|
|
市場調査レポート
商品コード
1361106
HIV診断薬および治療薬:世界市場Diagnostics and Therapeutics for HIV: Global Markets |
||||||
|
|||||||
| HIV診断薬および治療薬:世界市場 |
|
出版日: 2023年10月10日
発行: BCC Research
ページ情報: 英文 186 Pages
納期: 即納可能
|
- 全表示
- 概要
- 図表
- 目次
世界のHIV診断薬および治療薬の市場規模は、2023年の304億米ドルから、予測期間中は5.3%のCAGRで推移し、2028年末には393億米ドルの規模に成長すると予測されています。
ARV薬剤カテゴリー別で見ると、単錠レジメン (STR) /固定用量配合剤 (FDC) 薬剤の部門が、2023年の113億米ドルから、同期間中5.8%のCAGRで推移し、2028年末には149億米ドルの規模に成長すると予測されています。また、ヌクレオチド逆転写酵素阻害剤 (NRTI) の部門は、2023年の89億米ドルから、4.4%のCAGRで推移し、2028年末には110億米ドルの規模に成長すると予測されています。
当レポートでは、世界のHIV診断薬および治療薬の市場を調査し、市場および技術の概要、市場影響因子および市場機会の分析、市場規模の推移・予測、各種区分・地域別の詳細分析、技術および特許の動向、ESGの展開、競合情勢、主要企業のプロファイルなどをまとめています。
目次
第1章 イントロダクション
第2章 サマリー・ハイライト
第3章 市場および技術の背景
- ヒト免疫不全ウイルス (HIV) の概要
- レポート概要
- 疾患予後
- 症状
- 治療法
- HIVのタイムラインとマイルストーン
- HIVのライフサイクル:治療と作用機序
- HIVの予防
- 曝露前予防内服 (PrEP)
- 曝露後予防内服 (PEP)
- 薬剤耐性
- 服薬アドヒアランス
- WHOの取り組み
第4章 世界の産業動向
- 世界のHIV感染の動向
- 地域啓発プログラム
- 新規治療法の開発
- 官民パートナーシップ
- 患者の寿命延長に伴うHIV患者の併存疾患の増加
- HIV撲滅の世界のプログラム
- 母子感染の予防
- 課題
- 罹患リスクの過小評価
- STDに関連する社会的偏見
- 高額な治療費
第5章 市場内訳:HIV治療薬別
- 世界市場の予測
- 地域市場
- 北米
- 市場分析・予測
- 疫学
- HIVの検査・推奨事項
- 北米市場の関連規制
- 欧州のHIV治療薬市場
- 市場分析・予測
- 疫学
- HIVの検査・推奨事項
- 欧州のHIV市場に影響を与える規制
- アジア太平洋のHIV治療薬市場
- 東南アジア地域
- 西太平洋地域
- 予防およびスクリーニングプログラム
- アジア太平洋地域のHIV市場の規制
- ジェネリックメーカーの市場への影響
- 市場分析・予測
- その他の地域のHIV治療薬市場
- アフリカ地域
- HIV市場への世界の投資
第6章 HIV市場:ARV薬剤カテゴリー別
- 概要
- 治療のタイムライン
- 第一選択の治療計画
- 第二選択の治療計画
- 第三選択の治療計画
- 市場概要:医薬品カテゴリー別
- ヌクレオシド/ヌクレオチド逆転写酵素阻害剤 (NRTI)
- プロテアーゼ阻害剤 (PI)
- 単錠レジメン (STR) /固定用量配合剤 (FDC) 薬剤
- インテグラーゼ鎖転移阻害剤 (INSTI)
- 非ヌクレオチド逆転写酵素阻害剤 (NNRTI)
- 侵入阻害剤
第7章 新興技術
- 概要
- 分子診断学
- 次世代シーケンス (NGS)
- ポイントオブケア検査
- AI
- その他
第8章 HIV診断市場の評価
- HIV診断市場:検査タイプ別
- HIV検査の進化
- 抗原および抗体に基づく検査
- 将来の展望
- CD4テスト
- ウイルス負荷試験
- HIV診断製品のプロファイル:検査タイプ別
- CD4検査製品
- ウイルス量検査製品
第9章 特許
第10章 競合情勢
- M&A・提携
第11章 企業プロファイル
- ABBOTT
- ASTRAZENECA
- B. BRAUN SE
- BECTON, DICKINSON AND COMPANY
- BIOCON
- BIO-RAD LABORATORIES INC.
- BOEHRINGER INGELHEIM INTERNATIONAL GMBH
- DANAHER CORP.
- DEXCOM INC.
- INSULET CORP.
- JOHNSON & JOHNSON SERVICES INC.
- LIFESCAN IP HOLDINGS LLC
- LILLY
- MEDTRONIC
- MERCK & CO. INC.
- NOVA BIOMEDICAL
- NOVARTIS AG
- NOVO NORDISK A/S
- QIAGEN N.V.
- ROCHE (F. HOFFMANN-LA ROCHE LTD.)
- SANOFI
- SIEMENS HEALTHINEERS AG
- TERUMO CORP.
- THERMO FISHER SCIENTIFIC INC.
- YPSOMED AG
List of Tables
- Summary Table : Global Market for HIV Diagnostics and Therapeutics, by Drug Category, Through 2028
- Table 1 : Milestones in the History of HIV
- Table 2 : Life Cycle of HIV Therapeutics Target Points
- Table 3 : Global Market for HIV Therapeutics, by Drug Category, Through 2028
- Table 4 : Global Market for HIV Therapeutics, by Region, Through 2028
- Table 5 : Generic Drug Manufacturers in the Asia-Pacific Region
- Table 6 : WHO Recommendations for ART Treatment
- Table 7 : Approved NRTIs, 2022
- Table 8 : Approved Generic NRTIs, 2022
- Table 9 : Global Market for NRTIs, by Region, Through 2028
- Table 10 : Approved PIs, 2022
- Table 11 : Approved Generic PIs, 2022
- Table 12 : Global Market for Protease Inhibitors, by Region, Through 2028
- Table 13 : Approved STRs, 2022
- Table 14 : Approved STR FDC Generics, 2022
- Table 15 : Global Market for Single-Tablet Regimen FDC, by Region, Through 2028
- Table 16 : FDA Approved INSTIs, 2022
- Table 17 : Global Market for INSTIs, by Region, Through 2028
- Table 18 : Approved NNRTIs, 2022
- Table 19 : Approved Generic NNRTIs, 2022
- Table 20 : Global Market for NNRTIs, by Region, Through 2028
- Table 21 : Approved Entry Inhibitors, 2022
- Table 22 : Global Market for HIV Diagnostics, by Region, Through 2028
- Table 23 : Global Market for HIV Diagnostics, by Test Type, Through 2028
- Table 24 : Antibody HIV Tests, 2022
- Table 25 : FDA Approved Antigen-Antibody Combo Tests, 2012
- Table 26 : Point-of-Care CD4 Tests
- Table 27 : Viral Load Tests
- Table 28 : Select Patent Applications Filed on HIV Diagnostics and Therapeutics, 2023
- Table 29 : M&A Activity among Diagnostics and Therapeutics Manufacturing Companies, 2023
- Table 30 : Abbott: Financials, 2019-2022
- Table 31 : Abbott: Product Portfolio, by Business Segment
- Table 32 : Abbott: Recent Developments, 2023
- Table 33 : AstraZeneca: Financial Performance, 2020-2022
- Table 34 : AstraZeneca: Selected Key Developments, 2023
- Table 35 : Braun: Financials, 2021
- Table 36 : Becton, Dickinson and Company: Company Financials, 2022
- Table 37 : Becton, Dickinson and Company: Product Portfolio
- Table 38 : Biocon: Selected Key Developments, 2022
- Table 39 : Bio-Rad Laboratories Inc.: Company Financials, 2022
- Table 40 : Bio-Rad Laboratories Inc.: Product Portfolio
- Table 41 : Boehringer Ingelheim: Selected Key Developments, 2023
- Table 42 : Danaher Corp.: Company Financials, 2022
- Table 43 : Danaher Corp.: Product Portfolio
- Table 44 : Dexcom: Financials, 2021 and 2022
- Table 45 : Insulet Corp.: Revenue, by Segment, 2020-2022
- Table 46 : Johnson & Johnson: Financials, 2022
- Table 47 : Johnson & Johnson: Recent Developments and Strategies, 2017-2022
- Table 48 : Lilly: Financial Performance, 2018-2021
- Table 49 : Lilly: Key Developments, 2023
- Table 50 : Medtronic: Financials, 2020-2022
- Table 51 : Medtronic: Product Portfolio
- Table 52 : Medtronic: Patient Monitoring Device Product Portfolio
- Table 53 : Medtronic: Recent Developments, 2015-2022
- Table 54 : Merck & Co. Inc.: Financial Performance, 2020-2022
- Table 55 : Novartis: Key Financials, 2021-2022
- Table 56 : Novartis Key Developments, 2022
- Table 57 : Novo Nordisk: Key Financials, 2021-2022
- Table 58 : Novo Nordisk: Selected Key Developments, 2023
- Table 59 : Qiagen: Product Portfolio
- Table 60 : Roche: Financials, 2021-2022
- Table 61 : Roche: Revenue, by Business Segment, 2021 and 2022
- Table 62 : Roche: Product Portfolio
- Table 63 : Roche: Recent Developments, 2023
- Table 64 : Sanofi: Business Segment
- Table 65 : Sanofi: Financial Performance, 2021 and 2022
- Table 66 : Sanofi: Key Developments, 2023
- Table 67 : Siemens Healthineers AG: Product Portfolio
List of Figures
- Summary Figure : Global Market for HIV Diagnostics and Therapeutics, 2020-2028
- Figure 1 : Types of Drug Failure Encountered During HIV Treatment
- Figure 2 : Summary of the Global HIV Epidemic, 2022
- Figure 3 : Global Market for HIV Therapeutics, 2020-2028
- Figure 4 : Global Market Shares of HIV Therapeutics, by Drug Category, 2022
- Figure 5 : Global Market for HIV Therapeutics, by Region, 2020-2028
- Figure 6 : Number of People Living with HIV Globally, by Region, 2010-2022
- Figure 7 : North American Market for HIV Therapeutics, 2020-2028
- Figure 8 : HIV Diagnoses in the U.S., by Transmission Category, 2021
- Figure 9 : European Market for HIV Therapeutics, 2020-2028
- Figure 10 : European Market for HIV Therapeutics, by Country, 2020-2028
- Figure 11 : Asia-Pacific Market for HIV Therapeutics, 2020-2028
- Figure 12 : Asia-Pacific Market for HIV Therapeutics, by Country, 2020-2028
- Figure 13 : Rest of the World Market for HIV Therapeutics, 2020-2028
- Figure 14 : Treatment Switching
- Figure 15 : Global Market for NRTIs, 2020-2028
- Figure 16 : Global Market for NRTIs, by Region, 2020-2028
- Figure 17 : Global Market for Protease Inhibitors, 2020-2028
- Figure 18 : Global Market for Protease Inhibitors, by Region, 2020-2028
- Figure 19 : Global Market for Single-Tablet Regimen, 2020-2028
- Figure 20 : Global Market for Single-Tablet Regimen FDC, by Region, 2020-2028
- Figure 21 : Global Market for INSTIs, 2020-2028
- Figure 22 : Global Market for INSTIs, by Region, 2020-2028
- Figure 23 : Global Market for NNRTIs, 2020-2028
- Figure 24 : Global Market for NNRTIs, by Region, 2020-2028
- Figure 25 : HIV Diagnostics Test Types, by Category
- Figure 26 : Global Market for HIV Diagnostics, 2020-2028
- Figure 27 : Abbott: Revenue Share, by Country/Region, 2021 and 2022
- Figure 28 : Abbott: Revenue Share, by Business Segment, 2021 and 2022
- Figure 29 : AstraZeneca: Sales Share, by Segment, 2022
- Figure 30 : AstraZeneca: Revenue Share, by Region/Country, 2022
- Figure 31 : Braun: Revenue, 2015-2021
- Figure 32 : Braun: Revenue Share, by Country/Region, 2021
- Figure 33 : Braun: Revenue Share, by Segment, 2021
- Figure 34 : Becton, Dickinson and Company: Revenue Share, by Business Segment, 2022
- Figure 35 : Becton, Dickinson and Company: Revenue Share, by Region/Country, 2022
- Figure 36 : Bio-Rad Laboratories Inc.: Revenue Share, by Business Segment, 2022
- Figure 37 : Bio-Rad Laboratories Inc.: Revenue Share, by Region/Country, 2022
- Figure 38 : Boehringer Ingelheim: Annual Revenue, 2021 and 2022
- Figure 39 : Boehringer Ingelheim: Revenue Share, by Business Segment, 2021 and 2022
- Figure 40 : Boehringer Ingelheim: Revenue Share, by Region, 2021 and 2022
- Figure 41 : Danaher Corp.: Revenue Share, by Business Segment, 2022
- Figure 42 : Danaher Corp.: Revenue Share, by Region, 2022
- Figure 43 : Dexcom Inc.: Annual Revenue, 2019-2021
- Figure 44 : Dexcom: Revenue Share, by Region/Country, 2022
- Figure 45 : Dexcom: Revenue Share, by Sales Channel, 2022
- Figure 46 : Johnson & Johnson: Financials, 2017-2021
- Figure 47 : Johnson & Johnson: Revenue Share, by Country/Region, 2021
- Figure 48 : Johnson & Johnson: Revenue Share, by Segment, 2021
- Figure 49 : Lilly: Total Sales Share, by Product Segment, 2022
- Figure 50 : Medtronic: Financials, 2015-2022
- Figure 51 : Medtronic: Revenue Share, by Country/Region, 2022
- Figure 52 : Medtronic: Revenue Share, by Key Disease Segment, 2022
- Figure 53 : Merck & Co. Inc.: Revenue Share, by Business Segment, 2022
- Figure 54 : Merck & Co. Inc.: Revenue Share, by Region/Country, 2022
- Figure 55 : Novartis: Revenue Share, by Business Segment, 2022
- Figure 56 : Novartis: Total Revenue Share, by Region/Country, 2022
- Figure 57 : Novo Nordisk: Revenue Share, by Region, 2022
- Figure 58 : Novo Nordisk: Revenue Share, by Business Segment, 2022
- Figure 59 : Qiagen: Total Revenue, 2020-2022
- Figure 60 : Qiagen: Market Share, by Product Type, 2022
- Figure 61 : Roche: Revenue, by Country/Region, 2022
- Figure 62 : Sanofi: Net Sales Share, by Business Segment, 2022
- Figure 63 : Sanofi: Net Sales Share, by Region/Country, 2022
- Figure 64 : Siemens Healthineers AG: Total Revenue, 2020-2022
- Figure 65 : Siemens Healthineers AG: Market Share, by Business Segment, 2022
- Figure 66 : Thermo Fisher Scientific Inc.: Annual Revenue, 2020-2022
- Figure 67 : Thermo Fisher Scientific Inc.: Market Share, by Business Segment, 2022
Highlights:
The global market for HIV diagnostics and therapeutics is expected to increase from $30.4 billion in 2023 to $39.3 billion by the end of 2028, with compound annual growth rate (CAGR) of 5.3% during the forecast period of 2023-2028.
Single-tablet regimen FDC market for HIV diagnostics and therapeutics is expected to increase from $11.3 billion in 2023 to $14.9 billion by the end of 2028, at a CAGR of 5.8 during the forecast period of 2023-2028.
Nucleoside reverse transcriptase inhibitors market for HIV diagnostics and therapeutics is expected to increase from $8.9 billion in 2023 to $11.0 billion by the end of 2028, at a CAGR of 4.4% during the forecast period of 2023-2028.
Report Scope:
This report organizes information from diverse sources into sections such as an overview, industry structure, diagnostic test type, treatment by drug categories, leading drug profiles and historic drug sales, regulations, reimbursements, and patents. The scope of this report includes an overview of the global market scenario for the diagnosis and treatment of HIV, with base year data from 2022, estimations for 2023 and forecasts for 2028 that are derived using projections of CAGR during the forecast period. Market value data is provided at global, regional, and country levels for treatments by drug categories. Market data for HIV diagnostics is provided for regional levels, with details on test types. Estimated values are based on companies' total revenues. Projected and forecasted revenue values are in constant U.S. dollars that have not been adjusted for inflation.
The report forecasts the global market for HIV therapeutics by drug class. It discusses market data for nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs), protease inhibitors (PIs), single tablet regimen fixed-dose combination (STR FDC), HIV integrase strand transfer inhibitors (INSTIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs). The enzyme inhibitor drug class has been excluded when calculating the global HIV therapeutics market, as there are no late-stage pipeline molecules.
Diagnostic tests such as antigen- and antibody-based tests, CD4 tests and viral load tests are detailed with current and forecasted market data. Antigen- and antibody-based tests cover enzyme-linked immunosorbent assay (ELISA) tests, enzyme immunoassay (EIA) tests, rapid tests, and NAT tests; CD4 tests include tests conducted to monitor the CD4 load; and viral load tests include all PCR-based tests.
A detailed analysis illustrating market dynamics and market structure is incorporated into the report. A Porter's Five Forces analysis and the supply and distribution chain are discussed in detail to provide an in-depth understanding of this market. The report covers the current regulations and guidelines for diagnostics and therapeutics for HIV, and it provides a survey of the top market players, detailing their business operations, segment focus, revenue, and strategy analysis. The report also features a market share analysis of these leading players, including information about product launches and pipeline products.
In terms of geography, the report analyzes the market across the following regions: North America, Europe, Asia-Pacific and Rest of the World (RoW). Key countries like the U.S., Canada, Mexico, Germany, France, UK, Italy, China, India and Japan are given special focus due to the high concentration of HIV diagnostics and therapeutics manufacturing companies and contract manufacturing organizations located in these areas.
The base year for the market data in this report is 2022, historical data is provided for 2020 and forecast data is given for 2028. Historical, base year and forecast data are provided for each market segment of the report.
Report Includes:
- 27 data tables and 41 additional tables
- An overview of the global markets for diagnostics and therapeutics for HIV
- An estimate of the market's size and analyses of global market trends, with data from 2020-2022, estimates for 2023, and projections of compound annual growth rates (CAGRs) through 2028
- Quantification of the market potential of diagnostics and therapeutics for HIV by drug category, diagnostic test type, and region
- Coverage of HIV timeline and milestones, HIV life cycle, its treatment and mechanism of action, and description of HIV prophylaxis
- Evaluation of market dynamics such as drivers and restraints, current industry trends and patterns, and an assessment of the cost implications for treatments in many countries
- Information on regional awareness programs, WHO initiatives and development of novel therapies, and discussion on rising comorbidities in HIV patients and social stigma associated with STDs
- Coverage of ongoing research and predictions of the market's future direction
- Insights into the regulatory scenario for the U.S., Europe and Japan, to provide an overview of the regulations for new drug launches and diagnostic kits
- Descriptions of the competitive landscape, product mapping, the strategic initiatives of the leading companies, including their R&D activities
- Profiles of the key companies, including Medtronic, Abbott, Sanofi, Roche, Bio-Rad Laboratories Inc., and Danaher Corp.
Table of Contents
Chapter 1 Introduction
- Study Goals and Objectives
- Reasons for Doing This Study
- What's New in This Update?
- Scope of Report
- Information Sources and Methodology
- Secondary Research
- Primary Research
- Research Methodology
- Geographic Breakdown
Chapter 2 Summary and Highlights
- Overview
- Market Outlook
Chapter 3 Market and Technology Background
- Overview of Human Immunodeficiency Virus (HIV)
- Report Overview
- Disease Prognosis
- Symptoms
- Treatments
- HIV Timeline and Milestones
- HIV Life Cycle: Treatment and Mechanism of Action
- HIV Prophylaxis
- Pre-exposure Prophylaxis (PrEP)
- Post-exposure Prophylaxis (PEP)
- Drug Resistance
- Medication Adherence
- WHO Initiatives
Chapter 4 Global Industry Trends
- Trends in Global HIV Transmission
- Regional Awareness Programs
- Development of Novel Therapies
- Public-Private Partnerships
- Rising Comorbidities in HIV Patients with an Increase in the Longevity of Patients
- Global Programs to Counter HIV
- Preventing Transmission from Mother to Child
- Challenges
- Underestimating the Risk of Contracting the Disease
- Social Stigma Associated with STDs
- High Cost of Treatment
Chapter 5 Market Breakdown by HIV Therapeutic
- Global Market Forecast for HIV Therapeutics
- Regional Markets for HIV Therapeutics
- North America
- Market Analysis and Forecast
- Epidemiology
- HIV Testing and Recommendations
- Regulation of the North American Market for HIV Therapeutics
- European Market for HIV Therapeutics
- Market Analysis and Forecast
- Epidemiology
- HIV Testing and Recommendations
- Regulations Impacting the European HIV Market
- Asia-Pacific Market for HIV Therapeutics
- Southeast Asian Regions
- Western Pacific Regions
- Prevention and Screening Programs
- Regulation of the Asia-Pacific HIV Market
- Market Impact of Generic Manufacturers
- Market Analysis and Forecast
- Rest of the World (RoW) Market for HIV Therapeutics
- African Region
- Global Investments in the HIV Market
Chapter 6 HIV Market by ARV Drug Category
- Overview
- Timeline of Therapies
- First-Line Treatment Regimen
- Second-Line Treatment Regimen
- Third-Line/Salvage Treatment Regimen
- Market Overview by Drug Category
- Nucleoside/Nucleotide Reverse Transcriptase Inhibitors (NRTIs)
- Protease Inhibitors (PIs)
- Single-Tablet Regimen (STR)/Fixed-Dose Combination (FDC) Drugs
- Integrase Strand Transfer Inhibitors (INSTI)
- Non-Nucleotide Reverse Transcriptase Inhibitors (NNRTIs)
- Entry Inhibitors
Chapter 7 Emerging Technologies
- Overview
- Molecular Diagnostics
- Next-Generation Sequencing (NGS)
- Point-of-Care Testing
- Artificial Intelligence (AI)
- Other Emerging Techniques
Chapter 8 Evaluation of Market for HIV Diagnostics
- Market for HIV Diagnostics by Test Type
- Evolution of HIV Tests
- Antigen- and Antibody-Based Tests
- Future Perspective
- CD4 Testing
- Viral Load Testing
- HIV Diagnostic Product Profiles by Test Type
- CD4 Testing Products
- Viral Load Testing Products
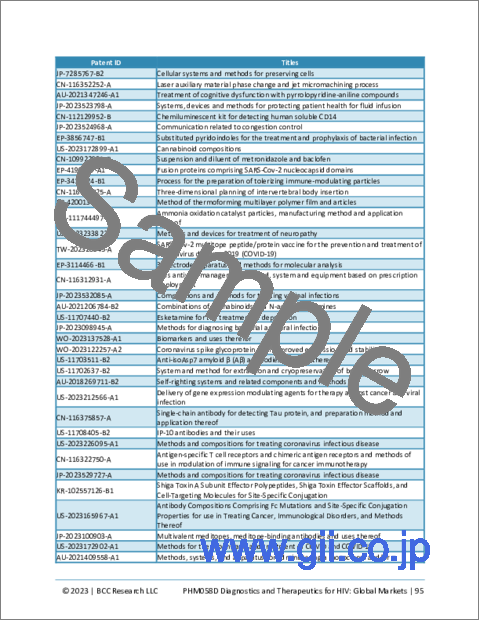
Chapter 9 Patents
- Overview
Chapter 10 Competitive Landscape
- Mergers, Acquisitions and Collaborations
Chapter 11 Company Profiles
- ABBOTT
- ASTRAZENECA
- B. BRAUN SE
- BECTON, DICKINSON AND COMPANY
- BIOCON
- BIO-RAD LABORATORIES INC.
- BOEHRINGER INGELHEIM INTERNATIONAL GMBH
- DANAHER CORP.
- DEXCOM INC.
- INSULET CORP.
- JOHNSON & JOHNSON SERVICES INC.
- LIFESCAN IP HOLDINGS LLC
- LILLY
- MEDTRONIC
- MERCK & CO. INC.
- NOVA BIOMEDICAL
- NOVARTIS AG
- NOVO NORDISK A/S
- QIAGEN N.V.
- ROCHE (F. HOFFMANN-LA ROCHE LTD.)
- SANOFI
- SIEMENS HEALTHINEERS AG
- TERUMO CORP.
- THERMO FISHER SCIENTIFIC INC.
- YPSOMED AG