|
|
市場調査レポート
商品コード
1494303
北米の感染症診断市場予測(~2030年):地域別分析 - 製品、技術、用途タイプ、検査タイプ、エンドユーザー別North America Infectious Disease Diagnostics Market Forecast to 2030 - Regional Analysis - By Product, Technology, Application Type, Testing Type, and End User |
||||||
|
|||||||
| 北米の感染症診断市場予測(~2030年):地域別分析 - 製品、技術、用途タイプ、検査タイプ、エンドユーザー別 |
|
出版日: 2024年03月14日
発行: The Insight Partners
ページ情報: 英文 115 Pages
納期: 即納可能
|
全表示
- 概要
- 図表
- 目次
北米の感染症診断の市場規模は、2022年に186億8,443万米ドルに達し、2022年~2030年にCAGR5.5%で成長し、2030年には287億1,807万米ドルに達すると予測されています。
感染症診断の研究開発への注力と資金調達の増加が北米の感染症診断市場を牽引
研究開発(R&D)は、製薬・バイオ医薬品企業の事業にとって不可欠な要素です。研究開発により、市場参加企業は、医学的・商業的に大きな可能性を秘めた様々な治療用途の新製品を開発することができます。さらに、新興国市場の大手企業は、先端技術を開発し、より多くの収益を上げるために研究開発に投資しています。感染症患者数の増加や検査室の能力不足、分子検査試薬の不足に対応するため、一部の診断検査メーカーは、検査室外での検査を容易にするため、迅速で使いやすい機器を提供しています。簡易検査キットは、検体から病原体のタンパク質を検出したり、血液や血清中の感染に反応して産生されるヒト抗体を検出したりします。
以下は、メーカーが行っているいくつかの主要な資金調達イニシアチブのリストです:
- 2022年3月、世界エイズ・マラリア・結核対策基金は、カナダが世界基金のCOVID-19対応メカニズム(C19RM)に6,000万カナダドル(4,399万米ドル)を拠出することを決定したと発表しました。この資金は、救命のための診断検査、治療、個人防護具(PPE)を中低所得国に提供するために役立てられます。
- 2021年9月、バイデン-ハリス政権は、米国の公衆衛生・ヘルスケア部門全体の感染制御・予防活動を強化するために21億米ドルを投資しました。バイデン-ハリス政権は、CDCを通じて、米国の救済プログラムに資金を投じ、米国の感染症について州、地方、地域の保健局やその他のパートナー組織に対処しました。
このように、感染症診断における研究開発・資金調達への注目の高まりは、北米の感染症診断市場の成長に有利な機会を生み出すと期待されています。
北米の感染症診断市場概要
米国における感染症診断市場の成長は、主に感染症の流行増加、高齢者人口の増加、主要企業による製品上市数の増加によってもたらされます。一般的に60歳以上の高齢者は免疫力が低下しているため、高齢化は感染症の顕著なリスク要因です。人口問題研究所が2020年に発表した調査によると、米国の65歳以上の人口は2020年には5,500万人、2060年には9,500万人に達すると予想されています。米国の規制当局は、さまざまな適応症の診断や治療のためのポイントオブケア(POC)製品の開発に有利な政策を課しています。例えば、2021年3月、米国食品医薬品局(FDA)はBinx Health IO CT/NG Assayを地域密着型クリニック、緊急医療環境、外来医療施設向けに認可しました。これはクラミジア感染症・淋菌感染症を診断する初のPOC検査製品です。2022年5月、世界有数の医療技術企業であるベクトン・ディッキンソンアンドカンパニー(BD)は、米国市場向けに新しい感染症用全自動ハイスループット分子診断プラットフォームの発売を発表しました。新しいBD COR MX装置は、BD CORプラットフォームの分析装置オプションとして、FDAから510(k)の認可を得て発売されました。BD CTGCTV2分子アッセイは、新システムで利用できる最初の検査で、最も流行している3つの非ウイルス性感染症(STI)、淋菌(GC)、クラミジア・トラコマティス(CT)、トリコモナス膣炎(TV)を検出する単一検査です。
北米の感染症診断市場の収益と2030年までの予測(金額)
北米の感染症診断市場セグメンテーション
北米の感染症診断市場は、製品、技術、用途タイプ、検査タイプ、エンドユーザー、国によって区分されます。
製品別では、北米の感染症診断市場はキット・試薬、機器、ソフトウェア・サービスに区分されます。2022年にはキット・試薬セグメントが最大シェアを占めました。
技術別では、北米の感染症診断市場はバイオセンサー、免疫診断、分子ベース診断技術、臨床生物学、その他に区分されます。2022年には免疫診断法が最大のシェアを占める。免疫診断法セグメントはさらに、酵素結合免疫吸着分析法(ELISA)、ウェスタンブロット分析法、免疫蛍光分析法に細分化されます。分子ベース診断技術セグメントは、さらにポリメラーゼ連鎖反応(PCR)、in-situハイブリダイゼーション、等温核酸増幅技術(INAAT)、次世代シーケンシング(NGS)、DNAマイクロアレイ、その他に細分化されます。
用途タイプ別に見ると、北米の感染症診断市場はラボ検査とポイントオブケア検査に二分されます。2022年はラボ検査セグメントが大きなシェアを占めています。
検査タイプ別では、北米の感染症診断市場はヒト用検査と動物用検査に二分されます。2022年にはヒト検査セグメントが大きなシェアを占めています。ヒト検査セグメントはさらにHIV、肝炎、HAI、HPV、結核、インフルエンザ、その他に細分化されます。
エンドユーザー別では、北米の感染症診断市場は診断ラボ、病院・診療所、研究機関、在宅医療、その他に区分されます。2022年には診断ラボセグメントが最大のシェアを占めました。
国別では、北米の感染症診断市場は米国、カナダ、メキシコに区分されます。2022年の北米の感染症診断市場は米国が支配的でした。
Abbott Laboratories、Bruker Corp、Cardinal Health Inc、F. Hoffmann-La Roche Ltd、Trinity Biotech Plc、Danaher Corp、Bio-Rad Laboratories Inc、ACON Laboratories Inc、DiaSorin SpAなどが北米の感染症診断市場で事業を展開する大手企業です。
目次
第1章 イントロダクション
第2章 エグゼクティブサマリー
- 主要洞察
- 北米の感染症診断市場:国別
第3章 調査手法
- 調査範囲
- 2次調査
- 1次調査
第4章 北米の感染症診断市場- 主要産業力学
- 市場促進要因
- 感染症罹患率の上昇
- 動物感染症における診断薬の用途拡大
- 市場抑制要因
- 不十分な償還シナリオ
- 市場機会
- 感染症診断における研究開発と資金調達の増加
- 今後の動向
- 製品の承認数と上市数の増加
- 影響分析
第5章 感染症診断市場:北米市場分析
- 北米の感染症診断市場収益(2022年~2030年)
第6章 北米の感染症診断市場 - 2030年までの収益と予測:製品別
- 北米の感染症診断市場の収益シェア:製品別(2022年・2030年)
- キット・試薬
- 機器
- ソフトウェア・サービス
第7章 北米の感染症診断市場 - 2030年までの収益と予測:技術別
- 北米の感染症診断市場の収益シェア:技術別(2022年・2030年)
- バイオセンサーベース
- 免疫診断技術
- 分子ベースの診断技術
- 臨床生物学
- その他
第8章 北米の感染症診断市場 - 2030年までの収益と予測:用途タイプ別
- 北米の感染症診断市場の収益シェア:用途タイプ別(2022年・2030年)
- ポイントオブケア検査
- ラボ検査
第9章 北米の感染症診断市場 - 2030年までの収益と予測:検査タイプ別
- 北米の感染症診断市場の収益シェア:検査タイプ別(2022年・2030年)
- ヒト用検査
- 動物用検査
第10章 北米の感染症診断市場 - 2030年までの収益と予測:エンドユーザー別
- 北米の感染症診断市場の収益シェア:エンドユーザー別(2022年・2030年)
- 診断研究所
- 病院・診療所
- 研究機関
- 在宅ケア環境
- その他
第11章 北米の感染症診断市場:国別分析
- 米国
- カナダ
- メキシコ
第12章 業界情勢
- 市場参入企業の成長戦略(%)
- 有機的発展
- 無機的発展
第13章 企業プロファイル
- Abbott Laboratories
- Bruker Corp
- Cardinal Health Inc
- F. Hoffmann-La Roche Ltd
- Trinity Biotech Plc
- Danaher Corp
- Bio-Rad Laboratories Inc
- ACON Laboratories Inc
- DiaSorin SpA
第14章 付録
List Of Tables
- Table 1. North America Infectious Disease Diagnostics Market Segmentation
- Table 2. Infectious Diseases and Fundings
- Table 3. North America Infectious Disease Diagnostics Market, for Immunodiagnostics by Technology - Revenue and Forecast to 2030 (US$ Million)
- Table 4. North America Infectious Disease Diagnostics Market, for Molecular-Based Diagnostic Techniques by Technology - Revenue and Forecast to 2030 (US$ Million)
- Table 5. North America Infectious Disease Diagnostics Market, for Human Testing by Testing Type - Revenue and Forecast to 2030 (US$ Million)
- Table 6. US: North America Infectious Disease Diagnostics Market, by Product - Revenue and Forecast to 2030 (US$ Million)
- Table 7. US: North America Infectious Disease Diagnostics Market, by Technology - Revenue and Forecast to 2030 (US$ Million)
- Table 8. US North America Infectious Disease Diagnostics Market, for Immunodiagnostics by Technology - Revenue and Forecast to 2030 (US$ Million)
- Table 9. US: North America Infectious Disease Diagnostics Market, for Molecular-Based Diagnostic Techniques by Technology - Revenue and Forecast to 2030 (US$ Million)
- Table 10. US: North America Infectious Disease Diagnostics Market, by Application Type - Revenue and Forecast to 2030 (US$ Million)
- Table 11. US: North America Infectious Disease Diagnostics Market, by Testing Type - Revenue and Forecast to 2030 (US$ Million)
- Table 12. US: North America Infectious Disease Diagnostics Market, for Human Testing by Testing Type - Revenue and Forecast to 2030 (US$ Million)
- Table 13. US: North America Infectious Disease Diagnostics Market, by End User - Revenue and Forecast to 2030 (US$ Million)
- Table 14. Canada: North America Infectious Disease Diagnostics Market, by Product - Revenue and Forecast to 2030 (US$ Million)
- Table 15. Canada: North America Infectious Disease Diagnostics Market, by Technology - Revenue and Forecast to 2030 (US$ Million)
- Table 16. Canada: North America Infectious Disease Diagnostics Market, for Immunodiagnostics by Technology - Revenue and Forecast to 2030 (US$ Million)
- Table 17. Canada: North America Infectious Disease Diagnostics Market, for Molecular-Based Diagnostic Techniques by Technology - Revenue and Forecast to 2030 (US$ Million)
- Table 18. Canada: North America Infectious Disease Diagnostics Market, by Application Type - Revenue and Forecast to 2030 (US$ Million)
- Table 19. Canada: North America Infectious Disease Diagnostics Market, by Testing Type - Revenue and Forecast to 2030 (US$ Million)
- Table 20. Canada: North America Infectious Disease Diagnostics Market, for Human Testing by Testing Type - Revenue and Forecast to 2030 (US$ Million)
- Table 21. Canada: North America Infectious Disease Diagnostics Market, by End User - Revenue and Forecast to 2030 (US$ Million)
- Table 22. Mexico: North America Infectious Disease Diagnostics Market, by Product - Revenue and Forecast to 2030 (US$ Million)
- Table 23. Mexico: North America Infectious Disease Diagnostics Market, by Technology - Revenue and Forecast to 2030 (US$ Million)
- Table 24. Mexico: North America Infectious Disease Diagnostics Market, for Immunodiagnostics by Technology - Revenue and Forecast to 2030 (US$ Million)
- Table 25. Mexico: North America Infectious Disease Diagnostics Market, for Molecular-Based Diagnostic Techniques by Technology - Revenue and Forecast to 2030 (US$ Million)
- Table 26. Mexico: North America Infectious Disease Diagnostics Market, by Application Type - Revenue and Forecast to 2030 (US$ Million)
- Table 27. Mexico: North America Infectious Disease Diagnostics Market, by Testing Type - Revenue and Forecast to 2030 (US$ Million)
- Table 28. Mexico: North America Infectious Disease Diagnostics Market, for Human Testing by Testing Type - Revenue and Forecast to 2030 (US$ Million)
- Table 29. Mexico: North America Infectious Disease Diagnostics Market, by End User - Revenue and Forecast to 2030 (US$ Million)
- Table 30. Organic Developments Done By Companies
- Table 31. Inorganic Developments Done By Companies
- Table 32. Glossary of Terms, North America Infectious Disease Diagnostics Market
List Of Figures
- Figure 1. North America Infectious Disease Diagnostics Market Segmentation, By Country
- Figure 2. North America Infectious Disease Diagnostics Market - Key Industry Dynamics
- Figure 3. Impact Analysis of Drivers and Restraints
- Figure 4. North America Infectious Disease Diagnostics Market Revenue (US$ Mn), 2022 - 2030
- Figure 5. North America Infectious Disease Diagnostics Market Revenue Share, by Product, 2022 & 2030 (%)
- Figure 6. Kits & Reagents: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 7. Instruments: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 8. Software & Services: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 9. North America Infectious Disease Diagnostics Market Revenue Share, by Technology, 2022 & 2030 (%)
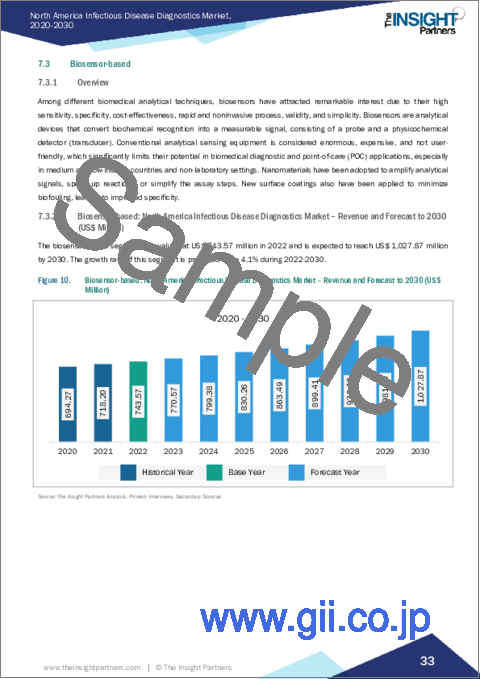
- Figure 10. Biosensor-based: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 11. Immunodiagnostics: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 12. Molecular-Based Diagnostic Techniques: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 13. Clinical Biology: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 14. Others: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 15. North America Infectious Disease Diagnostics Market Revenue Share, by Application Type, 2022 & 2030 (%)
- Figure 16. Point-of-Care Testing: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 17. Laboratory Testing: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 18. North America Infectious Disease Diagnostics Market Revenue Share, by Testing Type, 2022 & 2030 (%)
- Figure 19. Human Testing: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 20. Veterinary Testing: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 21. North America Infectious Disease Diagnostics Market Revenue Share, by End User, 2022 & 2030 (%)
- Figure 22. Diagnostic Laboratories: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 23. Hospitals and Clinics: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 24. Research Institutes: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 25. Homecare Settings: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 26. Others: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- Figure 27. North America: Infectious Disease Diagnostics Market, by Key Country - Revenue (2022) (US$ Million)
- Figure 28. North America Infectious Disease Diagnostics Market, By Key Countries, 2022 and 2030 (%)
- Figure 29. US: North America Infectious Disease Diagnostics Market Revenue and Forecast to 2030 (US$ Mn)
- Figure 30. Canada: North America Infectious Disease Diagnostics Market Revenue and Forecast to 2030 (US$ Mn)
- Figure 31. Mexico: North America Infectious Disease Diagnostics Market Revenue and Forecast to 2030 (US$ Mn)
- Figure 32. Growth Strategies Done by the Companies in the Market, (%)
The North America infectious disease diagnostics market was valued at US$ 18,684.43 million in 2022 and is expected to reach US$ 28,718.07 million by 2030; it is estimated to grow at a CAGR of 5.5% from 2022 to 2030.
Increasing Focus on R&D and Funding in Infectious Disease Diagnostics Drive North America Infectious Disease Diagnostics Market
Research and development (R&D) is an essential component of the business of pharmaceutical and biopharmaceutical companies. R&D enables market players to come up with new products for various therapeutic applications with significant medical and commercial potential. Moreover, leading market players invest in R&D to develop advanced technologies and generate more revenue. In response to the growing cases of infectious diseases and the shortage of laboratory capabilities and molecular testing reagents, some diagnostic testing manufacturers offer fast and easy-to-use devices to facilitate out-of-laboratory testing. The simple test kits detect proteins of pathogens from samples or human antibodies that are produced in response to infection in blood or serum.
Following is a list of a few key funding initiatives undertaken by the manufacturers:
- In March 2022, the Global Fund to Fight AIDS, Malaria, and Tuberculosis acclaimed the decision by Canada to contribute CAD 60 million (US$ 43.99 million) to the Global Fund's COVID-19 Response Mechanism (C19RM). The funding will help provide life-saving diagnostic tests, treatments, and personal protective equipment (PPE) to low- and middle-income countries.
- In September 2021, the Biden-Harris Administration invested US$ 2.1 billion to enhance infection control and prevention activities across the US public health and healthcare sectors. The Biden-Harris administration, operating through the CDC, invested funds in the US rescue programs to address state, local, and territorial health departments and other partner organizations about infectious diseases in the US.
Thus, rising focus on R&D and funding in infectious disease diagnostics is expected to create lucrative opportunities for the growth of the North America infectious disease diagnostics market.
North America Infectious Disease Diagnostics Market Overview
The infectious diseases diagnostics market growth in the US is primarily driven by the increasing prevalence of infectious diseases, rising geriatric population, and growing number of product launches by key players. Aging is a prominent risk factor for infectious diseases as people above 60 generally have compromised immunity. According to a study published by the Population Reference Bureau in 2020, the population of individuals aged 65 and above was 55 million in the US in 2020, and the number is expected to reach 95 million by 2060. Regulatory agencies in the US are imposing favorable policies for the development of point-of-care (POC) products for the diagnosis and treatment of various indications. For instance, in March 2021, the US Food Drug Administration (FDA) authorized Binx Health IO CT/NG Assay for community-based clinics, urgent care settings, and outpatient healthcare facilities; it is the first POC testing product for diagnosing chlamydial and gonorrheal infections. In May 2022, Becton Dickinson and Company (BD), one of the leading global medical technology companies, announced the launch of its new, fully automated, high-throughput molecular diagnostics platform for infectious diseases for the market in the US. The new BD COR MX instrument was launched as an analytical instrument option for the BD COR platform, with 510(k) clearance from the FDA. The BD CTGCTV2 molecular assay, the first test available on the new system, is a single test that detects the three most prevalent non-viral sexually transmitted infections (STIs)-Neisseria gonorrhoeae (GC), Chlamydia trachomatis (CT), and Trichomonas vaginalis (TV).
North America Infectious Disease Diagnostics Market Revenue and Forecast to 2030 (US$ Million)
North America Infectious Disease Diagnostics Market Segmentation
The North America infectious disease diagnostics market is segmented based on product, technology, application type, testing type, end user, and country.
Based on product, the North America infectious disease diagnostics market is segmented into kits & reagents, instruments, and software & services. The kits & reagents segment held the largest share in 2022.
By technology, the North America infectious disease diagnostics market is segmented into biosensor-based, immunodiagnostics, molecular-based diagnostic techniques, clinical biology, and others. The immunodiagnostics segment held the largest share in 2022. The immunodiagnostics segment is further subsegmented into enzyme-linked immunosorbent assay (ELISA), western blotting analysis, and immunofluorescence assay. The molecular-based diagnostic techniques segment is further subsegmented into polymerase chain reaction (PCR), in situ hybridization, isothermal nucleic acid amplification technology (INAAT), next-generation sequencing (NGS), DNA microarrays, and others.
By application type, the North America infectious disease diagnostics market is bifurcated into laboratory testing and point-of-care testing. The laboratory testing segment held a larger share in 2022.
By testing type, the North America infectious disease diagnostics market is bifurcated into human testing and veterinary testing. The human testing segment held a larger share in 2022. The human testing segment is further subsegmented into HIV, hepatitis, HAIs, HPV, tuberculosis, influenza, and others.
By end user, the North America infectious disease diagnostics market is segmented into diagnostic laboratories, hospitals & clinics, research institutes, homecare settings, and others. The diagnostic laboratories segment held the largest share in 2022.
Based on country, the North America infectious disease diagnostics market is segmented into the US, Canada, and Mexico. The US dominated the North America infectious disease diagnostics market in 2022.
Abbott Laboratories, Bruker Corp, Cardinal Health Inc, F. Hoffmann-La Roche Ltd, Trinity Biotech Plc, Danaher Corp, Bio-Rad Laboratories Inc, ACON Laboratories Inc, and DiaSorin SpA are some of the leading companies operating in the North America infectious disease diagnostics market.
Table Of Contents
1. Introduction
- 1.1 The Insight Partners Research Report Guidance
- 1.2 Market Segmentation
2. Executive Summary
- 2.1 Key Insights
- 2.2 North America Infectious Disease Diagnostics Market, by Country (US$ Million)
3. Research Methodology
- 3.1 Coverage
- 3.2 Secondary Research
- 3.3 Primary Research
4. North America Infectious Disease Diagnostics Market - Key Industry Dynamics
- 4.1 Market Drivers:
- 4.1.1 Rising Incidence of Infectious Diseases
- 4.1.2 Rising Application of Diagnostics in Veterinary Infectious Disease
- 4.2 Market Restraints
- 4.2.1 Inadequate Reimbursement Scenario
- 4.3 Market Opportunities
- 4.3.1 Increasing Focus on R&D and Funding in Infectious Disease Diagnostics
- 4.4 Future Trends
- 4.4.1 Rising Number of Product Approvals and Launches
- 4.5 Impact Analysis:
5. Infectious Disease Diagnostics Market - North America Market Analysis
- 5.1 North America Infectious Disease Diagnostics Market Revenue (US$ Mn), 2022 - 2030
6. North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 - by Product
- 6.1 Overview
- 6.2 North America Infectious Disease Diagnostics Market Revenue Share, by Product, 2022 & 2030 (%)
- 6.3 Kits & Reagents
- 6.3.1 Overview
- 6.3.2 Kits & Reagents: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- 6.4 Instruments
- 6.4.1 Overview
- 6.4.2 Instruments: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- 6.5 Software & Services
- 6.5.1 Overview
- 6.5.2 Software & Services: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
7. North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 - by Technology
- 7.1 Overview
- 7.2 North America Infectious Disease Diagnostics Market Revenue Share, by Technology, 2022 & 2030 (%)
- 7.3 Biosensor-based
- 7.3.1 Overview
- 7.3.2 Biosensor-based: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- 7.4 Immunodiagnostics
- 7.4.1 Overview
- 7.4.2 Immunodiagnostics: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- 7.4.2.1 North America Infectious Disease Diagnostics Market, for Immunodiagnostics by Technology, 2020-2030 (US$ Million)
- 7.5 Molecular-Based Diagnostic Techniques
- 7.5.1 Overview
- 7.5.2 Molecular-Based Diagnostic Techniques: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- 7.5.2.1 North America Infectious Disease Diagnostics Market, for Molecular-Based Diagnostic Techniques by Technology, 2020-2030 (US$ Million)
- 7.6 Clinical Biology
- 7.6.1 Overview
- 7.6.2 Clinical Biology: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- 7.7 Others
- 7.7.1 Overview
- 7.7.2 Others: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
8. North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 - by Application Type
- 8.1 Overview
- 8.2 North America Infectious Disease Diagnostics Market Revenue Share, by Application Type, 2022 & 2030 (%)
- 8.3 Point-of-Care Testing
- 8.3.1 Overview
- 8.3.2 Point-of-Care Testing: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- 8.4 Laboratory Testing
- 8.4.1 Overview
- 8.4.2 Laboratory Testing: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
9. North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 - by Testing Type
- 9.1 Overview
- 9.2 North America Infectious Disease Diagnostics Market Revenue Share, by Testing Type, 2022 & 2030 (%)
- 9.3 Human Testing
- 9.3.1 Overview
- 9.3.2 Human Testing: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- 9.3.2.1 North America Infectious Disease Diagnostics Market, for Human Testing by Testing Type, 2020-2030 (US$ Million)
- 9.4 Veterinary Testing
- 9.4.1 Overview
- 9.4.2 Veterinary Testing: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
10. North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 - by End User
- 10.1 Overview
- 10.2 North America Infectious Disease Diagnostics Market Revenue Share, by End User, 2022 & 2030 (%)
- 10.3 Diagnostic Laboratories
- 10.3.1 Overview
- 10.3.2 Diagnostic Laboratories: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- 10.4 Hospitals and Clinics
- 10.4.1 Overview
- 10.4.2 Hospitals and Clinics: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- 10.5 Research Institutes
- 10.5.1 Overview
- 10.5.2 Research Institutes: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- 10.6 Homecare Settings
- 10.6.1 Overview
- 10.6.2 Homecare Settings: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
- 10.7 Others
- 10.7.1 Overview
- 10.7.2 Others: North America Infectious Disease Diagnostics Market - Revenue and Forecast to 2030 (US$ Million)
11. North America Infectious Disease Diagnostics Market - Country Analysis
- 11.1 North America Infectious Disease Diagnostics Market - Country Analysis
- 11.1.1 North America Infectious Disease Diagnostics Market, by Country
- 11.1.1.1 US
- 11.1.1.1.1 Overview
- 11.1.1.1.2 US: North America Infectious Disease Diagnostics Market Revenue and Forecast to 2030 (US$ Mn)
- 11.1.1.1.3 US: North America Infectious Disease Diagnostics Market, by Product, 2020-2030 (US$ Million)
- 11.1.1.1.4 US: North America Infectious Disease Diagnostics Market, by Technology, 2020-2030 (US$ Million)
- 11.1.1.1.4.1 US: North America Infectious Disease Diagnostics Market, for Immunodiagnostics by Technology, 2020-2030 (US$ Million)
- 11.1.1.1.4.2 US: North America Infectious Disease Diagnostics Market, for Molecular-Based Diagnostic Techniques by Technology, 2020-2030 (US$ Million)
- 11.1.1.1.5 US: North America Infectious Disease Diagnostics Market, by Application Type, 2020-2030 (US$ Million)
- 11.1.1.1.6 US: North America Infectious Disease Diagnostics Market, by Testing Type, 2020-2030 (US$ Million)
- 11.1.1.1.6.1 US: North America Infectious Disease Diagnostics Market, for Human Testing by Testing Type, 2020-2030 (US$ Million)
- 11.1.1.1.7 US: North America Infectious Disease Diagnostics Market, by End User, 2020-2030 (US$ Million)
- 11.1.1.2 Canada
- 11.1.1.2.1 Overview
- 11.1.1.2.2 Canada: North America Infectious Disease Diagnostics Market Revenue and Forecast to 2030 (US$ Mn)
- 11.1.1.2.3 Canada: North America Infectious Disease Diagnostics Market, by Product, 2020-2030 (US$ Million)
- 11.1.1.2.4 Canada: North America Infectious Disease Diagnostics Market, by Technology, 2020-2030 (US$ Million)
- 11.1.1.2.4.1 Canada: North America Infectious Disease Diagnostics Market, for Immunodiagnostics by Technology, 2020-2030 (US$ Million)
- 11.1.1.2.4.2 Canada: North America Infectious Disease Diagnostics Market, for Molecular-Based Diagnostic Techniques by Technology, 2020-2030 (US$ Million)
- 11.1.1.2.5 Canada: North America Infectious Disease Diagnostics Market, by Application Type, 2020-2030 (US$ Million)
- 11.1.1.2.6 Canada: North America Infectious Disease Diagnostics Market, by Testing Type, 2020-2030 (US$ Million)
- 11.1.1.2.6.1 Canada: North America Infectious Disease Diagnostics Market, for Human Testing by Testing Type, 2020-2030 (US$ Million)
- 11.1.1.2.7 Canada: North America Infectious Disease Diagnostics Market, by End User, 2020-2030 (US$ Million)
- 11.1.1.3 Mexico
- 11.1.1.3.1 Overview
- 11.1.1.3.2 Mexico: North America Infectious Disease Diagnostics Market Revenue and Forecast to 2030 (US$ Mn)
- 11.1.1.3.3 Mexico: North America Infectious Disease Diagnostics Market, by Product, 2020-2030 (US$ Million)
- 11.1.1.3.4 Mexico: North America Infectious Disease Diagnostics Market, by Technology, 2020-2030 (US$ Million)
- 11.1.1.3.4.1 Mexico: North America Infectious Disease Diagnostics Market, for Immunodiagnostics by Technology, 2020-2030 (US$ Million)
- 11.1.1.3.4.2 Mexico: North America Infectious Disease Diagnostics Market, for Molecular-Based Diagnostic Techniques by Technology, 2020-2030 (US$ Million)
- 11.1.1.3.5 Mexico: North America Infectious Disease Diagnostics Market, by Application Type, 2020-2030 (US$ Million)
- 11.1.1.3.6 Mexico: North America Infectious Disease Diagnostics Market, by Testing Type, 2020-2030 (US$ Million)
- 11.1.1.3.6.1 Mexico: North America Infectious Disease Diagnostics Market, for Human Testing by Testing Type, 2020-2030 (US$ Million)
- 11.1.1.3.7 Mexico: North America Infectious Disease Diagnostics Market, by End User, 2020-2030 (US$ Million)
- 11.1.1.1 US
- 11.1.1 North America Infectious Disease Diagnostics Market, by Country
12. Industry Landscape
- 12.1 Overview
- 12.2 Growth Strategies Done by the Companies in the Market, (%)
- 12.3 Organic Developments
- 12.3.1 Overview
- 12.4 Inorganic Developments
- 12.4.1 Overview
13. Company Profiles
- 13.1 Abbott Laboratories
- 13.1.1 Key Facts
- 13.1.2 Business Description
- 13.1.3 Products and Services
- 13.1.4 Financial Overview
- 13.1.5 SWOT Analysis
- 13.1.6 Key Developments
- 13.2 Bruker Corp
- 13.2.1 Key Facts
- 13.2.2 Business Description
- 13.2.3 Products and Services
- 13.2.4 Financial Overview
- 13.2.5 SWOT Analysis
- 13.2.6 Key Developments
- 13.3 Cardinal Health Inc
- 13.3.1 Key Facts
- 13.3.2 Business Description
- 13.3.3 Products and Services
- 13.3.4 Financial Overview
- 13.3.5 SWOT Analysis
- 13.3.6 Key Developments
- 13.4 F. Hoffmann-La Roche Ltd
- 13.4.1 Key Facts
- 13.4.2 Business Description
- 13.4.3 Products and Services
- 13.4.4 Financial Overview
- 13.4.5 SWOT Analysis
- 13.4.6 Key Developments
- 13.5 Trinity Biotech Plc
- 13.5.1 Key Facts
- 13.5.2 Business Description
- 13.5.3 Products and Services
- 13.5.4 Financial Overview
- 13.5.5 SWOT Analysis
- 13.5.6 Key Developments
- 13.6 Danaher Corp
- 13.6.1 Key Facts
- 13.6.2 Business Description
- 13.6.3 Products and Services
- 13.6.4 Financial Overview
- 13.6.5 SWOT Analysis
- 13.6.6 Key Developments
- 13.7 Bio-Rad Laboratories Inc
- 13.7.1 Key Facts
- 13.7.2 Business Description
- 13.7.3 Products and Services
- 13.7.4 Financial Overview
- 13.7.5 SWOT Analysis
- 13.7.6 Key Developments
- 13.8 ACON Laboratories Inc
- 13.8.1 Key Facts
- 13.8.2 Business Description
- 13.8.3 Products and Services
- 13.8.4 Financial Overview
- 13.8.5 SWOT Analysis
- 13.8.6 Key Developments
- 13.9 DiaSorin SpA
- 13.9.1 Key Facts
- 13.9.2 Business Description
- 13.9.3 Products and Services
- 13.9.4 Financial Overview
- 13.9.5 SWOT Analysis
- 13.9.6 Key Developments
14. Appendix
- 14.1 About Us
- 14.2 Glossary of Terms