|
|
市場調査レポート
商品コード
1759271
低ホスファターゼ症の世界市場:地域・国別の分析・予測 (2025-2035年)Hypophosphatasia Market - A Global and Regional Analysis: Focus on Country and Region - Analysis and Forecast, 2025-2035 |
||||||
カスタマイズ可能
|
|||||||
| 低ホスファターゼ症の世界市場:地域・国別の分析・予測 (2025-2035年) |
|
出版日: 2025年06月30日
発行: BIS Research
ページ情報: 英文 100 Pages
納期: 1~5営業日
|
全表示
- 概要
- 図表
- 目次
低ホスファターゼ症 (HPP) 市場の主な成長要因の一つは、Strensiq (アスフォターゼ アルファ) のような酵素補充療法 (ERT) を中心とした標的治療への需要の高まりです。
この需要の増加には複数の要因があります。まず、遺伝子検査の進歩によりHPPの早期発見が可能となり、タイムリーな介入とより良好な疾患管理が実現できるようになりました。迅速な診断により、早期治療が行われ、患者の転帰が改善されます。さらに、希少疾患に対する規制支援 (希少疾病用医薬品指定や各種インセンティブ) が、HPPなどの疾患に対する治療薬の開発と商業化を後押ししています。政府は新しい治療法の承認を円滑にするための有利な政策を導入しており、それが患者へのアクセス改善につながっています。加えて、医療提供者および患者の間でのHPPに対する認知度の向上もこの需要増加を支えています。より多くの患者が診断され、効果的な治療を求めるようになった結果、市場が拡大しています。また、希少疾患に特化した専門治療センターの整備により、専門的なケアへのアクセスが向上し、患者は高度な治療と包括的な管理を受けられるようになっています。こうした要素が組み合わさることで、HPP治療市場は開発・成長のための有利な環境を整えており、標的治療の果たす役割が極めて重要であることが強調されています。
一方で、低ホスファターゼ症市場にはいくつかの課題が残っています。最大の課題の一つは、Strensiq (アスフォターゼアルファ) に代表される酵素補充療法 (ERT) の高額な治療費です。これらの治療法は非常に効果的である一方で、非常に高価であるため、特に医療資源が限られている地域や保険が十分でない患者にとっては、治療へのアクセスが困難です。HPPなどの慢性疾患に対する長期的な治療費は、患者および医療システムの双方に大きな経済的負担を与えます。このような費用の壁が、治療の遅れや質の低いケアを引き起こし、最終的には患者の健康転帰に悪影響を及ぼす可能性があります。さらに、疾患の希少性が研究開発の障壁となっており、代替治療法や低コストの選択肢の登場が難しい状況です。
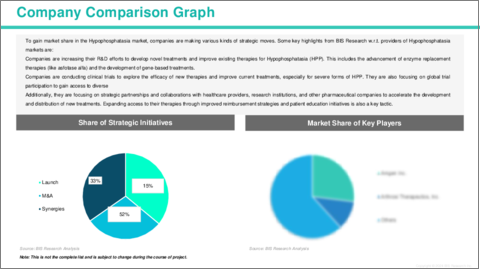
世界のHPP市場は競争が激しく、大手企業の数社が革新と市場成長を牽引しています。AstraZenecaは、Alexion Pharmaceuticalsの買収を通じてこの分野で重要な役割を果たしており、重症型HPPに対して承認された初かつ唯一の酵素補充療法Strensiq (アスフォターゼ アルファ) を提供しています。Mereo BioPharmaも希少疾患に注力している企業であり、HPPへの関与は限定的ではあるものの、注目されています。Amgenは骨の健康および代謝性疾患分野のリーダー企業であり、現時点でHPP向けの特定治療薬は保有していませんが、この領域への参入の潜在性があります。BridgeBio Pharmaは、子会社であるQED Therapeuticsを通じて遺伝子治療を推進しており、主に他の遺伝性疾患に焦点を当てている一方で、希少疾患に関する専門知識を活かしてHPP向けの今後の開発も期待されています。これらの企業は、治療の進歩、未充足ニーズへの対応、患者の転帰において重要な役割を果たしており、成長著しいHPP市場における中心的な存在です。
当レポートでは、世界の低ホスファターゼ症市場を調査し、主要動向、市場影響因子の分析、法規制環境、臨床試験の動向、市場規模の推移・予測、各種区分・地域/主要国別の詳細分析、競合情勢、主要企業のプロファイルなどをまとめています。
目次
エグゼクティブサマリー
第1章 世界の低ホスファターゼ症市場:業界展望
- 市場動向
- 規制の枠組み
- 疫学分析
- 臨床試験分析
- 市場力学
- 影響分析
- 市場促進要因
- 市場の課題
- 市場機会
第2章 世界の低ホスファターゼ症市場:地域別
- 北米
- 主な調査結果
- 市場力学
- 市場規模と予測
- 欧州
- 主な調査結果
- 市場力学
- 市場規模と予測
- アジア太平洋
- 主な調査結果
- 市場力学
- 市場規模と予測
第3章 世界の低ホスファターゼ症市場:競合情勢と企業プロファイル
- 主な戦略・展開
- M&A
- 相乗効果のある活動
- 事業拡大と資金調達
- 製品の発売と承認
- その他の活動
- 企業プロファイル
- AstraZeneca
- Mereo BioPharma
- Amgen
- Therachon (BridgeBio Pharma)
第4章 調査手法
List of Figures
- Figure: Global Hypophosphatasia Market (by Region), $Billion, 2024 and 2035
- Figure: Global Hypophosphatasia Market Key Trends, Analysis
List of Tables
- Table: Global Hypophosphatasia Market Dynamics, Impact Analysis
- Table: Global Hypophosphatasia Market (by Region), $Billion, 2024-2035
Global Hypophosphatasia Market, Analysis and Forecast: 2025-2035
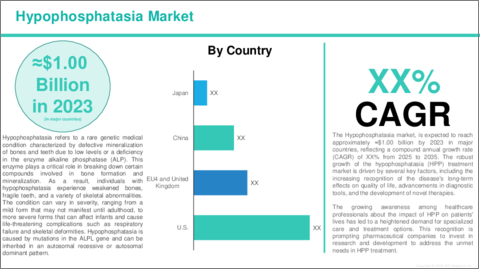
Hypophosphatasia is a rare genetic disorder caused by a deficiency in the enzyme alkaline phosphatase, which is essential for bone and tooth mineralization. This deficiency leads to weakened bones, skeletal deformities, and premature loss of teeth. The severity of hypophosphatasia varies, with the perinatal and infantile forms being life-threatening, while the childhood and adult forms are milder but still cause symptoms such as fractures, bone pain, and growth delays. Early diagnosis, often through blood tests and genetic testing, is crucial for effective treatment. The primary treatment for hypophosphatasia is enzyme replacement therapy, with Strensiq (asfotase alfa) being the most common option. This therapy helps restore enzyme levels, improving bone health and reducing fractures. While there is no cure for hypophosphatasia, advancements in treatment have significantly improved the quality of life for individuals with this condition, especially when diagnosed and treated early.
One of the key drivers of the Hypophosphatasia market is the increasing demand for targeted therapies, particularly enzyme replacement therapies (ERTs) like Strensiq (asfotase alfa). This growing demand is fueled by several factors. Advancements in genetic testing have enabled earlier detection of HPP, allowing for timely interventions and better disease management. The ability to diagnose HPP more quickly leads to improved patient outcomes through early treatment. Additionally, regulatory support for rare diseases, including orphan drug designations and incentives, has encouraged the development and commercialization of therapies for conditions like HPP. Governments have implemented favorable policies that facilitate the approval of new treatments, making them more accessible to patients. Increased disease awareness among healthcare providers and patients has further contributed to this demand, as more people are diagnosed and seek effective treatment options. Furthermore, the availability of specialized care centers dedicated to rare diseases has improved access to expert care, ensuring that patients receive advanced treatments and comprehensive management for HPP. Together, these factors have created a more favorable environment for the development and growth of the HPP treatment market, emphasizing the crucial role of targeted therapies in managing this rare and complex disease.
Despite the growth of the Hypophosphatasia market, several challenges continue to hinder its progress. One of the primary challenges is the high cost of treatment, particularly for enzyme replacement therapies (ERTs) such Strensiq (asfotase alfa). These therapies, while highly effective, are expensive, making access to treatment difficult for many patients, especially in regions with limited healthcare resources or without comprehensive insurance coverage. The high cost of long-term treatment for chronic conditions such hypophosphatasia can place a significant financial burden on both patients and healthcare systems. This barrier to access can result in delayed treatments or suboptimal care, ultimately impacting the outcomes for individuals with hypophosphatasia. Additionally, the rarity of the disease means that research and development in this field are limited, making it harder to introduce alternative therapies or lower-cost options.
The global Hypophosphatasia market is highly competitive, with several leading companies driving innovation and market growth. AstraZeneca, through its acquisition of Alexion Pharmaceuticals, is a major player, offering Strensiq (asfotase alfa), the first and only enzyme replacement therapy approved for severe forms of HYPOPHOSPHATASIA. Mereo BioPharma is another key player, with its focus on rare diseases, although its involvement in hypophosphatasia has been limited. Amgen, a leader in bone health and metabolic diseases, has the potential to expand its portfolio into the hypophosphatasia space, despite not currently offering a specific treatment for the condition. BridgeBio Pharma, through its subsidiary QED Therapeutics, is advancing genetic therapies, and while its primary focus is on other genetic diseases, its expertise in rare disorders could lead to future developments for hypophosphatasia. These companies are crucial in advancing treatments, addressing unmet medical needs, and enhancing patient outcomes in the rapidly growing hypophosphatasia market.
Market Segmentation:
Segmentation 1: by Region
- North America
- Europe
- Asia-Pacific
The global Hypophosphatasia market is undergoing significant transformation, fueled by emerging trends that are enhancing treatment options and patient outcomes. Key developments include the introduction of next-generation enzyme replacement therapies (ERTs), such as ALXN1850 (efzimfotase alfa) by AstraZeneca, which offer improved dosing schedules and greater bioavailability compared to earlier treatments such Strensiq (asfotase alfa).
Additionally, advancements in genetic testing have led to earlier and more accurate diagnoses, enabling personalized treatment plans that are tailored to individual patient profiles. The increasing adoption of precision medicine is further driving the development of therapies that target the underlying genetic causes of hypophosphatasia, moving beyond symptom management to potential curative approaches. Furthermore, global initiatives aimed at expanding healthcare infrastructure and providing financial support for rare disease treatments are improving access to care in underserved regions, thereby broadening the patient base and fostering market growth.
Table of Contents
Executive Summary
Scope and Definition
Market/Product Definition
Inclusion and Exclusion
Key Questions Answered
Analysis and Forecast Note
1. Global Hypophosphatasia Market: Industry Outlook
- 1.1 Introduction
- 1.2 Market Trends
- 1.3 Regulatory Framework
- 1.4 Epidemiology Analysis
- 1.5 Clinical Trial Analysis
- 1.6 Market Dynamics
- 1.6.1 Impact Analysis
- 1.6.2 Market Drivers
- 1.6.3 Market Challenges
- 1.6.4 Market Opportunities
2. Global Hypophosphatasia Market (Region), ($Billion), 2023-2035
- 2.1 North America
- 2.1.1 Key Findings
- 2.1.2 Market Dynamics
- 2.1.3 Market Sizing and Forecast
- 2.1.3.1 North America Hypophosphatasia Market, by Country
- 2.1.3.1.1 U.S.
- 2.1.3.1 North America Hypophosphatasia Market, by Country
- 2.2 Europe
- 2.2.1 Key Findings
- 2.2.2 Market Dynamics
- 2.2.3 Market Sizing and Forecast
- 2.2.3.1 Europe Hypophosphatasia Market, by Country
- 2.2.3.1.1 Germany
- 2.2.3.1.2 U.K.
- 2.2.3.1.3 France
- 2.2.3.1.4 Italy
- 2.2.3.1 Europe Hypophosphatasia Market, by Country
- 2.3 Asia Pacific
- 2.3.1 Key Findings
- 2.3.2 Market Dynamics
- 2.3.3 Market Sizing and Forecast
- 2.3.3.1 Asia Pacific Hypophosphatasia Market, by Country
- 2.3.3.1.1 China
- 2.3.3.1.2 Japan
- 2.3.3.1 Asia Pacific Hypophosphatasia Market, by Country
3. Global Hypophosphatasia Market: Competitive Landscape and Company Profiles
- 3.1 Key Strategies and Development
- 3.1.1 Mergers and Acquisitions
- 3.1.2 Synergistic Activities
- 3.1.3 Business Expansions and Funding
- 3.1.4 Product Launches and Approvals
- 3.1.5 Other Activities
- 3.2 Company Profiles
- 3.2.1 AstraZeneca
- 3.2.1.1 Overview
- 3.2.1.2 Top Products / Product Portfolio
- 3.2.1.3 Top Competitors
- 3.2.1.4 Target Customers/End-Users
- 3.2.1.5 Key Personnel
- 3.2.1.6 Analyst View
- 3.2.2 Mereo BioPharma
- 3.2.2.1 Overview
- 3.2.2.2 Top Products / Product Portfolio
- 3.2.2.3 Top Competitors
- 3.2.2.4 Target Customers/End-Users
- 3.2.2.5 Key Personnel
- 3.2.2.6 Analyst View
- 3.2.3 Amgen
- 3.2.3.1 Overview
- 3.2.3.2 Top Products / Product Portfolio
- 3.2.3.3 Top Competitors
- 3.2.3.4 Target Customers/End-Users
- 3.2.3.5 Key Personnel
- 3.2.3.6 Analyst View
- 3.2.4 Therachon (BridgeBio Pharma)
- 3.2.4.1 Overview
- 3.2.4.2 Top Products / Product Portfolio
- 3.2.4.3 Top Competitors
- 3.2.4.4 Target Customers/End-Users
- 3.2.4.5 Key Personnel
- 3.2.4.6 Analyst View
- 3.2.1 AstraZeneca