UK, in '000' (2020-2034)
- Figure 37 Allergen-specific Diagnosed Prevalent Cases of Allergic Rhinitis in EU4 and the UK, in '000' (2020-2034)
- Figure 38 Prevalent Cases of Allergic Rhinitis in Japan in '000s (2020-2034)
- Figure 39 Diagnosed Prevalent Cases of Allergic Rhinitis in Japan in '000s (2020-2034)
- Figure 40 Age-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Japan in '000s (2020-2034)
- Figure 41 Severity-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Japan in '000s (2020-2034)
- Figure 42 Allergen-specific Diagnosed Prevalent Cases of Allergic Rhinitis in Japan in '000s (2020-2034)
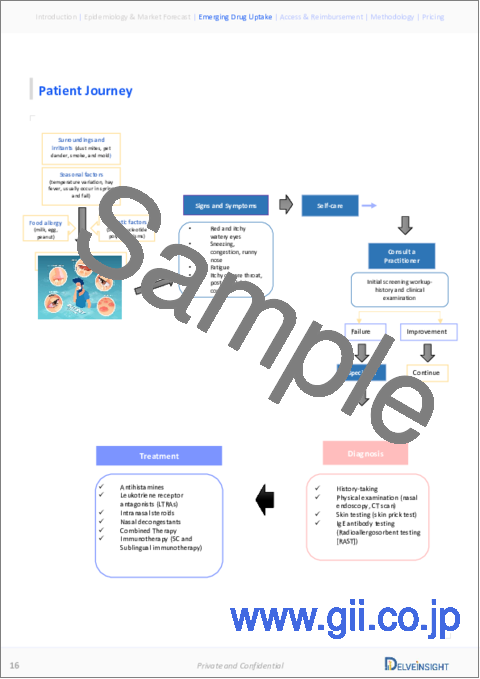
- Figure 43 Patient Journey
- Figure 44 Total Allergic rhinitis Market Size in the 7MM in USD million (2020-2034)
- Figure 45 Allergic rhinitis Market Size by Therapies in the 7MM in USD million (2020-2034)
- Figure 46 The United States Total Allergic rhinitis Market Size in USD million (2020-2034)
- Figure 47 Allergic rhinitis Market Size by Therapies in the US in USD million (2020-2034)
- Figure 48 The EU4 and the UK Total Allergic rhinitis Market Size, in USD million (2020-2034)
- Figure 49 Germany Total Allergic rhinitis Market Size, in USD million (2020-2034)
- Figure 50 France Total Allergic rhinitis Market Size, in USD million (2020-2034)
- Figure 51 Italy Total Allergic rhinitis Market Size, in USD million (2020-2034)
- Figure 52 Spain Total Allergic rhinitis Market Size, in USD million (2020-2034)
- Figure 53 The UK Total Allergic rhinitis Market Size, in USD million (2020-2034)
- Figure 54 Allergic rhinitis Market Size by Therapies in Germany in USD million (2020-2034)
- Figure 55 Allergic rhinitis Market Size by Therapies in France in USD million (2020-2034)
- Figure 56 Allergic rhinitis Market Size by Therapies in Italy in USD million (2020-2034)
- Figure 57 Allergic rhinitis Market Size by Therapies in Spain in USD million (2020-2034)
- Figure 58 Allergic rhinitis Market Size by Therapies in the UK in USD million (2020-2034)
- Figure 59 Allergic rhinitis Market Size by Therapies in the EU4 and the UK in USD million (2020-2034)
- Figure 60 Total Allergic rhinitis Market Size in Japan, in USD million (2020-2034)
- Figure 61 Allergic rhinitis Market Size by Therapies in Japan in USD million (2020-2034)
- Figure 62 Unmet Needs
- Figure 63 SWOT Analysis
Key Highlights:
- In 2023, the Allergic Rhinitis Market Size was highest in the US among the 7MM, accounting for approximately USD 3,600 million which is further expected to increase by 2034.
- In 2023, the prevalence of Allergic rhinitis was highest in Japan among the 7MM, accounting for approximately 65 million which is further expected to increase by 2034.
- The emerging drug REGN5713-5714-5715 is expected to launch in EU4 and the UK by 2025, and in Japan by 2026, which has the potential to reduce the disease burden of Allergic rhinitis in the forecasted years.
DelveInsight's "Allergic rhinitis Market Insights, Epidemiology, and Market Forecast - 2034" report deliver an in-depth understanding of the Allergic rhinitis, historical and forecasted epidemiology as well as the Allergic rhinitis market trends in the United States, EU4 and the UK (Germany, France, Italy, Spain) and the United Kingdom, and Japan.
The Allergic rhinitis market report provides current treatment practices, emerging drugs, and market share of the individual therapies, current and forecasted 7MM Allergic rhinitis market size from 2020 to 2034. The Allergic rhinitis Market Report also covers current Allergic rhinitis treatment practice, market drivers, market barriers, SWOT analysis, reimbursement and market access, and unmet medical needs to curate the best of the opportunities and assesses the underlying potential of the Allergic rhinitis market.
Allergic Rhinitis Treatment Market
Allergic rhinitis Overview
Allergic rhinitis is an atopic disease presenting symptoms of sneezing, nasal congestion, clear rhinorrhea, and nasal pruritis. It is an IgE-mediated immune response against inhaled antigens in the immediate phase, with a subsequent leukotriene-mediated late phase. This activity describes the evaluation and treatment of allergic rhinitis and highlights the role of the interprofessional team in improving care for patients with this condition.
Allergic rhinitis is classified based on triggering allergen (such as seasonal, perennial/year-round, or episodic), frequency of symptoms (intermittent allergic rhinitis and persistent allergic rhinitis), the severity of the symptoms (mild, mild intermittent, moderate, intermittent, moderate-severe, moderate intermittent, severe, severe intermittent, mild, severe persistent)
The risk factors for allergic rhinitis include pollens (tree, grass, and weed, including ragweed), indoor allergens (house dust mites and animal allergens), vegetal and animal proteins, chemicals, antibiotic use, self-reported air pollution, exposure to farm animals (only in LMICs), exposure to cats and/or dogs, maternal and paternal smoking, and vigorous physical activity in adolescents.
Typical initial symptoms include rhinorrhea, nasal itching, sneezing, and nasal congestion, although extra nasal symptoms such as allergic conjunctivitis, itchy ears and palate, and asthma are also commonly associated. A positive correlation between the clinical history and allergen sensitization is usually enough to support the diagnosis of allergic rhinitis.
Allergic rhinitis Diagnosis
Allergic rhinitis is often under-recognized due to low public awareness, limited allergist access, and confusing diagnoses such as the common cold. Diagnosis is largely based on the patient's medical history, physical examination, and in vivo IgE tests, although in vitro tests have found their way into routine allergy diagnostics. Polysensitization is frequent, and in vitro IgE assays, particularly those based on allergen components, provide additional useful information where the latter offers the possibility of distinguishing between genuine sensitization and cross-reactivity. Polysensitization and higher IgE levels against animal-derived allergens are linked to severe forms of other atopic complications like asthma. Nevertheless, many facts complicate prevention and treatment approaches pinpointing gold standard allergic rhinitis diagnosis. Future research is desirable to map the sensitization profiles for allergic rhinitis patients and find an approach with which this gained information can be adequately used. Although CRD offers information on the patient's sensitization profile, it is crucial for future diagnostics and treatments to filter out asymptomatic (poly) sensitized patients and to use the right tools from the allergy diagnostics collection only on those patients who require it.
Allergic rhinitis Treatment
The management of Allergic Rhinitis involves allergen avoidance, pharmacotherapy, allergen-specific immunotherapy, or a combination of all three. Pharmacologic options include intranasal corticosteroids (INSs), oral and intranasal antihistamines, intranasal chromones, oral and intranasal decongestants, oral and intranasal anticholinergic agents, and antileukotrienes. The Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines and the European Academy of Allergology and Clinical Immunology (EAACI) consensus statement note that INSs are a highly effective first-line treatment for moderate/severe or persistent Allergic Rhinitis because they relieve symptoms to a greater degree than other classes of drugs and are especially effective in controlling nasal congestion. The anti-inflammatory properties of INSs may explain their strong effect on clinical symptoms.
Allergic rhinitis Epidemiology
As the Allergic rhinitis market is derived using a patient-based model, the Allergic rhinitis epidemiology chapter in the report provides historical as well as forecasted epidemiology segmented by, Prevalent Cases of Allergic Rhinitis, Diagnosed Prevalent Cases of Allergic Rhinitis, Severity-Specific Diagnosed Prevalent Cases of Allergic Rhinitis, Age-specific Diagnosed Prevalent Cases of Allergic Rhinitis, Allergen-specific sensitivity of Diagnosed Prevalent Cases of Allergic Rhinitis in the 7MM covering, the United States, EU4 countries (Germany, France, Italy, and Spain), United Kingdom, and Japan from 2020 to 2034.
- In the assessment done by DelveInsight, the estimated total diagnosed prevalent cases of Allergic rhinitis in the 7MM were nearly 90 million in 2023.
- The highest total diagnosed prevalent cases of Allergic rhinitis were accounted by Japan in 2023 (~26 million), which are expected to show a rise in the future.
- Among the European countries, Germany had the highest diagnosed prevalent cases of Allergic rhinitis with ~10 million cases, followed by the United Kingdom, with ~9 million in 2023. Spain had the lowest cases (~5 million cases).
- Japan had 26 million total diagnosed prevalent cases of Allergic rhinitis in 2023, accounting for approximately 30% in 7MM.
- DelveInsight's analysis revealed that in 2023, about 20% of Allergic rhinitis cases in the 7MM were classified as mild, 60% as moderate, and 20% as severe.
- The age-specific diagnosed prevalent cases of allergic rhinitis were segmented in four age groups- 0-10 years, 10-17 years, 18-59 years, and 60 and above. In 2023, in the 7MM age group 18-59 years had the highest cases of allergic rhinitis (~60 million), followed by 60 and above, 10-17 years and 0-10 years.
- In 2023, in the 7MM, the allergen-specific diagnosed prevalent cases of allergic rhinitis were highest for grass pollen, followed by tree pollen, mites, weed pollen, animal dander, and fungal spores.
Allergic rhinitis Drug Chapters
The drug chapter segment of the Allergic rhinitis market report encloses a detailed analysis of Allergic rhinitis off-label drug and late-stage (Phase-III and Phase-II) pipeline drug. It also helps to understand the Allergic rhinitis clinical trial details, expressive pharmacological action, agreements and collaborations, approval and patent details, advantages and disadvantages of each included drug, and the latest news and press releases.
Allergic rhinitis Marketed Drugs
RYALTRIS (Olopatadine Hydrochloride and Mometasone Furoate Monohydrate Nasal Spray): Glenmark Pharmaceuticals Inc.
RYALTRIS is a metered, fixed-dose, aqueous suspension, prescription drug product nasal spray approved by the FDA for treating symptoms associated with Seasonal Allergic Rhinitis. Each unit of Ryaltris nasal spray contains 665 mcg of olopatadine hydrochloride, a histamine-1(H1)-receptor inhibitor, and 25 mcg of mometasone furoate, a corticosteroid. In January 2022, RYALTRIS was approved by the US FDA for treating symptoms associated with seasonal allergic rhinitis in adults and pediatric patients 12 years of age and older. In August 2021, Glenmark Pharmaceuticals received marketing approval for its fixed-dose combination nasal spray RYALTRIS in 13 countries across the European Union and the UK.
Emerging Allergic rhinitis Drugs
REGN5713-5714-5715: Regeneron Pharmaceuticals
REGN5713-5714-5715 is an investigational combination of three fully human monoclonal antibodies designed to treat allergic inflammatory conditions caused by the allergen Betv1, which is the main allergen responsible for birch pollen allergies. Birch allergy can trigger reactions such as allergic rhinitis and asthma. The Bet v1-specific mAbs (REGN5713, REGN5714, and REGN5715) were generated using Regeneron's VelocImmune platform. Preclinical studies demonstrated that the three mAbs bind independently and non-competitively to Bet v 1. All three together provided maximal inhibition of Bet v 1 binding to human polyclonal IgE and potently blocked basophil activation ex vivo and mast cell degranulation in vivo. The multi-antibody therapy is currently in Phase III of development.
Grass MATA MPL: Allergy Therapeutics
Grass MATA MPL contains an extract of 13 grass pollens modified with glutaraldehyde to form allergoids that reduce the reactivity with immunoglobulin E (IgE) antibodies without a reduction in other important immunological properties, such as T-cell reactivity. The allergoid is adsorbed to microcrystalline tyrosine as a depot adjuvant system formulation. Monophosphoryl lipid-A (MPL) is included as an adjuvant to increase the immunogenic effect of the immunotherapy and to enhance the switch from an allergen-specific helper T-cell Type 2 (Th2) to helper T-cell Type 1 (Th1) like an immune response. Grass MATA MPL is being developed as a pre-seasonal SC immunotherapy product for treating allergic rhinitis and/or rhinoconjunctivitis. In November 2023, Allergy Therapeutics completed the Phase III study to evaluate the efficacy and safety of PQ grass in subjects with seasonal allergic rhinitis and/or rhinoconjunctivitis induced by grass pollen.
Allergic rhinitis Market Outlook
The treatment goal for allergic rhinitis is to relieve symptoms. Therapeutic options to achieve this goal include avoidance measures, nasal saline irrigation, oral antihistamines, intranasal corticosteroids, combination intranasal corticosteroid/antihistamine sprays; leukotriene receptor antagonists (LTRAs), and allergen immunotherapy.
The complete spectrum of pharmacologic treatments for allergic rhinitis includes FDA-approved medications and off-label treatments. For mild AR, nasal washes help remove mucus. Off-label treatments for allergic rhinitis include antihistamines, which are effective for treating occasional allergy symptoms and can be used as nasal sprays. Corticosteroid nasal sprays are the most effective treatment for allergic rhinitis and work best when used continuously but are also helpful for shorter or intermittent use. These sprays are generally safe for children and adults and come in various brands, both off-label and prescription-based. Decongestants can also help reduce nasal stuffiness.
Other Allergic rhinitis medicines include leukotriene inhibitors, which are prescription drugs that block symptom-triggering chemicals. Allergy shots (immunotherapy) are sometimes recommended for difficult-to-control symptoms, involving regular doses of allergens to help the body adjust. Sublingual Immunotherapy Treatment (SLIT) involves placing medicine under the tongue to help with grass and ragweed allergies.
Allergic rhinitis Therapies approved in the 7MM include ODACTRA/ACARIZAX/MITICURE (SLIT-tablet) by ALK-Abello, GRASTEK/GRAZAX (Grass pollen allergy vaccine tablet) by ALK-Abello, Glenmark Pharmaceuticals' RYALTRIS, and others.
In light of the above, some developmental initiatives have been taken toward the management of Allergic rhinitis. The condition may evolve as some interesting therapies are heading down the pipeline. This would encourage reimbursement scenarios, doctors' adoption, and patient compliance. Some companies have initiated clinical trials investigating new treatment options, including Regeneron Pharmaceuticals' REGN5713-5714-5715 and Grass MATA by Allergy Therapeutics as the potential therapies lined up for forecast in the 7MM in the DelveInsight therapeutics market model.
In the upcoming Allergic rhinitis treatment landscape, there are plethora of companies investigating agents for use in the Allergic rhinitis which includes Allergy Therapeutics, Regeneron Pharmaceuticals and others. There are many more pharma companies which are conducting clinical trials for therapies of allergic rhinitis.
- The Allergic Rhinitis therapeutic Market Size in the 7MM was nearly USD 10,400 million in 2023.
- The United States accounted for the highest Allergic Rhinitis Market Size approximately 34% of the total market size in 7MM in 2023, in comparison to the other major markets i.e., EU4 countries (Germany, France, Italy, and Spain), and the United Kingdom, and Japan.
- Among the EU countries, Germany had the highest market size with USD 1,358 million in 2023, while Spain had the lowest Allergic Rhinitis Market Size with USD 604 million in 2023.
- The Allergic rhinitis market size in Japan was estimated to be approximately USD 2,347 million in 2023, which accounted for 23% of the total 7MM market.
- With the expected launch of upcoming Allergic rhinitis therapies, such as REGN5713-5714-5715 the total Allergic Rhinitis Market Size is expected to showcase growth in the upcoming years.
Allergic rhinitis Drugs Uptake
This section focuses on the uptake rate of potential Allergic rhinitis drugs expected to launch in the market during 2020-2034. For example, REGN5713-5714-5715 in the US is expected to be launched by 2024 with a peak shared of 0.1%. REGN5713-5714-5715 is anticipated to take 7 years to peak with a slow-medium uptake.
Allergic rhinitis Pipeline Development Activities
The Allergic rhinitis market report provides insights into Allergic rhinitis clinical trials within Phase III, Phase II, and Phase I stage. It also analyzes key players involved in developing targeted therapeutics.
Pipeline Development Activities
The Allergic rhinitis rmarket eport covers information on collaborations, acquisitions and mergers, licensing, and patent details for Allergic rhinitis emerging therapies.
KOL Views
To keep up with current Allergic rhinitis market trends, we take KOLs and SMEs' opinions working in the domain through primary research to fill the data gaps and validate the secondary research. Industry Experts were contacted for insights on Allergic rhinitis evolving treatment landscape, patient reliance on conventional therapies, patient therapy switching acceptability, and drug uptake along with challenges related to accessibility, including KOL from Division of Allergy, Immunology, and Rheumatology, Georgetown University Medical School,US; University Paris-Saclay, Suresnes, France; Royal College of Physicians of London, UK; Graduate School of Medicine, Chiba University, Japan; Department of Respiratory Medicine and Allergy, Tosei General Hospital, Japan; and others.
Delveinsight's analysts connected with 50+ KOLs to gather insights; however, interviews were conducted with 15+ KOLs in the 7MM. Their opinion helps understand and validate current and emerging therapies, treatment patterns, or Allergic rhinitis market trends. This will support the clients in potential upcoming novel treatments by identifying the overall scenario of the Allergic rhinitis market and the unmet needs.
Qualitative Analysis
We perform Qualitative and market Intelligence analysis using various approaches, such as SWOT and Conjoint Analysis. In the SWOT analysis, strengths, weaknesses, opportunities, and threats in terms of disease diagnosis, patient awareness, patient burden, competitive landscape, cost-effectiveness, and geographical accessibility of therapies are provided. These pointers are based on the Analyst's discretion and assessment of the patient burden, cost analysis, and existing and evolving treatment landscape.
Conjoint Analysis analyzes multiple approved and emerging Allergic rhinitis therapies based on relevant attributes such as safety, efficacy, frequency of administration, route of administration, and order of entry. Scoring is given based on these parameters to analyze the effectiveness of therapy.
Further, the therapies' safety is evaluated wherein the acceptability, tolerability, and adverse events are majorly observed, and it sets a clear understanding of the side effects posed by the drug in the trials. In addition, the scoring is also based on the route of administration, order of entry and designation, probability of success, and the addressable patient pool for each therapy. According to these parameters, the final weightage score and the ranking of the emerging therapies are decided.
Allergic rhinitis Market Access and Reimbursement
The high cost of therapies for the treatment is a major factor restraining the growth of the global drug market. Because of the high cost, the economic burden is increasing, leading the patient to escape from proper treatment.
The Allergic rhinitis market report further provides detailed insights on the country-wise accessibility and reimbursement scenarios, cost-effectiveness scenario of approved therapies, programs making accessibility easier and out-of-pocket costs more affordable, insights on patients insured under federal or state government prescription drug programs, etc.
Scope of the Allergic rhinitis Market Report
- The Allergic rhinitis market report covers a segment of key events, an executive summary, descriptive overview of Allergic rhinitis, explaining its causes, signs and symptoms, and currently available therapies.
- Comprehensive insight has been provided into the epidemiology segments and forecasts, the future growth potential of diagnosis rate, disease progression, and treatment guidelines.
- Additionally, an all-inclusive account of the current and emerging therapies and the elaborative profiles of late-stage and prominent therapies will impact the current treatment landscape.
- A detailed review of the Allergic rhinitis market, historical and forecasted market size, market share by therapies, detailed assumptions, and rationale behind the approach is included in the report covering the 7MM drug outreach.
- The Allergic rhinitis market report provides an edge while developing business strategies, by understanding trends, through SWOT analysis and expert insights/KOL views, patient journey, and treatment preferences that help shape and drive the 7MM Allergic rhinitis market.
Allergic Rhinitis Market Report Insights
- Allergic rhinitis Patient Population
- Allergic rhinitis Therapeutic Approaches
- Allergic rhinitis Pipeline Analysis
- Allergic rhinitis Market Size
- Allergic rhinitis Market Trends
- Existing and Future Allergic rhinitis Market Opportunity
Allergic rhinitis Market Report Key Strengths
- 11 years Forecast
- The 7MM Coverage
- Allergic rhinitis Epidemiology Segmentation
- Key Cross Competition
- Conjoint Analysis
- Allergic rhinitis Drugs Uptake
- Key Allergic rhinitis Market Forecast Assumptions
Allergic Rhinitis Market Report Assessment
- Current Treatment Practices
- Allergic rhinitis Unmet Needs
- Allergic rhinitis Pipeline Product Profiles
- Allergic rhinitis Market Attractiveness
- Qualitative Analysis (SWOT and Conjoint Analysis)
- Allergic rhinitis Market Drivers
- Allergic rhinitis Market Barriers
Key Questions Allergic rhinitis Answered In The Allergic rhinitis Market Report:
Allergic rhinitis Market Insights
- What was the Allergic rhinitis market share (%) distribution in 2020 and how it would look like in 2034?
- What would be the Allergic rhinitis market size as well as market size by therapies across the 7MM during the forecast period (2024-2034)?
- What are the key findings pertaining to the market across the 7MM and which country will have the largest Allergic rhinitis market size during the forecast period (2024-2034)?
- At what CAGR, the Allergic rhinitis market is expected to grow at the 7MM level during the forecast period (2024-2034)?
- What would be the Allergic rhinitis market outlook across the 7MM during the forecast period (2024-2034)?
- What would be the Allergic rhinitis market growth till 2034 and what will be the resultant market size in the year 2034?
- How would the market drivers, barriers, and future opportunities affect the Allergic rhinitis market dynamics and subsequent analysis of the associated trends?
Allergic rhinitis Epidemiology Insights
- What is the disease risk, burden, and unmet needs of Allergic rhinitis?
- What is the historical Allergic rhinitis patient population in the United States, EU5 (Germany, France, Italy, Spain, and the UK), and Japan?
- What would be the forecasted patient population of Allergic rhinitis at the 7MM level?
- What will be the growth opportunities across the 7MM with respect to the patient population pertaining to Allergic rhinitis?
- Out of the above-mentioned countries, which country would have the highest prevalent population of Allergic rhinitis during the forecast period (2024-2034)?
- At what CAGR the population is expected to grow across the 7MM during the forecast period (2024-2034)?
Current Allergic rhinitis Treatment Scenario, Marketed Drugs, and Emerging Therapies
- What are the current options for the treatment of Allergic rhinitis along with the approved therapy?
- What are the current treatment guidelines for the treatment of Allergic rhinitis in the US, Europe, And Japan?
- What are the Allergic rhinitis marketed drugs and their MOA, regulatory milestones, product development activities, advantages, disadvantages, safety, and efficacy, etc.?
- How many Allergic rhinitis companies are developing therapies for the treatment of Allergic rhinitis?
- How many emerging Allergic rhinitis therapies are in the mid-stage and late stages of development for the treatment of Allergic rhinitis?
- What are the key collaborations (Industry-Industry, Industry-Academia), Mergers and acquisitions, licensing activities related to the Allergic rhinitis therapies?
- What are the recent therapies, targets, mechanisms of action and technologies developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Allergic rhinitis and their status?
- What are the key designations that have been granted for the emerging therapies for Allergic rhinitis?
- What are the 7MM historical and forecasted Allergic rhinitis market?
Reasons to Allergic rhinitis Market Report Buy
- The Allergic rhinitis market report will help in developing business strategies by understanding the latest trends and changing treatment dynamics driving the Allergic rhinitis Market.
- Insights on patient burden/disease incidence, evolution in diagnosis, and factors contributing to the change in the epidemiology of the disease during the forecast years.
- To understand the existing Allergic rhinitis market opportunity in varying geographies and the growth potential over the coming years.
- Distribution of historical and current patient share based on real-world prescription data along with reported sales of approved products in the US, EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan.
- Identification of strong upcoming Allergic rhinitis companies in the market will help in devising strategies that will help in getting ahead of competitors.
- Detailed analysis and potential of current and emerging Allergic rhinitis therapies under the conjoint analysis section to provide visibility around leading emerging drugs.
- Highlights of Access and Reimbursement policies of approved therapies, barriers to accessibility of off-label expensive therapies, and patient assistance programs.
- To understand the perspective of Key Opinion Leaders around the accessibility, acceptability, and compliance-related challenges of existing treatment to overcome barriers in future.
- Detailed insights on the unmet need of the existing Allergic rhinitis market so that the upcoming Allergic rhinitis companies can strengthen their development and launch strategy.
Frequently Asked Questions:
1. What is the forecast period covered in the report?
The Allergic rhinitis Epidemiology and Market Insight report for the 7MM covers the forecast period from 2024 to 2034, providing a projection of market dynamics and trends during this timeframe.
2. Who are the key players in the Allergic rhinitis market?
The Allergic rhinitis market is quite robust. The major players are HAL Allergy Ltd., Glenmark Pharmaceuticals Inc., and others which are currently developing drugs for the treatment of Allergic rhinitis .
3. How is the market size estimated in the forecast report?
The market size is estimated through data analysis, statistical modeling, and expert opinions. It may consider factors such as incident cases, treatment costs, revenue generated, and market trends.
4. What is the key driver of the Allergic rhinitis market?
The increase in diagnosed prevalent cases of Allergic rhinitis and the launch of emerging therapies are attributed to be the key drivers for increasing Allergic rhinitis market.
5. What is the expected impact of emerging therapies or advancements in Allergic rhinitis treatment on the market?
Introducing new therapies, advancements in diagnostic techniques, and innovations in treatment approaches can significantly impact the Allergic rhinitis treatment market. Market forecast reports may provide analysis and predictions regarding the potential impact of these developments.
6. Does the report provide insights into the competitive landscape of the market?
The market forecast report may include information on the competitive landscape, profiling key market players, their product offerings, partnerships, and strategies, and helping stakeholders understand the competitive dynamics of the Allergic rhinitis market.
Table of Contents
1 Key Insights
2 Report Introduction
3 Allergic Rhinitis Market Overview at a Glance
- 3.1 Market Share (%) Distribution of Allergic Rhinitis in 2020
- 3.2 Market Share (%) Distribution of Allergic Rhinitis in 2034
4 Epidemiology and Market Forecast Methodology
5 Key Events
6 Executive Summary of of Allergic rhinitis
7 Disease Background and Overview of Allergic Rhinitis
- 7.1 Introduction
- 7.2 Classification of Rhinitis
- 7.3 Etiology
- 7.4 Pathogenesis
- 7.5 Signs and Symptoms
- 7.6 Complications of Allergic Rhinitis
- 7.7 Diagnosis
- 7.7.1 Differential Diagnosis
- 7.7.2 Diagnostic Guidelines
- 7.7.2.1 Allergic Rhinitis: Clinical Practice Guideline by American Academy of Otolaryngology-Head and Neck Surgery, (Endorsed 2014, Reaffirmed, April 2020)
- 7.7.2.2 Consensus Statement of the Italian Society of Pediatric Allergy and Immunology for the Pragmatic Management of Children And Adolescents With Allergic or Immunological Diseases During the COVID-19 Pandemic: 2020
- 7.7.2.3 Recommendation for Clinical Practice Diagnostic and Therapeutic Management of Allergic Rhinitis by ENT: French Society of Oto-Rhino-Laryngology and Face and Neck Surgery, 2020
- 7.7.2.4 Japanese Guidelines for Allergic Rhinitis: 2020
- 7.8 Tre tment
- 7.8.1 Treatment Guidelines
- 7.8.1.1 Allergic Rhinitis: Clinical Practice Guideline by American Academy of Otolaryngology Head and Neck Surgery, (Endorsed 2014, Reaffirmed, April 2020)
- 7.8.1.2 ARIA Guideline 2019: Treatment of Allergic Rhinitis in the German Health System
- 7.8.1.3 British Society of Allergy and Clinical Immunology (BSACI) Guideline for the Diagnosis and Management of Allergic and Non-allergic Rhinitis (Revised Edition 2017)
- 7.8.1.4 Consensus Statement of the Italian Society of Pediatric Allergy and Immunology for the Pragmatic Management of Children and Adolescents With Allergic or Immunological Diseases During the COVID-19 Pandemic: 2020
- 7.8.1.5 Recommendation for Clinical Practice-Diagnostic and Therapeutic Management of Allergic Rhinitis by ENT: French Society of Oto-Rhino-Laryngology and Face and Neck Surgery, 2020
- 7.8.1.6 Japanese Guidelines for Allergic Rhinitis: 2020
- 7.8.1 Treatment Guidelines
8 Epidemiology and Patient Population of Allergic Rhinitis
- 8.1 Key Findings
- 8.2 Assumptions and Rationale: The 7MM
- 8.2.1 The United States
- 8.2.2 EU4 and the United Kingdom
- 8.2.3 Japan
- 8.3 Total Prevalent Cases of Allergic Rhinitis in the 7MM
- 8.4 Diagnosed Prevalent Cases of Allergic Rhinitis in the 7MM
- 8.5 The United States
- 8.5.1 Prevalent cases of Allergic Rhinitis
- 8.5.2 Diagnosed prevalent cases of Allergic Rhinitis
- 8.5.3 Age-specific diagnosed prevalent cases of Allergic Rhinitis
- 8.5.4 Severity-specific diagnosed prevalent cases of Allergic Rhinitis
- 8.5.5 Allergen-specific diagnosed prevalent cases of Allergic Rhinitis
- 8.6 EU4 and the UK
- 8.6.1 Germany
- 8.6.1.1 Prevalent cases of Allergic Rhinitis
- 8.6.1.2 Diagnosed prevalent cases of Allergic Rhinitis
- 8.6.1.3 Age-specific diagnosed prevalent cases of Allergic Rhinitis
- 8.6.1.4 Severity-specific diagnosed prevalent cases of Allergic Rhinitis
- 8.6.1.5 Allergen-specific diagnosed prevalent cases of Allergic Rhinitis
- 8.6.2 France
- 8.6.2.1 Prevalent cases of Allergic Rhinitis
- 8.6.2.2 Diagnosed prevalent cases of Allergic Rhinitis
- 8.6.2.3 Age-specific diagnosed prevalent cases of Allergic Rhinitis
- 8.6.2.4 Severity-specific diagnosed prevalent cases of Allergic Rhinitis
- 8.6.2.5 Allergen-specific diagnosed prevalent cases of Allergic Rhinitis
- 8.6.3 Italy
- 8.6.3.1 Prevalent cases of Allergic Rhinitis
- 8.6.3.2 Diagnosed prevalent cases of Allergic Rhinitis
- 8.6.3.3 Age-specific diagnosed prevalent cases of Allergic Rhinitis
- 8.6.3.4 Severity-specific diagnosed prevalent cases of Allergic Rhinitis
- 8.6.3.5 Allergen-specific diagnosed prevalent cases of Allergic Rhinitis
- 8.6.4 Spain
- 8.6.4.1 Prevalent cases of Allergic Rhinitis
- 8.6.4.2 Diagnosed prevalent cases of Allergic Rhinitis
- 8.6.4.3 Age-specific diagnosed prevalent cases of Allergic Rhinitis
- 8.6.4.4 Severity-specific diagnosed prevalent cases of Allergic Rhinitis
- 8.6.4.5 Allergen-specific diagnosed prevalent cases of Allergic Rhinitis
- 8.6.5 The United Kingdom
- 8.6.5.1 Prevalent cases of Allergic Rhinitis
- 8.6.5.2 Diagnosed prevalent cases of Allergic Rhinitis
- 8.6.5.3 Age-specific diagnosed prevalent cases of Allergic Rhinitis
- 8.6.5.4 Severity-specific diagnosed prevalent cases of Allergic Rhinitis
- 8.6.5.5 Allergen-specific diagnosed prevalent cases of Allergic Rhinitis
- 8.6.1 Germany
- 8.7 Japan
- 8.7.1 Prevalent cases of Allergic Rhinitis in Japan
- 8.7.2 Diagnosed Prevalent Cases of Allergic Rhinitis in Japan
- 8.7.3 Age-specific diagnosed prevalent cases of Allergic Rhinitis in Japan
- 8.7.4 Severity-specific diagnosed prevalent cases of Allergic Rhinitis in Japan
- 8.7.5 Allergen-specific diagnosed prevalent cases of Allergic Rhinitis in Japan
9 Patient Journey
10 Marketed Drugs
- 10.1 Key Cross
- 10.2 RYALTRIS (Olopatadine Hydrochloride and Mometasone Furoate Monohydrate Nasal Spray): Glenmark Pharmaceuticals Inc.
- 10.2.1 Product Description
- 10.2.2 Regulatory Milestone
- 10.2.3 Clinical Development
- 10.2.4 Clinical Trial Information
- 10.2.5 Safety and Efficacy
- 10.2.6 Product Profile
11 Emerging Drugs
- 11.1 Key Cross Competition
- 11.2 REGN5713-5714-5715: Regeneron Pharmaceuticals
- 11.2.1 Product Description
- 11.2.2 Other Developmental Activities
- 11.2.3 Clinical Development
- 11.2.4 Clinical Trials Information
- 11.2.5 Product Profile
- 11.2.6 Analysts' Views
- 11.3 Grass MATA MPL: Allergy Therapeutics
- 11.3.1 Product Description
- 11.3.2 Clinical Developmental Activities
- 11.3.3 Clinical Trial Information
- 11.3.4 Product Profile
12 Allergic Rhinitis: Seven Major Market Analysis
- 12.1 Key Findings
- 12.2 Market Outlook
- 12.3 Key Market Forecast Assumptions
- 12.4 Conjoint Analysis
- 12.5 Total Market Size of Allergic Rhinitis in the 7MM
- 12.6 Market Size of Allergic Rhinitis by Therapies in the 7MM
- 12.7 Market Size of Allergic Rhinitis in the United States
- 12.7.1 Total Market Size of Allergic Rhinitis
- 12.7.3 Market size of Allergic Rhinitis by therapies
- 12.8 Market Size of Allergic Rhinitis in EU4 and the UK
- 12.8.1 Germany
- 12.8.1.1 Total Market Size of Allergic Rhinitis
- 12.8.1.2 Market size of Allergic Rhinitis by therapies
- 12.8.2 France
- 12.8.2.1 Total Market Size of Allergic Rhinitis
- 12.8.2.2 Market size of Allergic Rhinitis by therapies
- 12.8.3 Italy
- 12.8.3.1 Total Market Size of Allergic Rhinitis
- 12.8.3.2 Market size of Allergic Rhinitis by therapies
- 12.8.4 Spain
- 12.8.4.1 Total Market Size of Allergic Rhinitis
- 12.8.4.2 Market size of Allergic Rhinitis by therapies
- 12.8.5 The United Kingdom
- 12.8.5.1 Total Market Size of Allergic Rhinitis
- 12.8.5.2 Market size of Allergic Rhinitis by therapies
- 12.8.1 Germany
- 12.9 Market Size of Allergic Rhinitis in Japan
- 12.9.1 Total Market Size of Allergic Rhinitis
- 12.9.2 Market size of Allergic Rhinitis by therapies
13 Key Opinion Leaders' Views
14 SWOT Analysis
15 Unmet needs
16 Market Access and Reimbursement
- 16.1 The United States
- 16.1.1 Centers for Medicare & Medicaid Services (CMS)
- 16.2 In EU4 and the UK
- 16.2.1 Germany
- 16.2.2 France
- 16.2.3 Italy
- 16.2.4 Spain
- 16.2.5 United Kingdom
- 16.3 Japan
- 16.3.1 MHLW
17 Appendix
- 17.1 Bibliography
- 17.2 Acronyms and Abbreviations
- 17.3 Report Methodology