|
|
市場調査レポート
商品コード
1764708
非転移性前立腺がんの世界市場:地域・国別の分析・予測 (2025-2035年)Non-metastatic Prostate Cancer Market - A Global and Regional Analysis: Focus on Country and Region - Analysis and Forecast, 2025-2035 |
||||||
カスタマイズ可能
|
|||||||
| 非転移性前立腺がんの世界市場:地域・国別の分析・予測 (2025-2035年) |
|
出版日: 2025年07月09日
発行: BIS Research
ページ情報: 英文 100 Pages
納期: 1~5営業日
|
全表示
- 概要
- 図表
- 目次
非転移性前立腺がん市場の主な成長要因のひとつは、特に非転移性去勢抵抗性前立腺がん (nmCRPC) に対する分子標的治療の需要が高まっていることです。
前立腺がんが進行し、特にアンドロゲン除去療法 (ADT) などの標準的なホルモン療法に抵抗性を示す高リスク患者においては、より効果的で個別化された治療法が求められるようになっています。エンザルタミド (Xtandi) やアパルタミド (Erleada) といったアンドロゲン受容体阻害薬が、転移の遅延や生存率の改善に成功したことにより、このような治療薬に対する需要が急増しています。
さらに、PSA検査や多パラメトリックMRI (多項目MRI) といった早期診断技術の進歩と、前立腺がんに関する認知度の向上により、非転移性前立腺がんの早期発見が進んでいます。こうした早期診断によって革新的な治療を迅速に開始できるようになり、治療ニーズの増加に拍車をかけています。また、遺伝子検査やバイオマーカーに基づいた精密医療の普及により、患者一人ひとりに合わせた個別化治療が可能となり、市場成長をさらに加速させています。
全体として、より高度で毒性が少なく、効果的な治療法への需要、早期発見と患者の意識向上が、非転移性前立腺がん市場の拡大を力強く後押ししています。
一方で、いくつかの課題が依然として市場の進展を妨げています。主な課題の1つは、先進的な治療法のコストが高いことです。エンザルタミドやアパルタミドといったアンドロゲン受容体阻害薬や、免疫療法や分子標的治療などの新しい治療法は非常に高額であり、包括的な保険に加入していない患者にとってはアクセスが難しい状況です。これらの高額な治療は、医療制度全体にも大きな財政的負担をかけるため、特に医療資源が限られた発展途上国や地方では普及が進みにくいという問題もあります。
コストの壁に加え、もう一つの大きな課題は治療抵抗性です。エンザルタミドやアパルタミドなどの治療薬は、非転移性去勢抵抗性前立腺がん (nmCRPC) において高い有効性を示しているものの、長期使用により耐性が発現する患者も少なくありません。耐性が生じると、がんの進行リスクが高まり、医師は代替治療やより侵襲的な介入を検討せざるを得なくなります。しかし、これらの代替療法は常に有効とは限らず、選択肢が限られているケースもあります。去勢療法を継続しているにもかかわらず、去勢抵抗性が発現することは、前立腺がんの長期管理における最も深刻な課題のひとつです。
さらに、診断技術にも限界があります。多項目MRIや遺伝子検査などの技術進歩にもかかわらず、前立腺がんの早期段階は検出が難しい場合があります。また、広く使われているPSA検査は特異性に限界があり、偽陽性や偽陰性が発生する可能性があります。これにより、誤診や診断の遅れにつながり、適切な治療計画の立案が妨げられることがあります。
最後に、現在の治療法、特にアンドロゲン除去療法や化学療法に伴う副作用は、依然として患者のQOLに大きな影響を与えています。疲労感、性機能障害、骨粗鬆症、心血管系のリスクといった問題は、多くの患者にとって長期的な治療の継続を困難にする要因となっており、治療のアドヒアランスが低下し、非転移性前立腺がんの管理がさらに複雑化する可能性があります。
当レポートでは、世界の非転移性前立腺がんの市場を調査し、主要動向、市場影響因子の分析、法規制環境、技術・特許の分析、臨床試験の動向、市場規模の推移・予測、各種区分・地域/主要国別の詳細分析、競合情勢、主要企業のプロファイルなどをまとめています。
目次
エグゼクティブサマリー
第1章 世界の非転移性前立腺がん市場:業界の展望
- 市場動向
- 規制の枠組み
- 疫学分析
- 臨床試験分析
- 市場力学
- 影響分析
- 市場促進要因
- 市場の課題
- 市場機会
第2章 世界の非転移性前立腺がん市場:地域別
- 北米
- 主な調査結果
- 市場力学
- 市場規模・予測
- 欧州
- 主な調査結果
- 市場力学
- 市場規模・予測
- アジア太平洋
- 主な調査結果
- 市場力学
- 市場規模・予測
第3章 世界の非転移性前立腺がん市場:競合情勢と企業プロファイル
- 主要戦略と開発
- M&A
- 相乗効果のある活動
- 事業拡大と資金調達
- 製品の発売と承認
- その他の活動
- 企業プロファイル
- Johnson & Johnson (Janssen Pharmaceuticals)
- Astellas Pharma
- Bristol-Myers Squibb
- Pfizer Inc.
- Merck & Co.
- Bayer AG
- Sanofi
- Clovis Oncology
- AstraZeneca
- AbbVie Inc.
第4章 調査手法
List of Figures
- Figure: Global Non-metastatic Prostate Cancer Market (by Region), $Billion, 2024 and 2035
- Figure: Global Non-metastatic Prostate Cancer Market Key Trends, Analysis
List of Tables
- Table: Global Non-metastatic Prostate Cancer Market Dynamics, Impact Analysis
- Table: Global Non-metastatic Prostate Cancer Market (by Region), $Billion, 2024-2035
Global Non-metastatic Prostate Cancer Market, Analysis and Forecast: 2025-2035
Non-metastatic prostate cancer refers to a stage of prostate cancer where the disease remains confined to the prostate gland and has not spread to other parts of the body. This form of cancer is often diagnosed at an early stage through methods such as prostate-specific antigen (PSA) testing, digital rectal exams (DRE), and biopsy. Non-metastatic prostate cancer is typically classified as Stage I or Stage II in the TNM (Tumor, Node, Metastasis) staging system, where the tumor is localized within the prostate and there is no evidence of spread to nearby lymph nodes or distant organs. While the cancer may cause a slight or moderate rise in PSA levels, it is often still manageable with localized treatments. Common treatment options include active surveillance for less aggressive cases, radical prostatectomy (surgical removal of the prostate), or radiation therapy. In high-risk cases, androgen deprivation therapy (ADT) may be used to lower testosterone levels and prevent cancer progression. With early detection and appropriate treatment, non-metastatic prostate cancer generally has a favorable prognosis, but management varies depending on factors such as cancer aggressiveness, patient age, and overall health.
One of the key drivers of the Non-metastatic Prostate Cancer market is the increasing demand for targeted therapies, particularly for non-metastatic castration-resistant prostate cancer (nmCRPC). As prostate cancer evolves, particularly in high-risk patients who develop resistance to standard hormonal therapies like androgen deprivation therapy (ADT), there is a growing need for more effective and personalized treatment options. The success of androgen receptor inhibitors, such as Enzalutamide (Xtandi) and Apalutamide (Erleada), in delaying metastasis and improving survival rates has created a significant demand for such therapies.
Additionally, the rising awareness of prostate cancer, coupled with advancements in early diagnostic methods like PSA testing and multiparametric MRI, has led to earlier detection of non-metastatic prostate cancer. This early detection allows for timely intervention with innovative therapies, which are contributing to the growing demand for treatment options. Moreover, the increasing adoption of precision medicine, based on genetic testing and biomarkers, is enabling more personalized treatments that are tailored to the specific needs of each patient, further accelerating Non-metastatic prostate cancer market growth.
Overall, the demand for more advanced, less toxic, and more effective treatments for non-metastatic prostate cancer, along with early detection and increased awareness, is driving the expansion of the non-metastatic prostate cancer market.
Despite the growth of the Non-metastatic Prostate Cancer market, several challenges continue to hinder its progress. One of the primary challenges is the high cost of advanced therapies. Treatments such as androgen receptor inhibitors (e.g., Enzalutamide and Apalutamide) and newer therapies like immunotherapies or targeted treatments can be extremely expensive, making them less accessible for many patients, especially those without comprehensive insurance coverage. This high cost can also place a significant burden on healthcare systems, limiting widespread adoption, particularly in developing countries or regions with limited access to advanced medical care.
In addition to the cost barrier, another major challenge is treatment resistance. While therapies like Enzalutamide and Apalutamide have shown effectiveness in non-metastatic castration-resistant prostate cancer (nmCRPC), patients may eventually develop resistance to these treatments over time. This can lead to disease progression, forcing physicians to seek alternative therapies or more aggressive interventions, which may not always be available or as effective. The development of castration-resistant prostate cancer despite ongoing androgen deprivation therapy remains one of the most significant hurdles in the long-term management of prostate cancer.
Furthermore, there are limitations in diagnostic tools. Despite advances in imaging technologies like multiparametric MRI and genetic testing, early-stage prostate cancer is still difficult to detect in some cases. PSA testing, while widely used, has limitations in specificity, leading to false positives and false negatives, which can cause delays in accurate diagnosis and treatment planning.
Finally, side effects of current treatments, especially androgen deprivation therapy and chemotherapy, continue to affect patient quality of life. Issues like fatigue, sexual dysfunction, osteoporosis, and cardiovascular risks can make long-term adherence to treatment challenging for many patients, further complicating the management of non-metastatic prostate cancer.
Together, these factors present significant challenges in the Non-metastatic Prostate Cancer market, requiring continued innovation in both treatment options and diagnostic tools to overcome these barriers and improve patient outcomes.
The global Non-metastatic Prostate Cancer market is highly competitive, with several leading companies driving innovation and market growth, such as Johnson & Johnson (Janssen Pharmaceuticals), Astellas Pharma, Bristol-Myers Squibb, Pfizer Inc., Merck & Co., Bayer, Sanofi, Clovis Oncology, AstraZeneca, AbbVie, and Boehringer Ingelheim.
These companies are at the forefront of developing and commercializing treatments for non-metastatic prostate cancer, particularly non-metastatic castration-resistant prostate cancer (nmCRPC). Through next-generation androgen receptor inhibitors like Erleada (apalutamide) and Xtandi (enzalutamide), as well as androgen deprivation therapies and PARP inhibitors, they are offering innovative solutions to slow disease progression, delay metastasis, and improve survival rates.
With increased research investments and advancements in precision medicine, these companies are exploring new biomarkers, novel treatment combinations, and immunotherapies to provide more effective therapies tailored to individual patient needs. As global awareness and screening rates for prostate cancer rise, these companies continue to shape the landscape of non-metastatic prostate cancer treatment and contribute to the non-metastatic prostate cancer market's rapid growth.
Non-Metastatic Prostate Cancer Market Segmentation:
Segmentation 1: by Region
- North America
- Europe
- Asia-Pacific
The global Non-metastatic Prostate Cancer market is undergoing significant transformation, fueled by emerging trends that are reshaping the landscape of prostate cancer treatment. One of the key trends is the growing focus on precision medicine, where advancements in genomic testing and biomarker profiling are allowing healthcare providers to tailor treatments based on the specific genetic makeup of a patient's cancer.
This approach is leading to the development of targeted therapies, such as Enzalutamide (Xtandi) and Apalutamide (Erleada), which are more effective, and less toxic compared to traditional therapies. In addition, the use of combination therapies is becoming more prevalent, as treatments like androgen receptor inhibitors are being combined with chemotherapy, immunotherapy, and radiation therapy to enhance effectiveness and address resistance.
Furthermore, the continued advancements in diagnostic technologies, such as multiparametric MRI and liquid biopsy, are enabling earlier and more accurate detection of non-metastatic prostate cancer, allowing for timely intervention. As global awareness of prostate cancer increases and screening programs become more widespread, more men are being diagnosed at earlier stages, driving demand for innovative therapies. Together, these trends are not only transforming how non-metastatic prostate cancer is treated but also expanding the non-metastatic prostate cancer market and improving patient outcomes.
Table of Contents
Executive Summary
Scope and Definition
Market/Product Definition
Inclusion and Exclusion
Key Questions Answered
Analysis and Forecast Note
1. Global Non-metastatic Prostate Cancer Market: Industry Outlook
- 1.1 Introduction
- 1.2 Market Trends
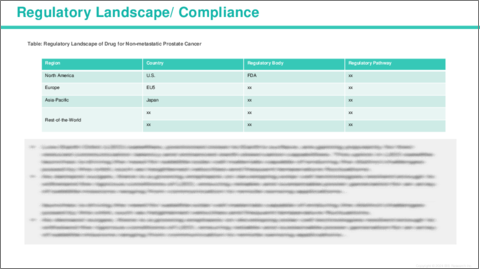
- 1.3 Regulatory Framework
- 1.4 Epidemiology Analysis
- 1.5 Clinical Trial Analysis
- 1.6 Market Dynamics
- 1.6.1 Impact Analysis
- 1.6.2 Market Drivers
- 1.6.3 Market Challenges
- 1.6.4 Market Opportunities
2. Global Non-metastatic Prostate Cancer Market (Region), ($Billion), 2023-2035
- 2.1 North America
- 2.1.1 Key Findings
- 2.1.2 Market Dynamics
- 2.1.3 Market Sizing and Forecast
- 2.1.3.1 North America Non-metastatic Prostate Cancer Market, by Country
- 2.1.3.1.1 U.S.
- 2.1.3.1 North America Non-metastatic Prostate Cancer Market, by Country
- 2.2 Europe
- 2.2.1 Key Findings
- 2.2.2 Market Dynamics
- 2.2.3 Market Sizing and Forecast
- 2.2.3.1 Europe Non-metastatic Prostate Cancer Market, by Country
- 2.2.3.1.1 Germany
- 2.2.3.1.2 U.K.
- 2.2.3.1.3 France
- 2.2.3.1.4 Italy
- 2.2.3.1 Europe Non-metastatic Prostate Cancer Market, by Country
- 2.3 Asia Pacific
- 2.3.1 Key Findings
- 2.3.2 Market Dynamics
- 2.3.3 Market Sizing and Forecast
- 2.3.3.1 Asia Pacific Non-metastatic Prostate Cancer Market, by Country
- 2.3.3.1.1 China
- 2.3.3.1.2 Japan
- 2.3.3.1 Asia Pacific Non-metastatic Prostate Cancer Market, by Country
3. Global Non-metastatic Prostate Cancer Market: Competitive Landscape and Company Profiles
- 3.1 Key Strategies and Development
- 3.1.1 Mergers and Acquisitions
- 3.1.2 Synergistic Activities
- 3.1.3 Business Expansions and Funding
- 3.1.4 Product Launches and Approvals
- 3.1.5 Other Activities
- 3.2 Company Profiles
- 3.2.1 Johnson & Johnson (Janssen Pharmaceuticals)
- 3.2.1.1 Overview
- 3.2.1.2 Top Products / Product Portfolio
- 3.2.1.3 Top Competitors
- 3.2.1.4 Target Customers/End-Users
- 3.2.1.5 Key Personnel
- 3.2.1.6 Analyst View
- 3.2.2 Astellas Pharma
- 3.2.2.1 Overview
- 3.2.2.2 Top Products / Product Portfolio
- 3.2.2.3 Top Competitors
- 3.2.2.4 Target Customers/End-Users
- 3.2.2.5 Key Personnel
- 3.2.2.6 Analyst View
- 3.2.3 Bristol-Myers Squibb
- 3.2.3.1 Overview
- 3.2.3.2 Top Products / Product Portfolio
- 3.2.3.3 Top Competitors
- 3.2.3.4 Target Customers/End-Users
- 3.2.3.5 Key Personnel
- 3.2.3.6 Analyst View
- 3.2.4 Pfizer Inc.
- 3.2.4.1 Overview
- 3.2.4.2 Top Products / Product Portfolio
- 3.2.4.3 Top Competitors
- 3.2.4.4 Target Customers/End-Users
- 3.2.4.5 Key Personnel
- 3.2.4.6 Analyst View
- 3.2.5 Merck & Co.
- 3.2.5.1 Overview
- 3.2.5.2 Top Products / Product Portfolio
- 3.2.5.3 Top Competitors
- 3.2.5.4 Target Customers/End-Users
- 3.2.5.5 Key Personnel
- 3.2.5.6 Analyst View
- 3.2.6 Bayer AG
- 3.2.6.1 Overview
- 3.2.6.2 Top Products / Product Portfolio
- 3.2.6.3 Top Competitors
- 3.2.6.4 Target Customers/End-Users
- 3.2.6.5 Key Personnel
- 3.2.6.6 Analyst View
- 3.2.7 Sanofi
- 3.2.7.1 Overview
- 3.2.7.2 Top Products / Product Portfolio
- 3.2.7.3 Top Competitors
- 3.2.7.4 Target Customers/End-Users
- 3.2.7.5 Key Personnel
- 3.2.7.6 Analyst View
- 3.2.8 Clovis Oncology
- 3.2.8.1 Overview
- 3.2.8.2 Top Products / Product Portfolio
- 3.2.8.3 Top Competitors
- 3.2.8.4 Target Customers/End-Users
- 3.2.8.5 Key Personnel
- 3.2.8.6 Analyst View
- 3.2.9 AstraZeneca
- 3.2.9.1 Overview
- 3.2.9.2 Top Products / Product Portfolio
- 3.2.9.3 Top Competitors
- 3.2.9.4 Target Customers/End-Users
- 3.2.9.5 Key Personnel
- 3.2.9.6 Analyst View
- 3.2.10 AbbVie Inc.
- 3.2.10.1 Overview
- 3.2.10.2 Top Products / Product Portfolio
- 3.2.10.3 Top Competitors
- 3.2.10.4 Target Customers/End-Users
- 3.2.10.5 Key Personnel
- 3.2.10.6 Analyst View
- 3.2.1 Johnson & Johnson (Janssen Pharmaceuticals)