|
|
市場調査レポート
商品コード
1738267
間葉系幹細胞市場- 世界の産業規模、シェア、動向、機会、予測、製品・サービス別、ワークフロー別、タイプ別、分離元別、適応症別、用途別、地域別、競合別、2020-2030年Mesenchymal Stem Cells Market - Global Industry Size, Share, Trends, Opportunity, and Forecast, Segmented By Product & Services, By Workflow, By Type, By Source of Isolation, By Indication, By Application, By Region & Competition, 2020-2030F |
||||||
カスタマイズ可能
|
|||||||
| 間葉系幹細胞市場- 世界の産業規模、シェア、動向、機会、予測、製品・サービス別、ワークフロー別、タイプ別、分離元別、適応症別、用途別、地域別、競合別、2020-2030年 |
|
出版日: 2025年05月30日
発行: TechSci Research
ページ情報: 英文 182 Pages
納期: 2~3営業日
|
全表示
- 概要
- 目次
間葉系幹細胞(MSCs)の世界市場は、2024年には27億1,000万米ドルと評価され、2030年までCAGR 8.54%で成長すると予測されています。
この市場は、再生医療における急速な進歩、臨床試験活動の活発化、MSCsの治療可能性に対する意識の高まりによって牽引力を増しています。骨、軟骨、脂肪組織など様々なタイプの細胞に分化する能力で知られるMSCは、様々な病状の治療に有望視されています。その免疫調節・抗炎症特性は、免疫原性が低いことと相まって、整形外科、神経学、心臓病、自己免疫疾患への応用に適しています。研究投資の増加と、慢性疾患の罹患率が上昇する高齢化人口を含む良好な人口動向は、市場をさらに推進しています。技術革新と臨床研究の拡大が、治療と研究の両領域におけるMSCの可能性を強調しています。
| 市場概要 | |
|---|---|
| 予測期間 | 2026-2030 |
| 市場規模:2024年 | 27億1,000万米ドル |
| 市場規模:2030年 | 44億米ドル |
| CAGR:2025年~2030年 | 8.54% |
| 急成長セグメント | アロジェニック |
| 最大市場 | 北米 |
市場促進要因
慢性疾患の増加
主な市場課題
規制上の課題
主要市場動向
技術の進歩
目次
第1章 概要
第2章 調査手法
第3章 エグゼクティブサマリー
第4章 顧客の声
第5章 世界の間葉系幹細胞市場展望
- 市場規模・予測
- 金額別
- 市場シェア・予測
- 製品とサービス別{製品(細胞と細胞株、キット、培地、試薬、その他)、サービス}
- ワークフロー別(細胞の調達と分離、培養と凍結保存、分化、特性評価)
- タイプ別(自家移植、同種移植)
- 分離源別(骨髄、臍帯血、末梢血、卵管、胎児肝臓、肺、脂肪組織)
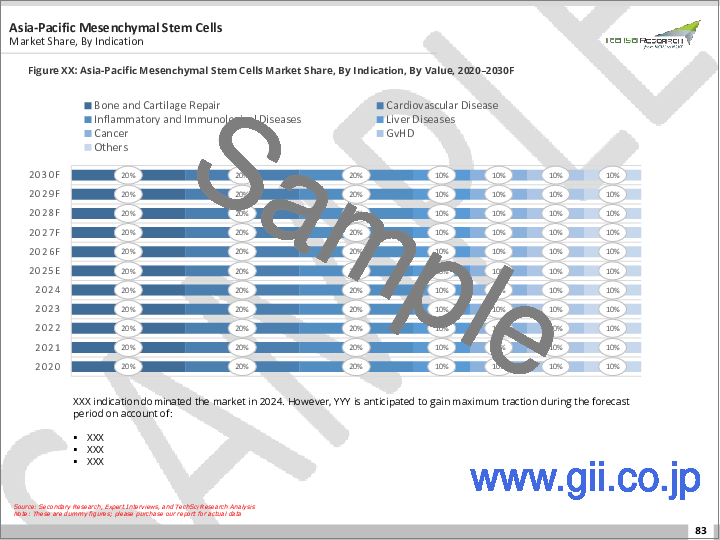
- 適応症別(骨および軟骨の修復、心血管疾患、炎症および免疫疾患、肝疾患、がん、GVHD、その他)
- 用途別(疾患モデリング、医薬品開発・発見、幹細胞バンキング、組織工学、毒性学研究、その他)
- 地域別
- 企業別(2024)
- 市場マップ
第6章 北米の間葉系幹細胞市場展望
- 市場規模・予測
- 市場シェア・予測
- 北米:国別分析
- 米国
- カナダ
- メキシコ
第7章 欧州の間葉系幹細胞市場展望
- 市場規模・予測
- 市場シェア・予測
- 欧州:国別分析
- ドイツ
- 英国
- イタリア
- フランス
- スペイン
第8章 アジア太平洋地域の間葉系幹細胞市場展望
- 市場規模・予測
- 市場シェア・予測
- アジア太平洋地域:国別分析
- 中国
- インド
- 日本
- 韓国
- オーストラリア
第9章 南米の間葉系幹細胞市場展望
- 市場規模・予測
- 市場シェア・予測
- 南米:国別分析
- ブラジル
- アルゼンチン
- コロンビア
第10章 中東・アフリカの間葉系幹細胞市場展望
- 市場規模・予測
- 市場シェア・予測
- 中東・アフリカ:国別分析
- 南アフリカ
- サウジアラビア
- アラブ首長国連邦
第11章 市場力学
- 促進要因
- 課題
第12章 市場動向と発展
- 合併と買収
- 製品開発
- 最近の動向
第13章 世界の間葉系幹細胞市場:SWOT分析
第14章 競合情勢
- Thermo Fisher Scientific, Inc
- Cell Applications, Inc
- Axol Biosciences Ltd
- Cytori Therapeutics Inc
- STEMCELL Technologies
- Cyagen Biosciences
- Celprogen Inc
- BrainStorm Cell Limited
- Stemedica Cell Technologies Inc
- Merck KGaA(MilliporeSigma)
- PromoCell GmbH
第15章 戦略的提言
第16章 調査会社について・免責事項
The Global Mesenchymal Stem Cells (MSCs) Market was valued at USD 2.71 billion in 2024 and is projected to grow at a CAGR of 8.54% through 2030. This market is gaining traction due to rapid advancements in regenerative medicine, increased clinical trial activity, and rising awareness of the therapeutic potential of MSCs. Known for their ability to differentiate into various cell types such as bone, cartilage, and adipose tissues, MSCs have shown promise in treating a wide range of medical conditions. Their immunomodulatory and anti-inflammatory properties, combined with low immunogenicity, make them suitable for applications in orthopedics, neurology, cardiology, and autoimmune diseases. Increasing research investments and favorable demographic trends, including an aging population with rising incidences of chronic conditions, are further propelling the market. The scope of MSC applications continues to broaden, encompassing emerging areas such as dermatology and oncology, while technological innovations and expanding clinical studies underline the potential for MSCs in both therapeutic and research domains.
| Market Overview | |
|---|---|
| Forecast Period | 2026-2030 |
| Market Size 2024 | USD 2.71 Billion |
| Market Size 2030 | USD 4.40 Billion |
| CAGR 2025-2030 | 8.54% |
| Fastest Growing Segment | Allogenic |
| Largest Market | North America |
Key Market Drivers
Rising Prevalence of Chronic Diseases
The growing burden of chronic illnesses such as diabetes, cardiovascular diseases, neurodegenerative conditions, and arthritis is significantly driving demand for mesenchymal stem cell-based therapies. As these long-term diseases impose substantial health and economic burdens globally, MSCs offer a regenerative and immunomodulatory approach that addresses underlying tissue damage and inflammation. Their application in chronic disease management is supported by increasing interest in cell-based therapies that promote healing and reduce dependency on symptomatic treatments. The World Health Organization attributes approximately 71% of global deaths to chronic diseases, underscoring the urgent need for innovative therapies like MSCs, which are becoming integral to modern medical approaches.
Key Market Challenges
Regulatory Challenges
Navigating the regulatory environment presents a key challenge for the MSC market. Regulations vary significantly across regions, affecting the approval, manufacturing, and commercialization of MSC-based products. Adhering to Good Manufacturing Practices (GMP) and gaining regulatory approvals can be time-consuming and financially burdensome. The lack of globally harmonized standards also complicates market entry for developers and manufacturers. As the demand for MSC therapies rises, the development of streamlined and unified regulatory pathways will be critical to facilitating wider access and accelerating growth in the global market.
Key Market Trends
Technological Advancements
Technological innovations are transforming the MSC landscape, enabling more efficient and scalable production, enhanced therapeutic efficacy, and targeted delivery. Advances in bioreactor technology, 3D scaffolds, and microfluidic systems have improved the reproducibility and yield of MSC cultures. Tools like CRISPR-Cas9 are being used to genetically modify MSCs to enhance regenerative or immunological capabilities. Progress in biomaterials and drug delivery platforms allows for precise MSC deployment, improving clinical outcomes. Automation and robotic systems are streamlining MSC manufacturing, while imaging techniques like MRI and bioluminescent imaging provide real-time tracking of therapeutic cells. These advancements collectively support the expansion of MSC-based applications and ensure consistency in product quality, aiding in broader adoption across healthcare systems.
Key Market Players
- Thermo Fisher Scientific, Inc
- Cell Applications, Inc
- Axol Biosciences Ltd
- Cytori Therapeutics Inc
- STEMCELL Technologies
- Cyagen Biosciences
- Celprogen Inc
- BrainStorm Cell Limited
- Stemedica Cell Technologies Inc
- Merck KGaA (MilliporeSigma)
- PromoCell GmbH
Report Scope:
In this report, the Global Mesenchymal Stem Cells Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:
Mesenchymal Stem Cells Market, By Product & Services:
- Product
- Services
Mesenchymal Stem Cells Market, By Workflow:
- Cell Sourcing & Isolation
- Culture & Cryopreservation
- Differentiation
- Characterization
Mesenchymal Stem Cells Market, By Type:
- Autologous
- Allogeneic
Mesenchymal Stem Cells Market, By Source of Isolation:
- Bone Marrow
- Cord Blood
- Peripheral Blood
- Fallopian Tube
- Fetal Liver
- Lung
- Adipose Tissues
Mesenchymal Stem Cells Market, By Indication:
- Bone And Cartilage Repair
- Cardiovascular Disease
- Inflammatory And Immunological Diseases
- Liver Diseases
- Cancer
- GvHD
- Others
Mesenchymal Stem Cells Market, By Application:
- Disease Modelling
- Drug Development & Discovery
- Stem Cell Banking
- Tissue Engineering
- Toxicology Studies
- Others
Mesenchymal Stem Cells Market, By Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Mesenchymal Stem Cells Market.
Available Customizations:
Global Mesenchymal Stem Cells market report with the given market data, TechSci Research offers customizations according to a company's specific needs. The following customization options are available for the report:
Company Information
- Detailed analysis and profiling of additional market players (up to five).
Table of Contents
1. Product Overview
- 1.1. Market Definition
- 1.2. Scope of the Market
- 1.2.1. Markets Covered
- 1.2.2. Years Considered for Study
- 1.2.3. Key Market Segmentations
2. Research Methodology
- 2.1. Objective of the Study
- 2.2. Baseline Methodology
- 2.3. Key Industry Partners
- 2.4. Major Association and Secondary Sources
- 2.5. Forecasting Methodology
- 2.6. Data Triangulation & Validation
- 2.7. Assumptions and Limitations
3. Executive Summary
4. Voice of Customer
5. Global Mesenchymal Stem Cells Market Outlook
- 5.1. Market Size & Forecast
- 5.1.1. By Value
- 5.2. Market Share & Forecast
- 5.2.1. By Product & Services {Products (Cells & Cell Lines, Kits, Media, & Reagents, Others), Services}
- 5.2.2. By Workflow (Cell Sourcing & Isolation, Culture & Cryopreservation, Differentiation, Characterization)
- 5.2.3. By Type (Autologous, Allogeneic)
- 5.2.4. By Source of Isolation (Bone Marrow, Cord Blood, Peripheral Blood, Fallopian Tube, Fetal Liver, Lung, Adipose Tissues)
- 5.2.5. By Indication (Bone and Cartilage Repair, Cardiovascular Disease, Inflammatory And Immunological Diseases, Liver Diseases, Cancer, GvHD, Others)
- 5.2.6. By Application (Disease Modelling, Drug Development & Discovery, Stem Cell Banking, Tissue Engineering, Toxicology Studies, Others)
- 5.2.7. By Region
- 5.2.8. By Company (2024)
- 5.3. Market Map
6. North America Mesenchymal Stem Cells Market Outlook
- 6.1. Market Size & Forecast
- 6.1.1. By Value
- 6.2. Market Share & Forecast
- 6.2.1. By Product & Services
- 6.2.2. By Workflow
- 6.2.3. By Type
- 6.2.4. By Source of Isolation
- 6.2.5. By Indication
- 6.2.6. By Application
- 6.2.7. By Country
- 6.3. North America: Country Analysis
- 6.3.1. United States Mesenchymal Stem Cells Market Outlook
- 6.3.1.1. Market Size & Forecast
- 6.3.1.1.1. By Value
- 6.3.1.2. Market Share & Forecast
- 6.3.1.2.1. By Product & Services
- 6.3.1.2.2. By Workflow
- 6.3.1.2.3. By Type
- 6.3.1.2.4. By Source of Isolation
- 6.3.1.2.5. By Indication
- 6.3.1.2.6. By Application
- 6.3.1.1. Market Size & Forecast
- 6.3.2. Canada Mesenchymal Stem Cells Market Outlook
- 6.3.2.1. Market Size & Forecast
- 6.3.2.1.1. By Value
- 6.3.2.2. Market Share & Forecast
- 6.3.2.2.1. By Product & Services
- 6.3.2.2.2. By Workflow
- 6.3.2.2.3. By Type
- 6.3.2.2.4. By Source of Isolation
- 6.3.2.2.5. By Indication
- 6.3.2.2.6. By Application
- 6.3.2.1. Market Size & Forecast
- 6.3.3. Mexico Mesenchymal Stem Cells Market Outlook
- 6.3.3.1. Market Size & Forecast
- 6.3.3.1.1. By Value
- 6.3.3.2. Market Share & Forecast
- 6.3.3.2.1. By Product & Services
- 6.3.3.2.2. By Workflow
- 6.3.3.2.3. By Type
- 6.3.3.2.4. By Source of Isolation
- 6.3.3.2.5. By Indication
- 6.3.3.2.6. By Application
- 6.3.3.1. Market Size & Forecast
- 6.3.1. United States Mesenchymal Stem Cells Market Outlook
7. Europe Mesenchymal Stem Cells Market Outlook
- 7.1. Market Size & Forecast
- 7.1.1. By Value
- 7.2. Market Share & Forecast
- 7.2.1. By Product & Services
- 7.2.2. By Workflow
- 7.2.3. By Type
- 7.2.4. By Source of Isolation
- 7.2.5. By Indication
- 7.2.6. By Application
- 7.2.7. By Country
- 7.3. Europe: Country Analysis
- 7.3.1. Germany Mesenchymal Stem Cells Market Outlook
- 7.3.1.1. Market Size & Forecast
- 7.3.1.1.1. By Value
- 7.3.1.2. Market Share & Forecast
- 7.3.1.2.1. By Product & Services
- 7.3.1.2.2. By Workflow
- 7.3.1.2.3. By Type
- 7.3.1.2.4. By Source of Isolation
- 7.3.1.2.5. By Indication
- 7.3.1.2.6. By Application
- 7.3.1.1. Market Size & Forecast
- 7.3.2. United Kingdom Mesenchymal Stem Cells Market Outlook
- 7.3.2.1. Market Size & Forecast
- 7.3.2.1.1. By Value
- 7.3.2.2. Market Share & Forecast
- 7.3.2.2.1. By Product & Services
- 7.3.2.2.2. By Workflow
- 7.3.2.2.3. By Type
- 7.3.2.2.4. By Source of Isolation
- 7.3.2.2.5. By Indication
- 7.3.2.2.6. By Application
- 7.3.2.1. Market Size & Forecast
- 7.3.3. Italy Mesenchymal Stem Cells Market Outlook
- 7.3.3.1. Market Size & Forecast
- 7.3.3.1.1. By Value
- 7.3.3.2. Market Share & Forecasty
- 7.3.3.2.1. By Product & Services
- 7.3.3.2.2. By Workflow
- 7.3.3.2.3. By Type
- 7.3.3.2.4. By Source of Isolation
- 7.3.3.2.5. By Indication
- 7.3.3.2.6. By Application
- 7.3.3.1. Market Size & Forecast
- 7.3.4. France Mesenchymal Stem Cells Market Outlook
- 7.3.4.1. Market Size & Forecast
- 7.3.4.1.1. By Value
- 7.3.4.2. Market Share & Forecast
- 7.3.4.2.1. By Product & Services
- 7.3.4.2.2. By Workflow
- 7.3.4.2.3. By Type
- 7.3.4.2.4. By Source of Isolation
- 7.3.4.2.5. By Indication
- 7.3.4.2.6. By Application
- 7.3.4.1. Market Size & Forecast
- 7.3.5. Spain Mesenchymal Stem Cells Market Outlook
- 7.3.5.1. Market Size & Forecast
- 7.3.5.1.1. By Value
- 7.3.5.2. Market Share & Forecast
- 7.3.5.2.1. By Product & Services
- 7.3.5.2.2. By Workflow
- 7.3.5.2.3. By Type
- 7.3.5.2.4. By Source of Isolation
- 7.3.5.2.5. By Indication
- 7.3.5.2.6. By Application
- 7.3.5.1. Market Size & Forecast
- 7.3.1. Germany Mesenchymal Stem Cells Market Outlook
8. Asia-Pacific Mesenchymal Stem Cells Market Outlook
- 8.1. Market Size & Forecast
- 8.1.1. By Value
- 8.2. Market Share & Forecast
- 8.2.1. By Product & Services
- 8.2.2. By Workflow
- 8.2.3. By Type
- 8.2.4. By Source of Isolation
- 8.2.5. By Indication
- 8.2.6. By Application
- 8.2.7. By Country
- 8.3. Asia-Pacific: Country Analysis
- 8.3.1. China Mesenchymal Stem Cells Market Outlook
- 8.3.1.1. Market Size & Forecast
- 8.3.1.1.1. By Value
- 8.3.1.2. Market Share & Forecast
- 8.3.1.2.1. By Product & Services
- 8.3.1.2.2. By Workflow
- 8.3.1.2.3. By Type
- 8.3.1.2.4. By Source of Isolation
- 8.3.1.2.5. By Indication
- 8.3.1.2.6. By Application
- 8.3.1.1. Market Size & Forecast
- 8.3.2. India Mesenchymal Stem Cells Market Outlook
- 8.3.2.1. Market Size & Forecast
- 8.3.2.1.1. By Value
- 8.3.2.2. Market Share & Forecast
- 8.3.2.2.1. By Product & Services
- 8.3.2.2.2. By Workflow
- 8.3.2.2.3. By Type
- 8.3.2.2.4. By Source of Isolation
- 8.3.2.2.5. By Indication
- 8.3.2.2.6. By Application
- 8.3.2.1. Market Size & Forecast
- 8.3.3. Japan Mesenchymal Stem Cells Market Outlook
- 8.3.3.1. Market Size & Forecast
- 8.3.3.1.1. By Value
- 8.3.3.2. Market Share & Forecast
- 8.3.3.2.1. By Product & Services
- 8.3.3.2.2. By Workflow
- 8.3.3.2.3. By Type
- 8.3.3.2.4. By Source of Isolation
- 8.3.3.2.5. By Indication
- 8.3.3.2.6. By Application
- 8.3.3.1. Market Size & Forecast
- 8.3.4. South Korea Mesenchymal Stem Cells Market Outlook
- 8.3.4.1. Market Size & Forecast
- 8.3.4.1.1. By Value
- 8.3.4.2. Market Share & Forecast
- 8.3.4.2.1. By Product & Services
- 8.3.4.2.2. By Workflow
- 8.3.4.2.3. By Type
- 8.3.4.2.4. By Source of Isolation
- 8.3.4.2.5. By Indication
- 8.3.4.2.6. By Application
- 8.3.4.1. Market Size & Forecast
- 8.3.5. Australia Mesenchymal Stem Cells Market Outlook
- 8.3.5.1. Market Size & Forecast
- 8.3.5.1.1. By Value
- 8.3.5.2. Market Share & Forecast
- 8.3.5.2.1. By Product & Services
- 8.3.5.2.2. By Workflow
- 8.3.5.2.3. By Type
- 8.3.5.2.4. By Source of Isolation
- 8.3.5.2.5. By Indication
- 8.3.5.2.6. By Application
- 8.3.5.1. Market Size & Forecast
- 8.3.1. China Mesenchymal Stem Cells Market Outlook
9. South America Mesenchymal Stem Cells Market Outlook
- 9.1. Market Size & Forecast
- 9.1.1. By Value
- 9.2. Market Share & Forecast
- 9.2.1. By Product & Services
- 9.2.2. By Workflow
- 9.2.3. By Type
- 9.2.4. By Source of Isolation
- 9.2.5. By Indication
- 9.2.6. By Application
- 9.2.7. By Country
- 9.3. South America: Country Analysis
- 9.3.1. Brazil Mesenchymal Stem Cells Market Outlook
- 9.3.1.1. Market Size & Forecast
- 9.3.1.1.1. By Value
- 9.3.1.2. Market Share & Forecast
- 9.3.1.2.1. By Product & Services
- 9.3.1.2.2. By Workflow
- 9.3.1.2.3. By Type
- 9.3.1.2.4. By Source of Isolation
- 9.3.1.2.5. By Indication
- 9.3.1.2.6. By Application
- 9.3.1.1. Market Size & Forecast
- 9.3.2. Argentina Mesenchymal Stem Cells Market Outlook
- 9.3.2.1. Market Size & Forecast
- 9.3.2.1.1. By Value
- 9.3.2.2. Market Share & Forecast
- 9.3.2.2.1. By Product & Services
- 9.3.2.2.2. By Workflow
- 9.3.2.2.3. By Type
- 9.3.2.2.4. By Source of Isolation
- 9.3.2.2.5. By Indication
- 9.3.2.2.6. By Application
- 9.3.2.1. Market Size & Forecast
- 9.3.3. Colombia Mesenchymal Stem Cells Market Outlook
- 9.3.3.1. Market Size & Forecast
- 9.3.3.1.1. By Value
- 9.3.3.2. Market Share & Forecast
- 9.3.3.2.1. By Product & Services
- 9.3.3.2.2. By Workflow
- 9.3.3.2.3. By Type
- 9.3.3.2.4. By Source of Isolation
- 9.3.3.2.5. By Indication
- 9.3.3.2.6. By Application
- 9.3.3.1. Market Size & Forecast
- 9.3.1. Brazil Mesenchymal Stem Cells Market Outlook
10. Middle East and Africa Mesenchymal Stem Cells Market Outlook
- 10.1. Market Size & Forecast
- 10.1.1. By Value
- 10.2. Market Share & Forecast
- 10.2.1. By Product & Services
- 10.2.2. By Workflow
- 10.2.3. By Type
- 10.2.4. By Source of Isolation
- 10.2.5. By Indication
- 10.2.6. By Application
- 10.2.7. By Country
- 10.3. MEA: Country Analysis
- 10.3.1. South Africa Mesenchymal Stem Cells Market Outlook
- 10.3.1.1. Market Size & Forecast
- 10.3.1.1.1. By Value
- 10.3.1.2. Market Share & Forecast
- 10.3.1.2.1. By Product & Services
- 10.3.1.2.2. By Workflow
- 10.3.1.2.3. By Type
- 10.3.1.2.4. By Source of Isolation
- 10.3.1.2.5. By Indication
- 10.3.1.2.6. By Application
- 10.3.1.1. Market Size & Forecast
- 10.3.2. Saudi Arabia Mesenchymal Stem Cells Market Outlook
- 10.3.2.1. Market Size & Forecast
- 10.3.2.1.1. By Value
- 10.3.2.2. Market Share & Forecast
- 10.3.2.2.1. By Product & Services
- 10.3.2.2.2. By Workflow
- 10.3.2.2.3. By Type
- 10.3.2.2.4. By Source of Isolation
- 10.3.2.2.5. By Indication
- 10.3.2.2.6. By Application
- 10.3.2.1. Market Size & Forecast
- 10.3.3. UAE Mesenchymal Stem Cells Market Outlook
- 10.3.3.1. Market Size & Forecast
- 10.3.3.1.1. By Value
- 10.3.3.2. Market Share & Forecast
- 10.3.3.2.1. By Product & Services
- 10.3.3.2.2. By Workflow
- 10.3.3.2.3. By Type
- 10.3.3.2.4. By Source of Isolation
- 10.3.3.2.5. By Indication
- 10.3.3.2.6. By Application
- 10.3.3.1. Market Size & Forecast
- 10.3.1. South Africa Mesenchymal Stem Cells Market Outlook
11. Market Dynamics
- 11.1. Drivers
- 11.2. Challenges
12. Market Trends & Developments
- 12.1. Merger & Acquisition
- 12.2. Product Development
- 12.3. Recent Developments
13. Global Mesenchymal Stem Cells Market: SWOT Analysis
14. Competitive Landscape
- 14.1. Thermo Fisher Scientific, Inc
- 14.1.1. Business Overview
- 14.1.2. Age Group Offerings
- 14.1.3. Recent Developments
- 14.1.4. Key Personnel
- 14.1.5. SWOT Analysis
- 14.2. Cell Applications, Inc
- 14.3. Axol Biosciences Ltd
- 14.4. Cytori Therapeutics Inc
- 14.5. STEMCELL Technologies
- 14.6. Cyagen Biosciences
- 14.7. Celprogen Inc
- 14.8. BrainStorm Cell Limited
- 14.9. Stemedica Cell Technologies Inc
- 14.10. Merck KGaA (MilliporeSigma)
- 14.11. PromoCell GmbH