|
|
市場調査レポート
商品コード
1578155
CRISPR技術:世界市場CRISPR Technology: Global Markets |
||||||
|
|||||||
| CRISPR技術:世界市場 |
|
出版日: 2024年10月23日
発行: BCC Research
ページ情報: 英文 122 Pages
納期: 即納可能
|
全表示
- 概要
- 図表
- 目次
世界のCRISPR技術の市場規模は、2023年の34億米ドル、2024年の38億米ドルから、予測期間中は14.4%のCAGRで推移し、2029年末には75億米ドルの規模に成長すると予測されています。
用途別では、医薬品開発用途の部門が2024年の19億米ドルから、15.5%のCAGRで推移し、2029年には38億米ドルに成長すると予測されています。農業用途の部門が、2024年の10億米ドルから、14.2%のCAGRで推移し、2029年には20億米ドルに成長すると予測されています。
当レポートでは、世界のCRISPR技術の市場を調査し、市場概要、市場影響因子および市場機会の分析、法規制環境、新興技術および技術開発の動向、市場規模の推移・予測、各種区分・地域別の詳細分析、競合情勢、主要企業のプロファイルなどをまとめています。
目次
第1章 エグゼクティブサマリー
- 市場見通し
- 調査範囲
- 市場概要
第2章 市場概要
- ゲノム編集
- CRISPR
- CRISPRのコンポーネント
- 作用機序
- CRISPR遺伝子編集ワークフロー
- CRISPR技術の利点と欠点
- 価格分析
- PESTLE分析
- ポーターのファイブフォース分析
- 規制状況:タイプ別
- ヒト
- 遺伝子ドライブ
- 農業
- 動物
- 規制状況:地域別
- 北米
- 欧州
- アジア太平洋
- その他の地域
第3章 市場力学
- 市場力学スナップショット
- 市場促進要因
- 慢性疾患の発症率の上昇
- 遺伝性疾患の有病率
- 政府と民間の資金
- 市場抑制要因
- 代替技術
- 市場機会
- 申請件数の増加
- 市場の課題
- 複雑かつ進化する規制状況
- CRISPRベースの治療の高コスト性
第4章 新興技術と開発
- 新興技術
- CRISPRの生体内送達
- 改良されたCasバリアント
- AI
- 臨床試験分析
- 臨床試験分析 (研究タイプ別)
- 臨床試験分析 (ステータス別)
- 臨床試験分析 (フェーズ別)
- 臨床試験
- 特許分析
- 特許 (年別)
- 特許 (出願人別)
- 特許 (主要所有者別)
- 特許 (管轄別)
- 知的財産紛争・特許問題
第5章 市場セグメンテーション分析
- セグメンテーションの内訳
- 市場内訳:用途別
- 医薬品開発
- 農業
- 診断
- その他
- 市場内訳:エンドユーザー別
- バイオテクノロジー・製薬企業
- 学術・政府研究機関
- CRO
- 地理的内訳
- 市場内訳:地域別
- 北米
- 欧州
- アジア太平洋
- その他の地域
第6章 競合情報
- 市場分析
- 戦略分析
第7章 CRISPR技術の持続可能性:ESGの観点
- ESG:イントロダクション
- CRISPR技術の持続可能性:ESGの観点
- 主要なESG問題
- CRISPR技術のESGパフォーマンス分析
- 環境パフォーマンス
- 社会的パフォーマンス
- ガバナンスパフォーマンス
- BCCによる総論
第8章 付録
- 調査手法
- 参考文献
- 略語
- 企業プロファイル
- AGILENT TECHNOLOGIES INC.
- BEAM THERAPEUTICS
- CARIBOU BIOSCIENCES INC.
- CRISPR THERAPEUTICS
- DANAHER CORP.
- EDITAS MEDICINE
- GENSCRIPT
- INTELLIA THERAPEUTICS INC.
- LONZA
- MERCK KGAA
- THERMO FISHER SCIENTIFIC INC.
List of Tables
- Summary Table : Global Market for CRISPR Technology, by Application, Through 2029
- Table 1 : Comparison of Genome Editing Techniques
- Table 2 : Comparison of sgRNA Formats
- Table 3 : Comparison of Cas Enzymes
- Table 4 : Pricing Analysis of CRISPR Technology Products, by Company
- Table 5 : Regulatory Rating: Human Gene Editing, by Country/Region
- Table 6 : Regulatory Rating: Gene Drives, by Country/Region
- Table 7 : Regulatory Rating: Crops/Food, by Country/Region
- Table 8 : Regulatory Rating: Animals, by Country/Region
- Table 9 : Regulatory Landscape in Asia-Pacific Countries
- Table 10 : Regulatory Landscape in South American Countries
- Table 11 : Chronic Diseases: Applications of CRISPR-based Therapy
- Table 12 : Genetic Disease: CRISPR-based Therapy
- Table 13 : CRISPR Technology: Venture Funding, 2021-2024
- Table 14 : Clinical Trials in CRISPR Technology, by Type of Study, August 2024
- Table 15 : Clinical Trials in CRISPR Technology, by Status, August 2024
- Table 16 : Clinical Trials in CRISPR Technology, by Phase, August 2024
- Table 17 : Select Clinical Trials, 2024
- Table 18 : Patents Granted on CRISPR Technology, by Top Applicant, 2020-2023
- Table 19 : Patents Granted on CRISPR Technology, by Top Owner, 2020-2023
- Table 20 : Patents Granted on CRISPR Technology, by Jurisdiction, 2020-2023
- Table 21 : Global Market for CRISPR Technology, by Application, Through 2029
- Table 22 : CRISPR-Based Genetic Therapy: Pipeline, 2024
- Table 23 : Global Market for Drug Development in CRISPR Technology, by Region, Through 2029
- Table 24 : CRISPR-Edited Crops
- Table 25 : Global Market for Agriculture in CRISPR Technology, by Region, Through 2029
- Table 26 : CRISPR-based Diagnostics
- Table 27 : Global Market for Diagnostics in CRISPR Technology, by Region, Through 2029
- Table 28 : Global Market for Other Applications in CRISPR Technology, by Region, Through 2029
- Table 29 : Global Market for CRISPR Technology, by End User, Through 2029
- Table 30 : Biotech and Pharmaceutical Partners of CRISPR Technology Companies
- Table 31 : Recent Collaborations Among Biotech, Pharma and CRISPR Technology Firms, 2023-2024
- Table 32 : Global Market for Biotech and Pharma Companies for CRISPR Technology, by Region, Through 2029
- Table 33 : Global Market for Academics and Government Research Institutes for CRISPR Technology, by Region, Through 2029
- Table 34 : Global Market for CRO for CRISPR Technology, by Region, Through 2029
- Table 35 : Global Market for CRISPR Technology, by Region, Through 2029
- Table 36 : North American Market for CRISPR Technology, by Country, Through 2029
- Table 37 : European Market for CRISPR Technology, by Country, Through 2029
- Table 38 : Asia-Pacific Market for CRISPR Technology, by Country, Through 2029
- Table 39 : Leading Companies in the CRISPR Technology Market
- Table 40 : Small and Medium-Sized Companies in the CRISPR Technology Market
- Table 41 : Recent Developments in the CRISPR Technology Market, 2022-2024
- Table 42 : Bioethical Issues and Risks Associated with CRISPR Technology
- Table 43 : ESG Rankings for CRISPR Technology Companies, 2024*
- Table 44 : ESG: Environmental Overview
- Table 45 : ESG: Social Overview
- Table 46 : ESG: Governance Overview
- Table 47 : Information Sources in this Report
- Table 48 : Abbreviations Used in this Report
- Table 49 : Agilent Technologies Inc.: Company Snapshot
- Table 50 : Agilent Technologies Inc.: Financial Performance, FY 2022 and 2023
- Table 51 : Agilent Technologies Inc.: Product Portfolio
- Table 52 : Agilent Technologies Inc.: News/Key Developments, 2021
- Table 53 : Beam Therapeutics: Company Snapshot
- Table 54 : Beam Therapeutics: Financial Performance, FY 2022 and 2023
- Table 55 : Beam Therapeutics: Product Portfolio
- Table 56 : Beam Therapeutics: News/Key Developments, 2021-2023
- Table 57 : Caribou Biosciences Inc.: Company Snapshot
- Table 58 : Caribou Biosciences Inc.: Financial Performance, FY 2022 and 2023
- Table 59 : Caribou Biosciences Inc.: Product Portfolio
- Table 60 : Caribou Biosciences Inc.: News/Key Developments, 2021-2023
- Table 61 : CRISPR Therapeutics: Company Snapshot
- Table 62 : CRISPR Therapeutics: Financial Performance, FY 2022 and 2023
- Table 63 : CRISPR Therapeutics: Product Portfolio
- Table 64 : CRISPR Therapeutics: News/Key Developments, 2022-2024
- Table 65 : Danaher Corp.: Company Snapshot
- Table 66 : Danaher Corp.: Financial Performance, FY 2022 and 2023
- Table 67 : Danaher Corp.: Product Portfolio
- Table 68 : Danaher Corp.: News/Key Developments, 2021-2024
- Table 69 : Editas Medicine: Company Snapshot
- Table 70 : Editas Medicine: Financial Performance, FY 2022 and 2023
- Table 71 : Editas Medicine: Product Portfolio
- Table 72 : Editas Medicine: News/Key Developments, 2022 and 2023
- Table 73 : GenScript: Company Snapshot
- Table 74 : GenScript: Financial Performance, FY 2022 and 2023
- Table 75 : GenScript: Product Portfolio
- Table 76 : GenScript: News/Key Developments, 2021
- Table 77 : Intellia Therapeutics Inc.: Company Snapshot
- Table 78 : Intellia Therapeutics Inc.: Financial Performance, FY 2022 and 2023
- Table 79 : Intellia Therapeutics Inc.: Product Portfolio
- Table 80 : Intellia Therapeutics Inc.: News/Key Developments, 2021-2023
- Table 81 : Lonza: Company Snapshot
- Table 82 : Lonza Financial Performance, FY 2022 and 2023
- Table 83 : Lonza: Product Portfolio
- Table 84 : Lonza: News/Key Developments, 2022-2024
- Table 85 : Merck KGaA: Company Snapshot
- Table 86 : Merck KGaA: Financial Performance, FY 2022 and 2023
- Table 87 : Merck KGaA: Product Portfolio
- Table 88 : Merck KGaA: News/Key Developments, 2021
- Table 89 : Thermo Fisher Scientific Inc.: Company Snapshot
- Table 90 : Thermo Fisher Scientific Inc.: Financial Performance, FY 2022 and 2023
- Table 91 : Thermo Fisher Scientific Inc: Product Portfolio
- Table 92 : Thermo Fisher Scientific Inc.: News/Key Developments, 2021 and 2022
List of Figures
- Summary Figure : Global Market for CRISPR Technology, by Application, 2021-2029
- Figure 1 : CRISPR: Mechanism of Action
- Figure 2 : CRISPR Gene Editing: Workflow
- Figure 3 : Advantages and Disadvantages of CRISPR Technology
- Figure 4 : PESTLE Analysis - CRISPR Technology
- Figure 5 : Market Dynamics of CRISPR Technology
- Figure 6 : Estimated Numbers of All Cancer Cases, Both Sexes, 2022-2050
- Figure 7 : Global Estimates: People with Diabetes (20 to 79), 2021-2045
- Figure 8 : Emerging Trends/Technologies in the CRISPR Technology Market
- Figure 9 : Patents Granted on CRISPR Technology, by Year, 2020-2022
- Figure 10 : Global Market Shares for CRISPR Technology, by Application, 2023
- Figure 11 : CRISPR Technology in Drug Development
- Figure 12 : Uses of CRISPR in Agriculture
- Figure 13 : Global Market Shares for CRISPR Technology, by End User, 2023
- Figure 14 : Number of Scientific Publications on CRISPR, by Year, 2020-2023
- Figure 15 : Global Market Shares for CRISPR Technology, by Region, 2023
- Figure 16 : North American Market Shares of CRISPR Technology, by Country, 2023
- Figure 17 : U.S. Market for CRISPR Technology, 2021-2029
- Figure 18 : Canadian Market for CRISPR Technology, 2021-2029
- Figure 19 : European Market Shares of CRISPR Technology, by Country, 2023
- Figure 20 : German Market for CRISPR Technology, 2021-2029
- Figure 21 : U.K. Market for CRISPR Technology, 2021-2029
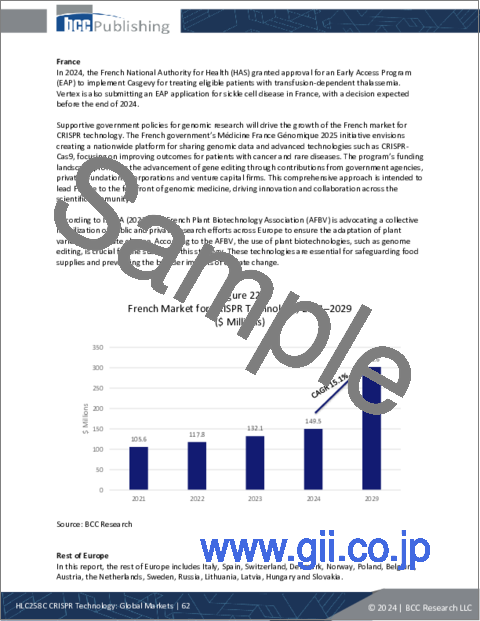
- Figure 22 : French Market for CRISPR Technology, 2021-2029
- Figure 23 : Rest of the European Market for CRISPR Technology, 2021-2029
- Figure 24 : Asia-Pacific Market Shares of CRISPR Technology, by Country, 2023
- Figure 25 : Chinese Market for CRISPR Technology, 2021-2029
- Figure 26 : Japanese Market for CRISPR Technology, 2021-2029
- Figure 27 : Indian Market for CRISPR Technology, 2021-2029
- Figure 28 : Rest of Asia-Pacific Market for CRISPR Technology, 2021-2029
- Figure 29 : RoW Market for CRISPR Technology, 2021-2029
- Figure 30 : Pillars of ESG
- Figure 31 : Advantages of ESG for Companies
- Figure 32 : Methodology Used in the CRISPR Technology Market
- Figure 33 : Agilent Technologies Inc.: Revenue Share, by Business Unit, FY 2023
- Figure 34 : Agilent Technologies Inc.: Revenue Share, by Region, FY 2023
- Figure 35 : Caribou Biosciences Inc.: Revenue Share, by Business Unit, FY 2023
- Figure 36 : Caribou Biosciences Inc.: Revenue Share, by Country/Region, FY 2023
- Figure 37 : Danaher Corp.: Market Share, by Business Unit, FY 2023
- Figure 38 : Danaher Corp.: Revenue Share, by Country/Region, FY 2023
- Figure 39 : GenScript: Revenue Share, by Business Unit, FY 2023
- Figure 40 : GenScript: Revenue Share, by Country/Region, FY 2023
- Figure 41 : Lonza: Revenue Share, by Business Unit, FY 2023
- Figure 42 : Lonza: Revenue Share, by Country/Region, FY 2023
- Figure 43 : Merck KGaA: Revenue Share, by Business Unit, FY 2023
- Figure 44 : Merck KGaA: Revenue Share, by Region, FY 2023
- Figure 45 : Thermo Fisher Scientific Inc.: Market Share, by Business Unit, FY 2023
- Figure 46 : Thermo Fisher Scientific Inc.: Revenue Share, by Region, FY 2023
The global market for CRISPR technology was valued at $3.4 billion in 2023. This market is expected to grow from $3.8 billion in 2024 to $7.5 billion by the end of 2029, at a compound annual growth rate (CAGR) of 14.4% from 2024 to 2029.
The global market for CRISPR technology in drug development application is expected to grow from $1.9 billion in 2024 to $3.8 billion by 2029, at a CAGR of 15.5% from 2024 to 2029.
The global market for CRISPR technology in agriculture application is expected to grow from $1.0 billion in 2024 to $2.0 billion by 2029, at a CAGR of 14.2% from 2024 to 2029.
Report Scope
This report on CRISPR technology provides market projections for 2029 and analyzes the market for CRISPR technology by application and end user. Applications covered include drug development, agriculture, diagnostics and others. End users include biotechnology and pharmaceutical companies, academics and government research institutes, and contract research organizations.
Report Includes
- 32 data tables and 61 additional tables
- An analysis of the global market for CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) technology
- Analyses of the global market trends, with data from 2021-2022, estimates for 2023, forecast for 2024, and projected CAGRs through 2029
- Discussion of the market potential for CRISPR technology
- Estimate of the market size and a revenue forecast for the global CRISPR technology market, and a corresponding market share analysis by application, end user, and region
- Discussion of the major market dynamics, regulations, industry challenges, and macroeconomic factors that will affect the demand for CRISPR technology market over the next five years
- A look at the recent breakthrough in CRISPR-Cas9 genome editing technology, and how it has propelled the rapid growth in genetic engineering and advanced pharmacological research
- A comparative study on CRISPR with TALE and ZFN nuclease as a gene editing tool and discussion on its higher adaptability over other nucleases
- Review of the patent filings and research publications for innovations in the CRISPR-Cas9 genome editing technology
- A discussion on ESG challenges and ESG practices of the industry participants
- A look at the proprietary technologies and strategic alliances of the companies best positioned in the CRISPR market
- Analysis of the industry structure, competitive landscape, clinical trials, ongoing research activities, and the impact of COVID-19
- Company profiles of the major players, including Merck KGaA, Thermo Fisher Scientific, Beam Therapeutics, CRISPR Therapeutics and Danaher Corp.
Table of Contents
Chapter 1 Executive Summary
- Market Outlook
- Scope of Report
- Market Summary
Chapter 2 Market Overview
- Genome Editing
- CRISPR
- Introduction
- Components of CRISPR
- Mechanism of Action
- CRISPR Gene Editing Workflow
- Advantages and Disadvantages of CRISPR Technology
- Pricing Analysis
- PESTLE Analysis
- Porter's Five Forces Analysis
- Regulatory Landscape, by Type
- Human
- Gene Drives
- Agriculture
- Animals
- Regulatory Landscape, by Region
- North America
- Europe
- Asia-Pacific
- Rest of the World
Chapter 3 Market Dynamics
- Market Dynamics Snapshot
- Market Drivers
- Rising Incidence of Chronic Diseases
- Prevalence of Genetic Disorders
- Government and Private Funding
- Market Restraints
- Alternative Technologies
- Market Opportunities
- Increase in the Number of Applications
- Market Challenges
- Complex and Evolving Regulatory Landscape
- High Cost of CRISPR-based Therapy
Chapter 4 Emerging Technologies and Developments
- Emerging Technologies
- In Vivo Delivery of CRISPR
- Improved Cas Variants
- Artificial Intelligence
- Clinical Trials Analysis
- Clinical Trials Analysis, by Type of Study
- Clinical Trials Analysis, by Status
- Clinical Trials Analysis, by Phase
- Clinical Trials
- Patent Analysis
- Patents, by Year
- Patents, by Top Applicant
- Patents, by Top Owner
- Patents, by Jurisdiction
- Intellectual Property Disputes and Patent Issues
Chapter 5 Market Segmentation Analysis
- Segmentation Breakdown
- Market Breakdown, by Application
- Drug Development
- Agriculture
- Diagnostics
- Other Applications
- Market Breakdown, by End User
- Biotech and Pharmaceutical Companies
- Academics and Government Research Institutes
- Contract Research Organizations
- Geographic Breakdown
- Market Analysis, by Region
- North America
- Europe
- Asia-Pacific
- Rest of the World
Chapter 6 Competitive Intelligence
- Market Analysis
- Strategic Analysis
Chapter 7 Sustainability in CRISPR Technology: An ESG Perspective
- Introduction to ESG
- Sustainability in CRISPR Technology: An ESG Perspective
- Key ESG Issues
- CRISPR Technology ESG Performance Analysis
- Environmental Performance
- Social Performance
- Governance Performance
- Concluding Remarks from BCC
Chapter 8 Appendix
- Methodology
- References
- Abbreviations
- Company Profiles
- AGILENT TECHNOLOGIES INC.
- BEAM THERAPEUTICS
- CARIBOU BIOSCIENCES INC.
- CRISPR THERAPEUTICS
- DANAHER CORP.
- EDITAS MEDICINE
- GENSCRIPT
- INTELLIA THERAPEUTICS INC.
- LONZA
- MERCK KGAA
- THERMO FISHER SCIENTIFIC INC.