|
|
市場調査レポート
商品コード
1781122
アジア太平洋の細胞シートによる遺伝子治療市場:技術タイプ・細胞シートタイプ・由来・用途・エンドユーザー・国別の分析 (2025-2035年)Asia-Pacific Cell Sheet-based Gene Therapy Market: Focus on Technology Type, Cell-sheet Type, Source Type, Application Type, End User, and Country - Analysis and Forecast, 2025-2035 |
||||||
カスタマイズ可能
|
|||||||
| アジア太平洋の細胞シートによる遺伝子治療市場:技術タイプ・細胞シートタイプ・由来・用途・エンドユーザー・国別の分析 (2025-2035年) |
|
出版日: 2025年07月31日
発行: BIS Research
ページ情報: 英文 51 Pages
納期: 1~5営業日
|
全表示
- 概要
- 図表
- 目次
アジア太平洋の細胞シートによる遺伝子治療の市場規模は、2024年の1億7,080万米ドルから、予測期間中はCAGR 16.06%で推移し、2035年には8億7,900万米ドルに成長すると予測されています。
世界の再生医療企業が積極的にこの地域での展開を進めており、地域の先駆的企業とともに、市場を牽引する主要企業となっています。CellSeed Inc. (日本) やJ-TEC (Japan Tissue Engineering Co., Ltd.) などの有力企業は、臨床開発パイプラインが充実しており、眼科、食道、心臓の再生を対象とした市場投入間近の治療法を有しています。これらの企業は、日本における再生医療への強力な政府支援と先進的な規制制度の恩恵を受けており、それが市場展開と技術革新を後押ししています。
| 主要市場統計 | |
|---|---|
| 予測期間 | 2025-2035年 |
| 2025年の評価 | 1億9,530万米ドル |
| 2035年予測 | 8億7,900万米ドル |
| CAGR | 16.06% |
アジアにおける細胞シートによる遺伝子治療市場は、組織工学の進歩と再生医療の需要拡大を背景に、着実に成長しています。この革新的な治療アプローチは、遺伝子改変技術と細胞シート技術を組み合わせることで、足場材を必要とせず、細胞外マトリックスと細胞間接着を保持したまま、標的組織の修復を可能にします。中国、韓国、日本といった国々は、先進的バイオ医薬品への投資増加や有利な規制環境を背景に、研究と商業化の最前線に立っています。
特に日本は、再生医療における先駆的な規制枠組みにより、皮膚再生、角膜修復、食道再建、心筋再生といった臨床応用を進展させてきました。また、韓国は精密製造と国際的なパートナーシップに注力しつつ、バイオテクノロジーのインフラ拡充を進めています。さらに中国では、国内イノベーションの促進と臨床パイプラインの開発を目的に、国際基準への適合が急速に進められています。
高齢化社会、慢性疾患の増加、バイオテクノロジー研究開発への政府投資の増加なども、市場拡大を後押ししています。一方で、複雑な製造工程、高コスト、地域間で異なる規制制度といった課題が拡張の妨げとなる可能性もあります。しかしながら、継続的な技術革新、産学連携、地域間での規制調和のイニシアティブにより、アジア太平洋地域は将来の遺伝子・細胞治療の主要拠点としての地位を確立しつつあります。
市場の分類:
セグメンテーション1:技術タイプ別
- 細胞シートによる工学技術
- 光誘導型細胞シート技術
- 温度応答性培養表面
- 足場不要技術 (スキャフォールドフリー)
- レイヤーバイレイヤー組立法
- その他の技術
- 遺伝子導入法
- ウイルスベクターによる方法 (例:レンチウイルス、アデノウイルス)
- 非ウイルスベクターによる方法 (例:リポソーム、ナノ粒子)
- CRISPR/Cas9
- その他の遺伝子導入法
セグメンテーション2:細胞シートタイプ別
- 単層細胞シート
- 共培養細胞シート
- 多層細胞シート
- その他
セグメンテーション3:由来別
- 自家由来
- 同種由来
- 幹細胞由来
セグメンテーション4:用途別
- 腫瘍
- 眼科
- 遺伝子疾患
- 心臓病学
- その他
セグメンテーション5:エンドユーザー別
- 病院および診療所
- 研究・学術機関
- バイオテクノロジーおよび製薬会社
- その他
セグメンテーション6:地域別
- アジア太平洋
- 日本
- インド
- 中国
- オーストラリア
- 韓国
- その他
当レポートでは、アジア太平洋の細胞シートによる遺伝子治療の市場を調査し、主要動向、市場影響因子の分析、法規制環境、技術・特許の分析、市場規模の推移・予測、各種区分・地域/主要国別の詳細分析、競合情勢、主要企業のプロファイルなどをまとめています。
目次
エグゼクティブサマリー
範囲と定義
第1章 細胞シートによる遺伝子治療市場:業界展望
- 細胞シートによる遺伝子治療市場の動向
- 再生医療と遺伝子工学の新たな融合
- 競合情勢
- 事業戦略
- 企業戦略
- 承認薬:細胞シートによる遺伝子治療
- パイプライン薬:細胞シートによる遺伝子治療
- 規制状況
- アジア太平洋
- 市場力学
- 動向、促進要因、課題、機会:現在および将来の影響評価
- 市場促進要因
- 市場抑制要因
- 市場機会
- 市場の課題
- 規制当局の承認と倫理的問題
第2章 細胞シートによる遺伝子治療市場:地域別
- 地域サマリー
- アジア太平洋
- 地域概要
- 市場成長の原動力
- 市場課題
- 日本
- 中国
- インド
- オーストラリア
- 韓国
- その他
第3章 調査手法
List of Figures
- Figure 1: Asia-Pacific Cell Sheet-based Gene Therapy Market (by Scenario), $Million, 2024, 2028, and 2035
- Figure 2: Asia-Pacific Cell Sheet-based Gene Therapy Market, 2024-2035
- Figure 3: Asia-Pacific Cell Sheet-based Gene Therapy Market Snapshot
- Figure 4: Asia-Pacific Cell Sheet-based Gene Therapy Market (by Technology Type), $Million, 2024, 2028, and 2035
- Figure 5: Asia-Pacific Cell Sheet-based Gene Therapy Market (by Cell-Sheet Type), $Million, 2024, 2028, and 2035
- Figure 6: Asia-Pacific Cell Sheet-based Gene Therapy Market (by Source Type), $Million, 2024, 2028, and 2035
- Figure 7: Asia-Pacific Cell Sheet-based Gene Therapy Market (by Application Type), $Million, 2024, 2028, and 2035
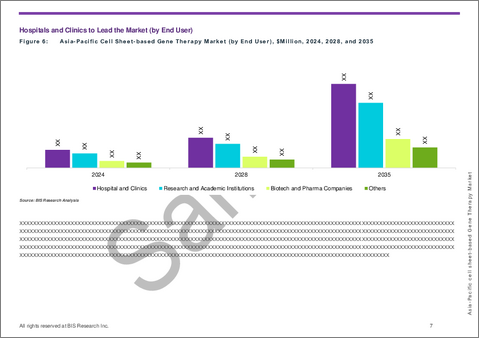
- Figure 8: Asia-Pacific Cell Sheet-based Gene Therapy Market (by End User), $Million, 2024, 2028, and 2035
- Figure 9: Japan Cell Sheet-based Gene Therapy Market, $Million, 2023-2035
- Figure 10: China Cell Sheet-based Gene Therapy Market, $Million, 2023-2035
- Figure 11: India Cell Sheet-based Gene Therapy Market, $Million, 2023-2035
- Figure 12: Australia Cell Sheet-based Gene Therapy Market, $Million, 2023-2035
- Figure 13: South Korea Cell Sheet-based Gene Therapy Market, $Million, 2023-2035
- Figure 14: Rest of Asia-Pacific Cell Sheet-based Gene Therapy Market, $Million, 2023-2035
- Figure 15: Inclusion and Exclusion Criteria for Cell Sheet-based Gene Therapy Market
- Figure 16: Data Triangulation
- Figure 17: Top-Down and Bottom-Up Approach
- Figure 18: Assumptions and Limitations
List of Tables
- Table 1: Market Snapshot
- Table 2: Approved Drugs in Cell-Sheet based Gene Therapy Market
- Table 3: Pipeline Drugs in Cell-Sheet based Gene Therapy Market
- Table 4: Cell Sheet-based Gene Therapy Market (by Region), $Million, 2023-2035
- Table 5: Asia-Pacific Cell Sheet-based Gene Therapy Market (by Technology Type), $Million, 2023-2035
- Table 6: Asia-Pacific Cell Sheet-based Gene Therapy Market (by Cell sheet-based Engineering Techniques Type), $Million, 2023-2035
- Table 7: Asia-Pacific Cell Sheet-based Gene Therapy Market (by Gene Delivery Methods), $Million, 2023-2035
- Table 8: Asia-Pacific Cell Sheet-based Gene Therapy Market (by Cell-sheet Type), $Million, 2023-2035
- Table 9: Asia-Pacific Cell Sheet-based Gene Therapy Market (by Source Type), $Million, 2023-2035
- Table 10: Asia-Pacific Cell Sheet-based Gene Therapy Market (by Application Type), $Million, 2023-2035
- Table 11: Asia-Pacific Cell Sheet-based Gene Therapy Market (by End User), $Million, 2023-2035
This report can be delivered in 2 working days.
Introduction to Asia-Pacific (APAC) Cell Sheet-based Gene Therapy Market
The Asia-Pacific cell sheet-based gene therapy market is projected to reach $879.0 million by 2035 from $170.8 million in 2024, growing at a CAGR of 16.06% during the forecast period 2025-2035. Global regenerative medicine businesses aggressively extending their footprint and regional pioneers are major players in the APAC cell sheet-based gene therapy market. Leading companies in the field, including CellSeed Inc. (Japan) and J-TEC (Japan Tissue Engineering Co., Ltd.), have robust clinical pipelines and treatments that are ready for the market that target ocular, esophageal, and cardiac regeneration. These businesses profit from Japan's robust government backing for regenerative medicine and its innovative regulatory framework.
| KEY MARKET STATISTICS | |
|---|---|
| Forecast Period | 2025 - 2035 |
| 2025 Evaluation | $195.3 Million |
| 2035 Forecast | $879.0 Million |
| CAGR | 16.06% |
Temperature-responsive culture dishes are used by CellSeed Inc. to produce autologous cell sheet products, and J-TEC has created and marketed a number of autologous cell-based goods, such as skin and cartilage repair products. Scalable clinical deployment throughout Asia is supported by their integrated R&D and GMP-compliant production facilities.
In the meantime, further funding and collaborations are being made in nations like China and South Korea with the goal of commercializing gene-modified cell sheets for uncommon ocular and dermatological disorders. Through strategic partnerships between academia, government, and the commercial sector, biotech firms and institutions around the area are placing an emphasis on translational research and industrial scalability.
These advancements establish APAC as a competitive center for next-generation cell sheet-based gene treatments and demonstrate the region's expanding significance in fostering innovation for complicated epithelium and tissue regeneration therapies.
Market Introduction
The market for cell sheet-based gene therapy in Asia is expanding steadily because to advances in tissue engineering and the growing need for regenerative medicine. This novel therapeutic strategy maintains the integrity of the extracellular matrix and cell-to-cell connections while enabling targeted tissue repair without the need for scaffolds through the combination of genetic alteration and cell sheet technology. With the help of advantageous regulatory environments and rising investments in cutting-edge biologics, nations like China, South Korea, and Japan are leading the way in research and commercialization.
Clinical applications in skin healing, corneal restoration, esophagus reconstruction, and cardiac regeneration have advanced due to Japan's innovative position in regulating regenerative medicine. With a focus on precision manufacturing and global partnerships, South Korea is also growing its biotech infrastructure. Furthermore, in an effort to foster domestic innovation and develop clinical pipelines, China is quickly bringing itself into compliance with international norms.
Market expansion is also supported by aging populations, the growing burden of chronic diseases, and greater government investment for biotechnology R&D. Scalability may be hampered by issues including intricate manufacturing procedures, exorbitant expenses, and regionally disparate regulations. Notwithstanding these obstacles, ongoing technological advancements, collaborations between academia and business, and regional harmonization initiatives are establishing Asia-Pacific as a major center for gene and cell therapies of the future.
Market Segmentation
Segmentation 1: By Technology Type
- Cell sheet-based Engineering Techniques
- Light-induced cell sheet technology
- Temperature-Responsive Culture Surfaces
- Scaffold-Free Techniques
- Layer-by-Layer Assembly
- Other Techniques
- Gene Delivery Methods
- Viral Vector-Based (e.g., Lentivirus, Adenovirus)
- Non-Viral Vector-Based (e.g., Liposomes, Nanoparticles)
- CRISPR/Cas9
- Other Gene Delivery Methods
Segmentation 2: By Cell-Sheet Type
- Monolayer Cell-sheet Type
- Co-culture Cell-sheet Type
- Multilayered Cell-sheet Type
- Others
Segmentation 3: By Source Type
- Autologous
- Allogenic
- Stem-cell Derived
Segmentation 4: By Application
- Oncology
- Ophthalmology
- Genetic Disorders
- Cardiology
- Others
Segmentation 5: By End-User
- Hospitals and Clinics
- Research and Academic Institutions
- Biotech and Pharma Companies
- Others
Segmentation 6: By Region
- Asia-Pacific
- Japan
- India
- China
- Australia
- South Korea
- Rest-of-Asia-Pacific
APAC Cell Sheet-Based Gene Therapy Market Trends, Drivers and Challenges
Market Trends
- Rapid growth of the cell and gene therapy sector in the
APAC region, supported by increasing clinical trials and commercial interest.
- Rising adoption of autologous therapies, especially for oncology, genetic, and regenerative applications.
- Strong innovation pipeline in countries like Japan, South Korea, and China for late-stage cell sheet therapies targeting cartilage, ocular, and skin tissues.
Market Drivers
- Growing burden of chronic, genetic, and age-related diseases is increasing demand for regenerative therapies.
- Supportive regulatory initiatives and fast-track approval mechanisms in key APAC countries are encouraging development and commercialization.
- Expanding public-private partnerships and infrastructure investments are boosting manufacturing capacity and clinical translation.
Market Challenges
- High production and operational costs for cell sheet-based therapies limit affordability and accessibility.
- Fragmented regulatory requirements across the region create hurdles for cross-border clinical and commercial integration.
- Shortage of skilled talent in advanced cell therapy manufacturing and quality control remains a key bottleneck.
Market Opportunities
- Integration of automation and AI-driven quality control to streamline production and reduce time to market.
- Expansion of contract manufacturing organizations (CMOs) and academic-industry collaborations to scale innovation.
- Increasing focus on personalized medicine and precision therapies is creating demand for novel cell-based platforms.
How can this report add value to an organization
Product/Innovation Strategy: The report offers in-depth insights into the latest technological advancements in APAC cell sheet-based gene therapy, enabling organizations to drive innovation and develop cutting-edge products tailored to market needs.
Growth/Marketing Strategy: By providing comprehensive market analysis and identifying key growth opportunities, the report equips organizations with the knowledge to craft targeted marketing strategies and expand their market presence effectively.
Competitive Strategy: The report includes a thorough competitive landscape analysis, helping organizations understand their competitors' strengths and weaknesses and allowing them to strategize effectively to gain a competitive edge in the market.
Regulatory and Compliance Strategy: It provides updates on evolving regulatory frameworks, approvals, and industry guidelines, ensuring organizations stay compliant and accelerate market entry for new APAC cell sheet-based gene therapy.
Investment and Business Expansion Strategy: By analyzing market trends, funding patterns, and partnership opportunities, the report assists organizations in making informed investment decisions and identifying potential M&A opportunities for business growth.
Table of Contents
Executive Summary
Scope and Definition
1 Cell sheet-based Gene Therapy Market: Industry Outlook
- 1.1 Trends in Cell Sheet-based Gene Therapy Market
- 1.1.1 Emerging Convergence of Regenerative Medicine and Genetic Engineering
- 1.2 Competitive Landscape
- 1.2.1 Business Strategies
- 1.2.1.1 Product Developments
- 1.2.2 Corporate Strategies
- 1.2.2.1 Partnerships and Joint Ventures
- 1.2.1 Business Strategies
- 1.3 Approved Drugs, Cell sheet-based Gene Therapy
- 1.4 Pipeline Drugs, Cell sheet-based Gene Therapy
- 1.5 Regulatory Landscape
- 1.5.1 Asia-Pacific
- 1.5.1.1 Japan
- 1.5.1.2 China
- 1.5.1.3 South Korea
- 1.5.1 Asia-Pacific
- 1.6 Market Dynamics
- 1.6.1 Trends, Drivers, Challenges, and Opportunities: Current and Future Impact Assessment
- 1.6.2 Market Drivers
- 1.6.2.1 Advancement in Regenerative Medicine
- 1.6.2.2 Growing Investment in Personalized Medicine
- 1.6.2.3 Technological Advancements in Cell Sheet Therapy
- 1.6.3 Market Restraints
- 1.6.3.1 Rising Cost of Development and Manufacturing
- 1.6.4 Market Opportunities
- 1.6.4.1 Substantial Surge in the Rise of Cell Sheet Approaches
- 1.6.4.2 Rising Application for Localized and Minimally Invasive Treatments
- 1.7 Market Challenges
- 1.7.1 Regulatory Approval and Ethical Issues
2 Cell Sheet-based Gene Therapy Market (by Region), Value ($million), 2023-2035
- 2.1 Regional Summary
- 2.2 Asia-Pacific
- 2.2.1 Regional Overview
- 2.2.2 Driving Factors for Market Growth
- 2.2.3 Factors Challenging the Market
- 2.2.4 Japan
- 2.2.5 China
- 2.2.6 India
- 2.2.7 Australia
- 2.2.8 South Korea
- 2.2.9 Rest-of-Asia-Pacific
3 Research Methodology
- 3.1 Data Sources
- 3.1.1 Primary Data Sources
- 3.1.2 Secondary Data Sources
- 3.1.3 Inclusion and Exclusion
- 3.1.4 Data Triangulation
- 3.2 Market Estimation and Forecast